Abstract
This cross-sectional study aimed to study animal, farm, and within-farm seroprevalence of C. burnetii and to identify associated risk factors in goat and sheep farm in northern Jordan. Questionnaire was developed to collect information about risk factors and farms management practices. Blood samples from 730, ≥ 1-year-old females (goat n = 250; sheep n = 480) were randomly collected from 20 goat herds and 40 sheep flocks. IDEXX ELISA Kit was used to detect C. burnetii antibodies. The overall goat and sheep seroprevalence level was 32.5% (237/730) and was significantly higher in goats (43.3%, 108/250; 95% CI 37–49.6) than sheep (27%, 129/480; 95% CI 29.1–36.2) (χ2 test, p ≤ 0.001). Eighty percent (16/20) of goat herds and 60% (24/40) of sheep flocks had at least one seropositive animal (p ≥ 0.05). The average within goat herds and sheep flock seroprevalence were 36.4% (ranged: 0–91%) and 23.4% (ranged: 0–82%), respectively. Multivariate logistic regression model revealed that seroprevalence increased 1.79 times in goat herds compared with sheep flocks, 3.2 times more in farms containing ≥ 100 animals, and 1.7 times higher in farms with their animals that were ≥ 2 years of age than in farms with their animals that are < 2 years of age. In addition, seroprevalence significantly increased 1.52 times in farms loaning bucks or rams during breeding season and 1.63 times in farms containing cats on premises (p ≤ 0.05). Farm biosecurity measures are essential to prevent introduction and minimize transmission of C. burnetii infection to humans and animals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Q fever is caused by Coxiella burnetii (C. burnetii), a widely spread zoonotic bacterial disease. It is a Gram-negative, obligate intracellular rickettsial microorganism that can infect a wide range of hosts including ruminants, dogs, cats, birds, arthropods as well as humans (Angelakis and Raoult 2010; Van den Brom et al. 2015; Mori and Roest 2018). Infected cattle and small ruminants are considered to be the primary reservoir of C. burnetii and usually shed the bacteria in feces, urine, colostrum, milk, vaginal excretion, placenta, and uterine fluids (Muskens et al. 2011). Clinical signs in infected cattle, goats, and sheep include late-term abortion, stillbirth, infertility, and low birth weight of newborn animals (Van den Brom et al. 2015).
Q fever has been reported worldwide as an endemic zoonotic disease (Meadows et al. 2015 a,b; Ezatkhah et al. 2015). Outbreaks in humans have been reported in slaughterhouses, ruminant farms, and institutions with intensive sheep research and teaching programs (Graham et al. 1989). Sporadic human Q fever cases have also been reported in individuals helping dogs and cats during parturition (Marrie et al. 1988). Humans typically are infected with Q fever through the inhalation of contaminated particles shed by infected animals or ingestion of unpasteurized milk or dairy products (Rabinowitz and Conti 2009). The disease in humans is typically an acute febrile illness without specific clinical signs in majority of cases. However, hepatitis and atypical pneumonia were reported in about 40% of the cases, while a small percentage (5%) of cases may develop chronic infection and life-threatening valvular endocarditis (Limonard et al. 2010; Mori and Roest 2018).
There is a lack of information about the seroprevalence and possible risk factors contributed to the occurrence of Q fever in goat and sheep farms in Jordan. Therefore, this cross-sectional study aimed to determine the animal, farm, and within-farm seroprevalence of C. burnetii in goat and sheep and to identify associated risk factors with goat and sheep seroprevalence in northern Jordan.
Materials and methods
Study population and farm management
According to the report published by the Jordanian Department of Statistics in 2015 (DOS 2015), there were about 0.86 million goats and 2.57 million sheep in Jordan of which 36 % were present in the study area. The most common goat breed in Jordan is the mixed breed (local breed x Damascus breed), while Awassi is the most common sheep breed. The production system for goat and sheep is mainly of the seminomadic type as animals move out from their raising farms to other parts of Jordan for grazing during early March until August when pastures are mostly available. During fall and winter months, animals are housed and group fed approximately 600–700 g barley, 200–250 g wheat-bran, and 1 kg straw, and per head per day.
Study design and sampling of goat and sheep farms
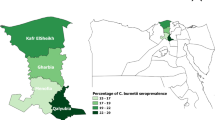
In this cross-sectional study, the outcome variable was classified as either seropositive or seronegative for C. burnetii. The study target population was all goat and sheep farms located in the four governorates (Irbid, Mafraq, Jarash, and Ajloun) in northern Jordan. There were about 8500 registered goat and sheep farms present in the study area (DOS 2015).
A previously published study conducted in the central region of Jordan by Aldomy et al. (1998) revealed an estimate of about 19% and 8% C. burnetii seroprevalence between and within goat and sheep farms, respectively. These estimates were used to determine the number of goat and sheep farms and the number of animals within each selected farm needed for the study.
The number of farms was determined using Martin et al. (1987) formula: n = (1.96)2 PQ/L2, where the 1.96 is 95% confidence level, p is the expected prevalence of positive seroprevalence animals (19%), Q is the expected prevalence of negative seroprevalence animals (81%), and L is the allowable error (required precision) around the true population mean (10%). In order to recruit the necessary number of farms (20 goat herds and 40 sheep flocks), farm owners or managers from 36 goat herds and 65 sheep flocks with at least 30 adult female animals (≥ 1 year old) were contacted and asked to participate in the study.
The number of animals within each selected farm needed for this study was calculated using Martin’s et al. formula: n = [1- (1-α 1/d)] [N – (D – 1)/2], where n is the minimum number of animals required from each farm, α is the probability (confidence level, 90%) of observing at least one diseased animal in the selected sample when the disease affects at least D/N animals in the farm, D is the number of diseased animals in the farm, and N is the total number of animals present in the farm. A total of 250 goats and 480 sheep were randomly selected and sampled. None of the participating goat herds or sheep flocks was vaccinated for C. burnetii.
Questionnaire
A questionnaire was constructed based on recent literature to collect information about risk factors possibly associated with C. burnetii seroprevalence and farm management practices (Schimmer et al. 2011; Van den Brom et al. 2015; Meadows et al. 2015a, 2015b; Zahid et al. 2016). The questionnaire was written in Arabic language and available upon request from the corresponding author. The questionnaire consisted of 22 questions and was clustered into four main categories: status of animal’s health, reproductive management, animal feeding, and other farm management practices. It had 17 questions of a close-ended type with 2 to 3 options. The remaining 5 questions were open-ended type, and similar answers were grouped and coded. In order to identify potential sources for misunderstanding of the questions and to further refine the questionnaire, a pilot testing was performed on seven nonparticipating sheep and goat farm owners or managers. One member of the research team was responsible to administer the questionnaire and to conduct the personal interview with the farm owner or manager. Each interview lasted about 20 min. Animal sampling and questionnaire administration took place between November 2015 and September 2017. Two randomly selected questions repeated at the end of the questionnaire related to feed suppliers and animal replacement practices were used to examine the repeatability of the questionnaire. The two questions were nominal responses with 2 or 3 categories, and their repeatability was assessed using kappa (κ) statistic. The external validity was checked using records of Veterinary Services Department, Jordan Ministry of Agriculture.
Blood analysis
Blood samples from the jugular vein were collected into10-ml vacutainer sterile plain tubes. Collected blood samples were kept on ice at 4–7oC until transported to the lab within 1 to 2 hours. Samples were centrifuged at 3000 rpm for 10 min, and serum was harvested and stored at – 20 oC until analysis. The CHEKIT C. burnetii Antibody ELISA Test Kit (IDEXX Laboratories, Broomfield, CO, USA) was used according to the manufacturer’s guidelines to analyze serum samples. Both phase I and phase II antibodies can be detected by the kit. The kit also uses the 9-mile antigen to provide a cumulative serological outcome. Test results were expressed as the ratio between sample (S) optical density (OD) and positive control (P). An S:P ratio ≥ 40% was considered seropositive.
Statistical analysis
Data obtained from the questionnaire and serology results were entered into a Microsoft Excel and carefully checked for errors. The statistical analysis was performed using SPSS 2016, version 24 (SPSS Corp., IBM, Armonk., NY, USA). Initially, associations between seroprevalence to C. burnetii (outcome variable) and its hypothesized risk factors were screened using the univariate analysis (χ2 test). Potential risk factors with p< 0.2 (χ2; two-tailed test) and collinearity r < 0.6 were considered in building the final multivariate logistic regression model using manual stepwise forward logistic-regression analysis. Spearman’s rank correlation test was used to assess the collinearity between covariate risk factors. All risk factors that were not significant in the logistic regression model were reentered whenever a new risk factor became significant or a risk factor was removed from the model. Potential confounder variables were evaluated in every model until the final model was constructed. If the point estimates of the regression coefficients in a model changed ≥ 10% with the potential confounder present, the risk factor was considered as a confounder. Risk factors with a p value < 0.05 were statistically significant and kept in the final model. The Hosmer and Lemeshow (1989) goodness of fit test was used to evaluate the fit of the final model
Results
For the participating goat herds and sheep flocks, the mean herd size was 68 (SD + 41) and 98 (SD + 45), respectively. Table 1 shows animal–level C. burnetii seroprevalence in goat (n = 250) and sheep (n = 480) in four governorates of northern Jordan. The overall animal-level seroprevalence was 32.5% (237/730) and was significantly higher in goats (43.3%, 108/250; 95% CI 37–49.6) than sheep (27%, 129/480; 95% CI 29.1–36.2) (p < 0.001).
Presence of one positive animal in either goat or sheep farm was used to classify the farm as positive. The overall farm-level seroprevalence of C. burnetii was 80% for goat herds (16/20; 95% CI: 62.5–97.5) and 60% for sheep flocks (24/40; 95% CI: 44.8–75.2). The difference in seroprevalence between goat and sheep farms was not statistically significant (Table 2). The mean seroprevalence of C. burnetii within goat farms was 36.4% (ranged: 0–91%) and within sheep farms was 23.4% (ranged: 0–82%). Table 2 shows results of the univariate analysis of farm-level risk factors (p ≤ 0.20) associated with C. burnetii seroprevalence in goat herds and sheep flocks.
Table 3 shows risk factors (p ≤ 0.05) associated with C. burnetii seroprevalence in goat herds and sheep flocks (farm-level). Goat farms were 1.79 times more likely to be C. burnetii seropositive compared with sheep farms. Farms with ≥ 100 adult animals were 3.2 times more likely to be C. burnetii seropositive compared with farms contained less than 100 animals. Farms that had ≥ 60% of their animals 2 years or older were 1.7 times higher to be C. burnetii seropositive than farms with their animals were less than 2 years old. Loaning bucks or rams during the breeding season and presence of cats on farms increased the probability of C. burnetii seroprevalence by 1.52 and 1.63 times more, respectively.
Discussion
In our study, the overall animal-level seroprevalence of C. burnetii was significantly higher in goats (43.3%) than sheep (27%) while the overall farm-level seroprevalence was not significant between goat and sheep farms (80% and 60% respectively). A previous study conducted in central Jordan in goat and sheep farms with a history of abortions during the last kidding and lambing season reported that the seroprevalence of C. burnetii was 10.7% (12/112) in goat and 12.1% (41/340) in sheep using the complement fixation test (Aldomy et al. 1998). The increase in seroprevalence in our study might be due to the different serological test used and/or differences in the antigenicity and virulence of the C. burnetii genotype present in the area.
Results of our study revealed that animal seroprevalence of C. burnetii in goats was significantly higher than that in sheep which agrees with other recent studies (Rodrıguez et al. 2010; Van den Brom et al. 2015; Jarelnabi et al. 2018). However, other studies reported a higher seroprevalence in sheep (Kshash 2012; Anastacio et al. 2013). Rizzo et al. (2016) reported no difference was observed in animal seroprevalence between goat and sheep, but at the farm level, the seroprevalence was higher in sheep. These reported differences in the seroprevalence might be due to variation in susceptibility of goat and sheep to different C. burnetii genotypes (Chochlakis et al. 2018). In Turkey, goat and sheep farm seroprevalence was 81% and 20%, respectively (Kennerman et al. 2010). In Ontario Canada, the reported goat and sheep-level seroprevalence was 32.5% and 14.7%, respectively, and the seroprevalence at the farm-level for goats and sheep was 63.2% and 48.6%, respectively (Meadows et al. 2015 a,b). In northern Spain, C. burnetii seroprevalence was 74% in goat herds and 11.8% in sheep flock (Ruiz-Fons et al. 2010). In Pakistan, seroprevalence of 33.2% and 28.4% was reported in goats and sheep, respectively, while herd seroprevalence was 69.2% for goats and 77% for sheep (Zahid et al. 2016). In the Netherlands, goat seroprevalence was 21.4%, and farm seroprevalence was 43.1% (Schimmer et al. 2011). Goat seroprevalence was 27% in Egypt (khalifa et al. 2016), 34% In Saudi Arabia (Jarelnabi et al. 2018), and 48.2% in Greece (Pape et al. 2009). The animal and farm seroprevalence levels of C. burnetii estimates observed in our study fall within the range of seroprevalence studies published in the region and elsewhere. Interpretation of studies comparing seroprevalence of C. burnetii should be done with caution due to differences in study design, animal breed, and serological test used (Kittelberger et al. 2009).
In our study, the average seroprevalence of C. burnetii within goat farms was 36.4% (ranged: 0–91%) and within sheep farms was 23.4% (ranged: 0–82%). This finding indicates a wide spread of C. burnetii infection within goat and sheep farms. In France, it has been reported that within dairy goat herds the seroprevalence was over 40% (Dubuc-Forfait et al. 2011). In Canada, the average seroprevalence within meat and dairy goat farms was 12.9% (range: 0 to 70%) and 43.1% (range: 0 to 91.4%), respectively, while in dairy sheep farms was 23.5% (range: 0 to 74.3%) and in meat sheep farms was 9.5% (range: 0 to 65.7%) (Meadows et al. 2015 a,b). Most goat herds and sheep flocks in Jordan are seminomadic and pass long distances every day during the grazing season. This type of production system might contribute to the horizontal spreading of the pathogen to large areas of the country.
The univariate risk factor analysis at the farm level revealed several factors to be significantly (p ≤ 0.05) associated with C. burnetii seroprevalence. However, the multivariate logistic regression model identified five risk factors; animal species, herd/flock size, age (more than 60% of flock ≥ 2 years of age), loaning males during breeding season, and presence of cats on farms (p ≤ 0.05).
The logistic regression model revealed that goat herds were 1.79 times more likely to be seropositive than sheep flocks. However, the univariate analysis revealed a nonsignificant difference at the farm level. This difference in the significant level in the two statistical tests might be due to the smaller number of goat herds compared with number of sheep flocks and adjustments with the other four risk factors present in the final multivariate logistic regression model, which supports the statistically significant findings of C. burnetii seroprevalence at the animal-level.
In this study, the odd of goat and sheep farms seroprevalence increased with increasing the herd or flock size. This positive association might be due to lack of proper hygienic practices as only about 27% of farms used disinfectants and about 22% of farms disposed placentas and aborted fetuses properly. Other studies attributed the high seroprevalence in large farms to large population at risk, larger amounts of feed needed, and more veterinarians visiting and working at large farms (Schimmer et al. 2011; Rizzo et al. 2016; Meadows et al. 2015b).
Our study showed that goat and sheep farms with ≥ 60% of their animals were 2 years or older were 1.7 times more likely to be C. burnetii seropositive than farms with their animals that were less than 2 years old. Previous studies found positive association between increased age and seroprevalence (Ruiz-Fons et al. 2010; Rizzo et al. 2016). As the animal gets older, the risk of acquiring infection due to contacts with other infected animals or exposure to the bacterium becomes higher (Ruiz-Fons et al. 2010).
Results of the final logistic regression model indicated that goat and sheep farms had higher probability of being seropositive if they loaned males during the breeding season. Meadows et al. (2015a) reported that there was an 8-fold increase in C. burnetii seroprevalence in sheep farms used to loan sheep to other farms.
The presence of cats in goat and sheep farms was another risk factor associated with a significant increase in seroprevalence to C. burnetii. Presence of cats may introduce C. burnetii infection to the farm and spread infection within farms. Houwers et al. (1992) reported that 10.4% of the examined cats were C. burnetii seropositive. Cantas et al. (2011) reported that dogs and cats’ presence on goat farms was a significant risk factor for C. burnetii infection. In addition, Marrie et al. (1988) reported that exposure to cats during birth was a risk factor for causing Q fever infection in humans. Our study concluded that implementation of strict biosecurity measures is essential to prevent introduction and minimize transmission of Coxiella infection to humans and to other animal farms. Further studies on C. burnetii infection and determination of the bacteria genotypes in other animal species and humans in Jordan are recommended.
References
Aldomy, F. M. M., Wilsmore, A. J., Safi, S. H., 1998. Q fever and abortion in sheep and goats in Jordan. Pakistan Veterinary Journal. 18 (1), 43-45.
Anastacio, S., Tavares, N., Carolino, N., Sidi-Boumedine, K., da Silva, G.J., 2013. Serological evidence of exposure to Coxiella burnetii in sheep and goats in central Portugal. Veterinary Microbiology, (16), 500–505.
Angelakis, E., Raoult, D., 2010. Q fever. Veterinary Microbiology, 140: 297-309.
Cantas, H., Muwonge, A., Sareyyupoglu, B., Yardimci, H., Skjerve, E., 2011. Q fever abortions in ruminants and associated on-farm risk factors in northern Cyprus. BMC Veterinary Research, 7-13.
Chochlakis, D., Santos, A. S., Giadinis, N. D., Papadopoulos, D., Boubaris, L., Kalaitzakis, E., Psaroulaki, A., Kritas, S. K., Petridou, E. I., 2018. Genotyping of Coxiella burnetii in sheep and goat abortion samples. BMC Microbiology, 18(1), 204.
Department of Statistics (DOS), The Hashemite Kingdom of Jordan Agricultural Surveys Annual report 2015. In: http://dosweb.dos.gov.jo/agriculture/livestock/number-of-livestock-by-governorate/ Amman, Jordan. Accessed date November 2015.
Dubuc-Forfait, C., Prigent, M., Delcont, A., Champion, J. L., Marois, M., Kouji, M., Dufour, P., Sidi-Boumedine, K., Rousset, E., 2011. Evaluation of Coxiella burnetii transmission risk levels in goat herds without apparent Q fever (P067) (abstract), Proceeding of the 6th International Meeting on Rickettsia and Rickettsial Diseases.
Ezatkhah, M., Alimolaei, M., Khalili, M., Sharifi, H., 2015. Seroepidemiological study of Q fever in small ruminants from southeast Iran, Journal of Infection and Public Health, (8), 170-176.
Graham, C. J., Yamauchi, T., Rountree, P., 1989. Q fever in animal laboratory workers: An outbreak and its investigation. American Journal of Infection Control, 17 (6), 345-348.
Hosmer, D. W., Lemeshow, S., 1989. Applied Logistic Regression. Wiley, New York.
Houwers, D. J., Van der Meer, M., Van Dijk, A. A., Qssewaarde, J. M., 1992. Prevalence of infections with C. burnetii in dogs and cats in the Netherlands and the central region of the Netherlands, 1991-1992. Research report 91-102, Utrecht.
Jarelnabi, A. A., Alshaikh, M. A., Bakhiet, A. O., Omer, S. A., Aljumaah, R. S., Harkiss, G. D., Mohammed, O. B., Hussein, M. F., 2018. Seroprevalence of Q fever in farm animals in Saudi Arabia. Biomedical Research, 29 (5), 895-900.
Kennerman, E., Rousset, E., Golcu, E., Dufour, P., 2010. Seroprevalence of Q fever (coxiellosis) in sheep from the Southern Marmara Region, Turkey. Comparative Immunology, Microbiology and Infectious Diseases, 33:37–45.
Khalifa, N. O., Elhofy, F. I., Fahmy, H. A., Sobhy, M. M., Agag, M. A., 2016. Seroprevalence and molecular detection of Coxiella burnetii in sheep, goats and human in Egypt. Journal of Microbiology, Biotechnology and Food Science, 2 (1), 1-7.
Kittelberger, R., Mars, J., Wibberley, G., Sting, R., Henning, K., Horner, G. W., Garnett, K. M., Hannah, M. J., Jenner, J. A., Pigott, C. J., O'Keefe, J. S., 2009. Comparison of the Q-fever complement fixation test and two commercial enzyme-linked immunosorbent assays for the detection of serum antibodies against Coxiella burnetii (Q-fever) in ruminants: Recommendations for use of serological tests on imported animals in New Zealand, New Zealand Veterinary Journal, 57(5), 262-268.
Kshash, Q. H., 2012. Prevalence of Q-fever in small ruminants in Al Qassim city. Basrah Journal of Veterinary Research, (11), 342–348.
Limonard, G. J. M., Nabuurs-Franssen, M. H., Weers-Pothoff, G., Wijkmans, C., Besselink, R., Horrevorts, A. M., Schneeberger, P. M., Groot, C. A. R., 2010. One-year follow-up of patients of the ongoing Dutch Q fever outbreak: Clinical, serological and echocardiographic findings. Infection, 38 (6), 471-477.
Marrie, T. J., Durant, H., Williams, J. C., Minte, E., Waag, D., 1988. Exposure to parturient cats is a risk factor for acquisition of Q fever in maritieme Canada. Journal of Infectious Diseases, (158), 101-108.
Martin, S. W., Meek, A. H., Willeberg, P., 1987. Veterinary Epidemiology. Iowa: Iowa State University, p 22-42.
Meadows, S., Jones-Bitton, A., McEwen, S., Jansen, J., Menzies, P., 2015a. Coxiella burnetii seropositivity and associated risk factors in sheep in Ontario, Canada. Preventive Veterinary Medicine, 122 (1-2), 129-134.
Meadows, S., Jones-Bitton, A., McEwen, S., Jansen, J., Menzies, P., 2015b. Coxiella burnetii seropositivity and associated risk factors in goats in Ontario, Canada. Preventive Veterinary Medicine, 121 (3-4), 199-205.
Mori, M., Roest, H. J., 2018. Farming, Q fever and public health: agricultural practices and beyond. Archives Public Health. (6), 76:2.
Muskens, J. H., Van Engelen, C., Van Maanen, C., Bartels, C., Lam, T. L., 2011. Prevalence of Coxiella burnetii infection in Dutch dairy herds based on testing bulk tank milk and individual samples by PCR and ELISA.168:79.
Pape, M., Bouzalas, E. G., Koptopoulos, G. S., Mandraveli, K., Arvanitidou-Vagiona, M., Nikolaidis, P., Alexiou-Daniel, St., 2009. The serological prevalence of Coxiella burnetii antibodies in sheep and goats in northern Greece. Clinical Microbiology and Infection. (2), 146-147.
Rabinowitz, P. M., Conti, A. A., 2009. Human-Animal Medicine Clinical Approach to Zoonoses, Toxicants and Other Shared Health Risk. First edn. Missouri, USA: Mosby and Saunders.
Rizzo, F., Vitale, N., Ballardini, M., Borromeo, V., Luzzago, C., Chiavacci, L., Mandola, M. L., 2016. Q fever seroprevalence and risk factors in sheep and goats in northwest Italy. Preventive Veterinary Medicine, (130), 10-17.
Rodrıguez, N. F., Carranza, C., Bolanos, M., Perez-Arellano, J. L., et al., 2010. Seroprevalence of Coxiella burnetii in domestic ruminants in Gran Canaria Island, Spain. Transboundary Emerging Diseases, (57), 66–67.
Ruiz-Fons, F., Astobiza, I., Barandika, J. F., Hurtado, A., Atxaerandio, R., Juste, R. A., Garcia-Perez, A. L., 2010. Seroepidemiological study of Q fever in domestic ruminants in semi-extensive grazing systems. MBC Veterinary Research, (6), 3.
Schimmer, B., Luttikholt, S., Hautvast, J. L., Graat, E. A., Vellema, P., Van Dynhoven, Y. T., 2011. Seroprevalence and risk factors of Q fever in goats on commercial dairy goat farms in the Netherlands, 2009-2010. MBC Veterinary Research. (7), 81.
Van den Brom, R., Van Engelen, E., Roest, H. I. J., Van der Hoek, W., Vellema, P., 2015. Coxiella burnetii infections in sheep or goats: an opinionated review. Veterinary Microbiology. (18), 119-129.
Zahid, M. U., Hussain, M. H., Saqip, M., Neubauer, H., Abbas, G., Khan, I., Mansoor, M. K., Asi, M. N., Ahmad, T., Muhammad, G., 2016. Seroprevalence of Q fever (Coxiellosis) in small ruminants of two districts in Punjab, Pakistan. Vector-Borne and Zoonotic Diseases. 16(7), 449-454.
Acknowledgments
This study was financially supported by the Deanship of Research at Jordan University of Science and Technology (research grant number: 487/2014).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lafi, S.Q., Talafha, A.Q., Abu-Dalbouh, M.A. et al. Seroprevalence and associated risk factors of Coxiella burnetii (Q fever) in goats and sheep in northern Jordan. Trop Anim Health Prod 52, 1553–1559 (2020). https://doi.org/10.1007/s11250-019-02153-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-019-02153-0