Abstract
A cross-sectional study was conducted to determine the seroprevalence and the risk factors associated with C. burnetii infection in cattle in the state of Setif in northeastern Algeria from March 2016 to April 2018. A total of 678 cows animals aged at least 24 months and belonging to 90 herds were randomly selected. A serum sample from each cow was tested for antibodies against C. burnetii using an indirect enzyme-linked immunosorbent assay (ELISA). A structured questionnaire focusing on risk factors for C. burnetii infection was administered to farm owners involved in the study. The individual animal prevalence was 11.36% (77/678) (95%CI 8.97–13.75%), the herd prevalence was 45.56% (41/90) (95%CI 35.27–55.84%), and the within-herd prevalence ranged from 9.09 to 57.14% (mean 23.71%; Q1 11.11%, Q2 or median 20%, Q3 30%). Multivariable logistic regression analysis revealed that contact with other herds (odds ratio (OR) 1.95, 95 CI 1.12–3.42) and purchased animals (OR 2.05, 95 CI 1.14–3.68) was identified as risk factors for seropositivity to C. burnetii, while the use of disinfectants (OR 0.32, 95 CI 0.14–0.72) was identified as protective factor. The results from the present study indicate that C. burnetii is circulating into cattle herds in the region of Setif in Northeastern of Algeria. It is recommended to implement good hygienic practices and measures of biosecurity to reduce the spread of infection between cattle herds and possible exposure of humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Q fever in humans or coxiellosis in animals is a ubiquitous worldwide zoonosis with the exception of New Zealand. The causal agent is Coxiella burnetii, which is a Gram-negative obligate intracellular bacterium, belonging to Coxiellaceae family, order Legionellales of the gamma subdivision of Proteobacteria (Bielawska-Drózd et al. 2013).
C. burnetii can infect a wide range of animals, including mammalian and non-mammalian animals (Parker et al. 2006). Domestic ruminants are recognized as the primary reservoirs of C. burnetii for human infection (Kirkan et al. 2008; Roest et al. 2011; Alvarez et al. 2012), which shed the bacteria mainly with birth products, vaginal discharges, urine, milk, and feces (Guatteo et al. 2006, 2007; Rousset et al. 2009; Angelakis and Raoult 2010; EFSA 2010). C. burnetii transmits mainly to humans or animals through inhalation of infected aerosols or dust, while its oral transmission remains controversial (Porter et al. 2011). Furthermore, ticks play a role in the maintenance of C. burnetii infection among wildlife and in the transmission of C. burnetii from wildlife to domestic ruminants (EFSA 2010). However, its role in transmission of Q fever to humans is rarely documented (Porter et al. 2011). The infection is mostly asymptomatic in ruminants. However, during clinical expression, it is mainly manifested by reproductive disorders including abortion, stillbirth, premature delivery, and delivery of weak offspring, particularly in small ruminants, as well as, infertility, metritis, and mastitis in cattle (Agerholm 2013; Porter et al. 2011). In humans, Q fever can be asymptomatic, as an acute form with fever, atypical pneumonia, and hepatitis, or it can progress to chronic form with long-term sequelae including fatigue, abortion, and heart disease (Vanderburg et al. 2014; Wielders et al. 2014).
Several surveys have been performed in many countries to evaluate the prevalence of C. burnetii in cattle, which ranges from 0 to 100% for animal level and from 4.4 to 100% for herd level (Guatteo et al. 2011). Limited serological studies on bovine coxiellosis were carried out in different Algerian regions targeting a small number of cows and adopting different sampling strategies (Dechicha et al. 2010; Abdelhadi et al. 2015; Agag et al. 2017; Derdour et al. 2017). To date, no epidemiological survey has targeted the Setif region in Algeria. However, Lacheheb and Raoult (2009) showed a high seroprevalence among the human inhabitants of Setif (15.5%) with a significantly higher seroprevalence among inhabitants of rural areas (20%). Therefore, the main objectives of this study are to estimate the apparent seroprevalence of C. burnetii infection in cows at herd and animal levels and to identify risk factors associated with C. burnetii seropositivity in the Setif region of Algeria.
Material and methods
Study area
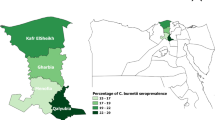
The present study was conducted from March 2016 to April 2018 in the Setif high plains in northeastern Algeria. The region covers about 6550 km2, lies between eastern longitudes of 4° 73′–6° 02′ and northern latitudes of 35° 61′–36° 59′, and has an altitude that vary between 900 and 2000 m above sea level. The climate is semi-arid Mediterranean, characterized by cold rainy winters and hot dry summers. The temperatures often exceed 40 °C in summer and fall below 0 °C in winter, with frequent snowfall and frequent frost. The mean annual rainfall was of 350 mm from 1984 to 2014. The study area contains about 161,952 cattle, of which 79,354 are dairy cows, distributed across 4465 dairy herds (Agricultural Services direction of Setif 2015) (Fig. 1).
Study design and sampling
The study was designed as cross-sectional targeting a convenient sample of cows aged 24 months old and over selected by simple random sampling method from a cattle population that exists in the Setif area. Firstly, to estimate the number of sampled animals, we used the formula for simple random samples recommended by Thrusfield (2007):
where N was the sample size, 1.96 was the Z value for the selected confidence level (95%), P was the individual disease prevalence, and L is the desired absolute precision. A minimum sample size of 600 animals was obtained using 50% expected individual prevalence (since there was no previous study in this area), an absolute precision of 4%, and a confidence level of 95%. However, a total of 678 animals were included in this study to increase the precision.
Secondly, to determine the minimal number of cows to be selected within each dairy herd, we adopted the formula described by Thrusfield (2007):
where “n” is the sample size, “p” is the probability of detection of at least one seropositive cow, “N” is the herd size, and “d” is the number of seropositive cows in the herd. The probability of detection of at least one seropositive cow in a herd was determined at 95% (P = 0.95), and the number of seropositive cows in each herd “d” was calculated assuming within-herd prevalence of 25% (Carbonero et al. 2015; Guatteo et al. 2011). For this purpose, a minimum sample size of 11 animals per herd was used. On farms with a herd size up to 11 animals, all animals were included. Finally, a total of 678 female cows from 90 herds were randomly selected. The herds and animals within herds were randomly selected using the RAND function of Microsoft Excel® 2013. In the case, where the owner of the selected herd refused to participate, we looked for the closest neighbor herd. The blood samples were collected from the coccygeal vein of each cow into plain vacutainer tubes using disposable needles, and immediately transported on ice to the laboratory. The sera were separated by centrifuging the tubes at 1000×g for 10 min and stored at − 20 °C until use.
Data collection
A structured questionnaire focusing on risk factors for C. burnetii infection was administered to farm owners on the day of sample collection. The questionnaire was divided into two parts:
-
1.
The first part involved farm characteristics and herd management: management system (intensive or semi-intensive), herd size (≤ 11 cows or > 11 cows), breeding type (dairy or mixed), use of disinfectants (yes or no), contact with other herds (yes or no), source of water (well water or groundwater or tap water), presence of ticks (yes or no), presence of small ruminants (yes or no), presence of horses (yes or no), presence of dogs (yes or no), presence of cats (yes or no), type of reproduction (only natural or only artificial insemination or both types), use of calving pens (yes or no) and milking (mechanic or manual).
-
2.
The second part involved individual characteristics and reproduction disorders collected from each cow such as breed (imported breeds; Prim’holstein, Montbeliarde, and Fleckvieh, or local breeds mainly brown of atlas, or crossed breeds between imported and local breeds), age in years (> 2 to ≤ 5 or > 5 to ≤ 8 or > 8), animal origin (homebred or purchased), history of abortion in previous year (yes or no), history of stillbirths in previous year (yes or no), and history of infertility in previous year (yes or no).
Laboratory analysis
Serological analysis
Determination of antibodies against phase I and phase II antigens of C. burnetii from each serum sample was screened by employing a commercial ELISA “ID Screen Q Fever Indirect Multi-species Kit” (IDvet, Grabels, France) following the protocol prescribed by the manufacturer. This test uses native antigens isolated from an aborted bovine placenta and purified from culture of phases I and II C. burnetii. The manufacturer’s internal validation report indicates a specificity of 100% based on the negative serological results obtained by this assay on 167 bovine serum from free Breton herds (no abortion was recorded for 3 years, and no positive result was obtained, either by ELISA or fixation complement over the last 3 years), and a 100% sensitivity based on positive serological results on 52 sera of aborted cows and positive for C. burnetii by complement fixation or by PCR on placenta. The optical density percent (% OD) was calculated according to the formula:
Samples with a % OD greater than 50% were considered positive; % OD between 40 and 50% were considered as doubtful, and those less than 40% were determined to be negative. Doubtful results were considered negative in this study.
Statistical analysis
The apparent prevalence (AP) of antibodies to C. burnetii at individual level was estimated from the ratio of seropositive cows to the total number of cows examined. Prevalence of positive herds was estimated from the ratio of positive herds to the total number of herds investigated; herds that contain at least one seropositive cow were considered positive, with the exact binomial CI of 95% (Thrusfield 2007). Analysis of risk factors potentially associated with C. burnetii seropositivity was evaluated in two steps. Firstly, we conducted a univariable analysis of each variable using a chi-square test and those variables that presented P ≤ 0.25 were subjected to multivariable logistic regression analysis. The multivariable analysis was then performed using backward stepwise selection using a likelihood ratio test at each step with a significance level of 0.05 for entry and 0.1 for removal. All variable with a P < 0.05 was considered statistically significant. The fit of the model was assessed using the Hosmer and Lemeshow goodness-of-fit (Hosmer and Lemeshow 2000). Spearman’s correlation test was used to check a correlation among the independent variables, and if higher collinearity (correlation coefficient > 0.9) was found between those variables, one of them was excluded from the multivariable analysis according to the biological plausibility (Dohoo et al. 1996). A variable is considered as a confounding factor if its removal changed the regression coefficient of the other variables by more than 25%. Finally, all pairwise interactions were tested for significance (P ≤ 0.05). The statistical analysis was performed using SPSS v25.0 software (SPSS Inc., Chicago, IL, USA).
Results
Seroprevalence of Coxiella burnetii
Out of the 678 cows tested, 77 were found positive for C. burnetii phase I and phase II antigens antibodies with an individual seroprevalence of 11.36% (95%CI 8.97–13.75%). At the herd level, 41 of 90 selected cattle herds had at least one seropositive cow to C. burnetii infection, giving a herd seroprevelence of 45.56% (95%CI 35.27–55.84%). Regarding the within-herd prevalence, the prevalence of seropositive cows per herd ranged from 9.09 to 57.14% (mean 23.71%, Q1 11.11%; median 20%, Q3 30%).
Risk factor analysis
Regarding the risk factor analysis, the six factors herd size, contact with other herds, presence of small ruminants in farm, use of disinfectants, origin of cows, and history of infertility in previous year were all significant on the univariable analyses (P < 0.25) and were selected for multivariable logistic regression analysis (Table 1). When these independent variables were subjected to the multivariable analysis, contact with other herds (OR 1.95, 95 CI 1.12–3.42) and purchased animals (OR 2.05, 95 CI 1.14–3.68) were identified as risk factors for seropositivity to C. burnetii, while the use of disinfectants (OR 0.32, 95 CI 0.14–0.72) was identified as a protective factor (Table 2). The final model had a good fit (Hosmer and Lemeshow test: χ2 = 5.006; P = 0.287).
Discussion
Our work is the first study conducted with an appropriate sampling design to determine the individual and cattle herd prevalence, as well as, risk factors for the infection of C. burnetii in the state of Setif in the northeastern of Algeria. In this study, we chose the ELISA test instead of other serological tests to detect reactive antibodies to C. burnetii in serum samples for its higher sensitivity and for practical reasons because it is rapid, inexpensive, easy to perform in laboratories, and has higher throughout (OIE 2018). ELISA is a method of indirect diagnosis that highlights a past exposure to C. burnetii by the detection of their specific antibodies; therefore, a positive result does not confirm an active infection because this requires the use of direct diagnostic methods such as ELISA antigen or PCR (Muskens et al. 2011; Alvarez et al. 2012). The sensitivity and specificity of the commercial kit ELISA used are 100% (Seo et al. 2017; IDvet, internal validation report), which indicates an identical value of apparent and true seroprevalence. Since the vaccination against C. burnetii is not practiced in Algeria, the results of this serological study are a response to the natural infection.
The individual prevalence of 11.36% obtained in this study is similar to the 10.6% reported in Bejaïa state northern of the study area (Agag et al. 2017), but lower than a value of 23.91% reported in the region of Tiaret located in western Algeria (Abdelhadi et al. 2015), and 29% observed in one farm that suffered an abortion problem, located in the state of Blida in the center of the country (Dechicha et al. 2010). On the other hand, our seroprevalence was higher than that found in control case study between infectious causes of abortion seropositivity and cow abortion in Algiers, capital of Algeria, 1.66% (Derdour et al. 2017). This difference in prevalence between these regions might be partially attributed to the sampling strategies that are different.
Bovine coxiellosis has been reported in many countries with different prevalence rates (Guatteo et al. 2011). Compared with other serological investigations carried out in some African and Mediterranean countries, our individual seroprevalence seems to be lower than 14.5% in Nigeria (Tukur et al. 2014), 16.21% in Tunisia (Elandalousi et al. 2015), 16.3% in the East of Turkey (Ceylan et al. 2009), 14.4% in Italy (Capuano et al. 2001), 19.3% in Egypt (Klemmer et al. 2018), 29.92% in Sudan (Hussien et al. 2017), and 31.3% in Cameroon (Scolamacchia et al. 2010). Nevertheless, it was comparable with 10.5% in Kenya (Wardrop et al. 2016). Our study showed a higher seroprevalence than those observed 6.8% in another study in Nigeria (Adamu et al., 2018), 4% in Chad (Schelling et al. 2003), 3.6% in Senegal (Kamga-Waladjo et al. 2010), and 6.76% in Spain (Alvarez et al. 2012). This variation in prevalence rates between regions and countries may be linked to several factors such as local ecological factors and type of management which may influence the transmission of C. burnetii (Hussien et al. 2017).
This study concluded that 45.56% of herds had at least one seropositive animal. This result is higher than 22% in the state of Bejaïa (Agag et al. 2017), which demonstrates the widespread of C. burnetii among herds in the examined area. Several studies reported considerable variation in the seroprevalence of C. burnetii in herd cattle such as Spain (30%) (Alvarez et al. 2012), Nigeria (57.1%) (Tukur et al. 2014), Cameroon (68.1%) (Scolamacchia et al. 2010), and Italy (68%) (Capuano et al. 2001).
The within-herd prevalence obtained in the current work that ranged from 9.09 to 57.14% with mean of 23.71% (Q1 11.11%, median 20%, Q3 30%) is close to the mean values estimated from many studies in the whole world by Guatteo et al. (2011) (median 26.3%, Q1 21.8%, Q3 38.2%).
In the risk factor analysis, a positive association exists between seropositivity of C. burnetii and contact with other herds through the sharing of the same grazing fields and or the same source of water (P < 0.01). This can be explained on the one hand by the facts that contact with other herds increases the chance of meeting with infected cattle favoring the direct transmission of C. burnetii between animals, and on the other hand, by the contamination of the grazing and watering environment. Especially this bacterium is characterized by a very high stability towards environmental conditions and can stay infectious for many months (Gürtler et al. 2014). The environment can be contaminated either by abortion and birth products, feces, urine, milk, and vaginal mucus from infected animals at the time of grazing or watering (Guatteo et al. 2006, 2007; Angelakis and Raoult 2010; EFSA 2010; Astobiza et al. 2011) or by dissemination of C. burnetii from contaminated farms through soil, animal skin, and wastewater (Kersh et al. 2013; Villari et al. 2018), as well as by wind (Nusinovici et al. 2015).
In this study, the purchased cows were also identified as a risk factor for C. burnetii infection. The seroprevalence of purchased cows (20.71%) was significantly higher than for cows whose origin was the farm (8.92%) (P < 0.05). This is in agreement with the study of Obaidat and Kersh (2017), who reported a significant association between the addition of new cattle to the herd and C. burnetii antibody positivity in bulk milk tank (BTM) of Jordanian dairy cattle herds (Obaidat and Kersh 2017), and the study of van Engelen et al. (2014), who showed that the purchase of cattle from at least two addresses in 2009 in the Netherlands was significantly correlated with the presence of both C. burnetii antibodies and DNA in BTM of dairy cattle herds (van Engelen et al. 2014). Furthermore, it has been revealed that the lack of quarantine of newly purchased animals is a factor that increased the risk of C. burnetii seropositivity for dairy cows in Denmark (Paul et al. 2012). This emphasizes the importance of taking biosecurity measures like quarantine and screening of newly purchased animals to prevent the introduction of infected animals into the herds.
In fact, both risk factors identified in this study whether the contact between herds or the introduction of new cows in the herd support the spread of infection from one herd to the other, which explains the high herd seroprevalence obtained in this study (45.56%).
However, the use of disinfectants was identified as the factors that protect against bovine coxiellosis. A similar result was reported recently in domestic ruminants in Lebanon (Dabaja et al. 2019). In addition, it was revealed that the prevalence of C. burnetii antibodies in cattle decreases in farms where the cleaning and disinfection of equipment after use (Tukur et al. 2014), the cleaning of the bedding in the cubicles at least once per day (van Engelen et al. 2014), and the frequent cleaning of the feeders (Obaidat and Kersh 2017) were realized, hence, the interest of good hygiene practices in the reduction of exposure to C. burnetii in livestock. C. burnetii or more precisely its infectious form small cell variant (SCV) is known to be resistant to environmental factors and chemical disinfectants (Cantas et al. 2011; Pexara et al. 2018). However, it is completely inactivated following exposure to Quaternary ammonium or 70% ethanol during 30 min contact time (Plummer et al. 2018). It has been revealed also that exposure to 1% Peroxygen or 1:100 dilution of hypchlorite during 30 min contact time reduced more than 90% of infectivity (Plummer et al. 2018). The disinfectant can destroy a wide range of pathogens and minimize the risk of infection in cattle; therefore, it indirectly helps the immune system of animals to fight against pathogens resistant to disinfectants like C. burnetii, in particular, the destruction of pathogens with immunosuppressive effects such as bovine herpesvirus-1 (BHV-1) and bovine viral diarrhea virus (BVDV) that predispose cattle to secondary infections (Potgieter 1995; Srikumaran et al. 2007; Biswas et al. 2013; Molina et al. 2013; Lanyon et al. 2014).
In conclusion, the results from the current study indicate the presence and circulation of C. burnetii infection in cattle herd in Setif state of Northeastern Algeria. Consequently, some hygiene and biosecurity measures must be implemented mainly focusing on risk factors identified in this work, such as limiting contact between herds, quarantine of newly purchased animals, and the use of disinfectants that can reduce the spread of infection and possible transmission to humans. Finally, more epidemiological surveys in animals and human are needed to better understand and control of this disease in Algeria.
References
Abdelhadi, F.Z., Abdelhadi, S.A., Niar, A., Benallou, B., Meliani, S., Smail, N.L., Mahmoud, D., 2015. Abortions in Cattle on the Level of Tiaret Area ( Algeria ). Global Veterinaria, 14, 638–645.
Adamu, S.G., Kabir, J., Umoh, J.U., Raji, M.A., 2018. Seroprevalence of brucellosis and Q fever (Coxiellosis) in cattle herds in Maigana and Birnin Gwari agro-ecological zone of Kaduna State, Nigeria. Tropical Animal Health and Production, 50, 1583–1589.
Agag, S., Kaidi, R., Khelef, D., 2017. Séroprévalence de la fièvre Q chez les bovins de la région de Bejaïa (Algérie). Revue d’élevage et de médecine vétérinaire des pays tropicaux, 69, 155–159.
Agerholm, J.S., 2013. Coxiella burnetii associated reproductive disorders in domestic animals-a critical review. Acta Veterinaria Scandinavica, 55, 13.
Alvarez, J., Perez, A., Mardones, F.O., Pérez-Sancho, M., García-Seco, T., Pagés, E., Mirat, F., Díaz, R., Carpintero, J., Domínguez, L., 2012. Epidemiological factors associated with the exposure of cattle to Coxiella burnetii in the Madrid region of Spain. Veterinary Journal, 194, 102–107.
Angelakis, E., Raoult, D., 2010. Q fever. Veterinary Microbiology, 140, 297–309.
Astobiza, I., Barandika, J. F., Ruiz-Fons, F., Hurtado, A., Povedano, I., Juste, R. A., & García-Pérez, A. L. 2011. Coxiella burnetii shedding and environmental contamination at lambing in two highly naturally-infected dairy sheep flocks after vaccination. Research in veterinary science, 91, e58-e63.
Bielawska-Drózd, A., Cieślik, P., Mirski, T., Bartoszcze, M., Knap, J.P., Gaweł, J., Zakowska, D., 2013. Q fever - selected issues. Annals of Agricultural and Environmental Medicine, 20, 222–232.
Biswas, S., Bandyopadhyay, S., Dimri, U., H. Patra, P., 2013. Bovine herpesvirus-1 (BHV-1) - a re-emerging concern in livestock: a revisit to its biology, epidemiology, diagnosis, and prophylaxis. Veterinary Quarterly, 33, 68–81.
Cantas, H., Muwonge, A., Sareyyupoglu, B., Yardimci, H., Skjerve, E., 2011. Q fever abortions in ruminants and associated on-farm risk factors in northern Cyprus. BMC Veterinary Research, 7, 13.
Capuano, F., Landolfi, M.C., Monetti, D.M., 2001. Influence of three types of farm management on the seroprevalence of Q fever as assessed by an indirect immunofluorescence assay. Veterinary Record, 149, 669–671.
Carbonero, A., Guzmán, L.T., Montaño, K., Torralbo, A., Arenas-Montes, A., Saa, L.R., 2015. Coxiella burnetii seroprevalence and associated risk factors in dairy and mixed cattle farms from Ecuador. Preventive Veterinary Medecine, 118, 427–435.
Ceylan, E., Berktas, M., Keles, I., Agaoglu, Z., 2009. Seroprevalence of Q Fever in Cattle and Sheep in the East of Turkey. Asian Journal of Animal and Veterinary Advances (AJAVA), 4, 114–121.
Dabaja, M.F., Greco, G., Villari, S., Vesco, G., Bayan, A., Bazzal, B. El, Ibrahim, E., Gargano, V., Sciacca, C., Lelli, R., Ezzedine, M., Mortada, H., Tempesta, M., Mortada, M., 2019. Occurrence and risk factors of Coxiella burnetii in domestic ruminants in Lebanon. Comparative Immunology, Microbiology and Infectious Diseases, 64, 109–116
Dohoo, I.R., Ducroc, C., Fourichon, C., Donald, A., Hurnik, D., 1996. An overview of techniques for dealing with large numbers of independent variables in epidemiologic studies. Preventive Veterinary Medecine, 29, 221–239
Dechicha, A., Gharbi, S., Kebbal, S., Chatagnon, G., Tainturier, D., Ouzrout, R., Guetarni, D., 2010. Serological survey of etiological agents associated with abortion in two Algerian dairy cattle breeding farms. Journal of Veterinary Medecine and Animal Health, 2, 001–005.
Derdour, S.-Y., Hafsi, F., Azzag, N., Tennah, S., Laamari, A., China, B., Ghalmi, F., 2017. Prevalence of the main infectious causes of abortion in dairy cattle in Algeria. Journal of Veterinary Research. 61,337–343.
EFSA, Panel on Animal Health and Welfare (AHAW), 2010. Scientific Opinion on Q fever., EFSA Journal, 8, 1–114. http://www.efsa.europa.eu/en/efsajournal/pub/1595. (Accessed 15 sep 2018)
Elandalousi, R.B., Ghram, A., Maaroufi, A., Mnif, W., 2015. Séroprévalence des maladies abortives zoonotiques chez les ruminants au nord de la Tunisie. Research fr, 2, 1419.
Guatteo, R., Beaudeau, F., Berri, M., Rodolakis, A., Joly, A., Seegers, H., 2006. Shedding routes of Coxiella burnetii in dairy cows: implications for detection and control. Veterinary Research, 37, 827–833.
Guatteo, R., Beaudeau, F., Joly, A., Seegers, H., 2007. Coxiella burnetii shedding by dairy cows. Veterinary Research, 38, 849–860.
Guatteo, R., Seegers, H., Taurel, A.F., Joly, A., Beaudeau, F., 2011. Prevalence of Coxiella burnetii infection in domestic ruminants: A critical review. Veterinary Microbiology, 149, 1–16.
Gürtler. L., Bauerfeind, U., Blümel, J., Burger, R., Drosten, C., Gröner, A., Heiden M, Hildebrandt M., Jansen B., Offergeld R., Pauli G., Seitz R., Schlenkrich U., Schottstedt V., Strobel, J., Willkommen, H., 2014. Coxiella burnetii - Pathogenic Agent of Q (Query) Fever. transfusion medicine hemotherapy, 41,60–72
Hosmer, D.W., Lemeshow, S., 2000. Applied logistic regression, (John Wiley & Sons, New York) 375.
Hussien, M.O., Enan, K.A., Alfaki, S.H., Gafar, R.A., Taha, K.M., Rahim, A., El, M., 2017. Seroprevalence of Coxiella burnetii in Dairy Cattle and Camel in Sudan. International Journal of Infection, 4, e42945.
Kamga-Waladjo, A.R., Gbati, O.B., Kone, P., Lapo, R.A., Chatagnon, G., Bakou, S.N., Pangui, L.J., Diop, P.E.H., Akakpo, J.A., Tainturier, D., 2010. Seroprevalence of Neospora caninum antibodies and its consequences for reproductive parameters in dairy cows from Dakar-Senegal, West Africa. Tropical Animal Health and Production, 42, 953–959.
Kersh, G.J., Fitzpatrick, K.A., Self, J.S., Priestley, R.A., Kelly, A.J., Ryan Lash, R., Marsden-Haug, N., Nett, R.J., Bjork, A., Massung, R.F., Andersona, A.D., 2013. Presence and Persistence of Coxiella burnetii in the environments of goat farms associated with a Q fever outbreak. Applied and Environmental Microbiology, 79, 1697–1703
Kirkan, Ş., Kaya, O., Tekbiyik, S., Parin, U., 2008. Detection of Coxiella burnetii in cattle by PCR. Turkish Journal of Veterinary and Animal Science, 32, 215–220.
Klemmer, J., Njeru, J., Emam, A., El-Sayed, A., Moawad, A.A., Henning, K., Elbeskawy, M.A., Sauter-Louis, C., Straubinger, R.K., Neubauer, H., El-Diasty, M.M., 2018. Q fever in Egypt: Epidemiological survey of Coxiella burnetii specific antibodies in cattle, buffaloes, sheep, goats and camels. PLoS One. 13, e0192188.
Lacheheb, A., Raoult, D., 2009. Seroprevalence of Q- fever in Algeria. Clinical Microbiology and Infection, 15, 167–168
Lanyon, S.R., Hill, F.I., Reichel, M.P., Brownlie, J., 2014. Bovine viral diarrhoea: Pathogenesis and diagnosis. Veterinary Journal, 199, 201–209.
Molina, V., Risalde, M.A., Sánchez-Cordón, P.J., Pedrera, M., Romero-Palomo, F., Luzzago, C., Gómez-Villamandos, J.C., 2013. Effect of infection with BHV-1 on peripheral blood leukocytes and lymphocyte subpopulations in calves with subclinical BVD. Research in Veterinary Science, 95, 115–122.
Muskens, J., Van Engelen, E., Van Maanen, C., Bartels, C., Lam, T.J.G.M., 2011. Paper: Prevalence of Coxiella burnetii infection in Dutch dairy herds based on testing bulk tank milk and individual samples by PCR and ELISA. Veterinary Record, 168, 79.
Nusinovici, S., Hoch, T., Brahim, M.L., Joly, A., Beaudeau, F., 2015. The Effect of Wind on Coxiella burnetii Transmission Between Cattle Herds: a Mechanistic Approach. transboundary and emerging diseases, 64, 585–592.
Obaidat, M.M., Kersh, G.J., 2017. Prevalence and Risk Factors of Coxiella burnetii Antibodies in Bulk Milk from Cattle, Sheep, and Goats in Jordan. Journal of Food Protection, 80, 561–566.
World Organization for Animal Health (OIE), 2018. Chapter 2.1.16. Q fever. in Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. OIE, Paris. http://www.oie.int/fileadmin/Home/fr/Health_standards/tahm/2.01.16_Q_FEVER .pdf Accessed 18 September 2018.
Parker, N.R., Barralet, J.H., Bell, A.M., 2006. Seminar Q fever. Lancet, 367, 679–88.
Paul, S., Agger, J. F., Markussen, B., Christoffersen, A. B., Agerholm, J. S., 2012. Factors associated with Coxiella burnetii antibody positivity in Danish dairy cows. Preventive Veterinary Medecine.107, 57–64.
Pexara, A., Solomakos, N., Govaris, A., 2018. Q fever and prevalence of Coxiella burnetii in milk. Trends in Food Science and Technology, 71, 65–72.
Plummer, P.J., McClure, J.T., Menzies, P., Morley, P.S., Van den Brom, R., Van Metre, D.C., 2018. Management of Coxiella burnetii infection in livestock populations and the associated zoonotic risk: A consensus statement. Journal of Veterinary Internal Medicine, 32, 1481–1494.
Porter, S.R., Czaplicki, G., Mainil, J., Guattéo, R., Saegerman, C., 2011. Q fever: Current state of knowledge and perspectives of research of a neglected zoonosis. International Journal of Microbiology, 2011.
Potgieter, L.N., 1995. Immunology of bovine viral diarrhea virus. The Veterinary clinics of North America. Food animal practice, 11, 501–520.
Roest, H.I.J., Tilburg, J.J.H.C., Van Der Hoek, W., Vellema, P., Van Zijderveld, F.G., Klaassen, C.H.W., Raoult, D., 2011. The Q fever epidemic in the Netherlands: History, onset, response and reflection. Epidemiology and Infection, 139, 1–12.
Rousset, E., Berri, M., Durand, B., Dufour, P., Prigent, M., Delcroix, T., Touratier, A., Rodolakis, A., 2009. Coxiella burnetii shedding routes and antibody response after outbreaks of Q fever-induced abortion in dairy goat herds. Applied and Environmental Microbiology, 75, 428–433.
Schelling, E., Diguimbaye, C., Daoud, S., Nicolet, J., Boerlin, P., Tanner, M., Zinsstag, J., 2003. Brucellosis and Q-fever seroprevalences of nomadic pastoralists and their livestock in Chad. Preventive Veterinary Medecine, 61, 279–293.
Scolamacchia, F., Handel, I.G., Fèvre, E.M., Morgan, K.L., Tanya, V.N., Bronsvoort, B.M.D.C., 2010. Serological patterns of brucellosis, leptospirosis and Q fever in Bos indicus cattle in Cameroon. PLoS One, 5, e8623.
Seo, M. G., Ouh, I. O., Lee, S. H., Kim, J. W., Rhee, M. H., Kwon, O. D., , Kim, T.H., Kwak, D., 2017. Prevalence of Coxiella burnetii in cattle at South Korean national breeding stock farms. PloS one, 12, e0177478.
Srikumaran, S., Kelling, C.L., Ambagala, A., 2007. Immune evasion by pathogens of bovine respiratory disease complex. Animal Health Research Reviews, 8, 215–229.
Thrusfield, M. 2007. Veterinary Epidemiology, 3rd edn., Blackwell Science Ltd, Oxford, UK. pp. 230–238.
Tukur, H.B., Ajogi, I., Kabir, J., Umoh, J.U., 2014. Seroprevalence of Coxiella Burnetti in Cattle and Its Risk Factors in Kaduna Metropolis , Kaduna State, Nigeria. IOSR Journal of Agriculture and Veterinary Science (IOSR-JAVS), 7, 1–5.
Van Engelen, E., Schotten, N., Schimmer, B., Hautvast, J. L. A., Van Schaik, G., van Duijnhoven, Y. T. H. P., 2014. Prevalence and risk factors for Coxiella burnetii (Q fever) in Dutch dairy cattle herds based on bulk tank milk testing. Preventive Veterinary Medecine, 117, 103–109.
Vanderburg, S., Rubach, M.P., Halliday, J.E.B., Cleaveland, S., Reddy, E.A., Crump, J.A., 2014. Epidemiology of Coxiella burnetii Infection in Africa: A OneHealth Systematic Review. PLOS Neglected Tropical Diseases, 8, e2787.
Villari, S., Galluzzo, P., Arnone, M., Alfano, M., Geraci, F., Chiarenza, G., 2018. Seroprevalence of Coxiella burnetii infection (Q fever) in sheep farms located in Sicily (Southern Italy) and related risk factors. small ruminant research, 164, 82–86.
Wardrop, N.A., Thomas, L.F., Cook, E.A.J., de Glanville, W.A., Atkinson, P.M., Wamae, C.N., Fèvre, E.M., 2016. The Sero-epidemiology of Coxiella burnetii in Humans and Cattle, Western Kenya: Evidence from a Cross-Sectional Study. PLOS Neglected Tropical Diseases, 10, e0005032.
Wielders, C.C.H., Wuister, A.M.H., de Visser, V.L., de Jager-Leclercq, M.G., Groot, C.A.R., Dijkstra, F., van Gageldonk-Lafeber, A.B., van Leuken, J.P.G., Wever, P.C., van der Hoek, W., 2014. Characteristics of hospitalized acute Q fever patients during a large epidemic, The Netherlands. PLoS One, 9, e91764.
Acknowledgments
We are very grateful to the Zoonosis Laboratory of Istituto Zooprofilattico Sperimentale della Sardegna for funding all the laboratory analyses, and the staff for scientific and technical support. Also, we acknowledge the farmers for their willingness to participate and to provide information about their farms and animals.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
All animal owners declared their oral consent before the collection of the blood samples as well to the related survey questions. The cattle were sampled by a qualified veterinarian following all applicable guidelines for the care and use of animal.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Menadi, S.E., Mura, A., Santucciu, C. et al. Seroprevalence and risk factors of Coxiella burnetii infection in cattle in northeast Algeria. Trop Anim Health Prod 52, 935–942 (2020). https://doi.org/10.1007/s11250-019-02083-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-019-02083-x