Abstract
Q-fever is a worldwide spread zoonotic disease associated with severe illness in humans and abortions and stillbirths in ruminants. Ruminants are major sources of human infection where subclinical carriers shed the bacteria in various secretions and excreta. The goal of the current study was to investigate the prevalence and risk factors of Coxiella burnetii infection among cattle, sheep, and goats in the eastern province of the Kingdom of Saudi Arabia (KSA). A total of 1310 serum samples were collected through a designed cross-sectional study from private farms and slaughterhouses in the study area and examined against antibodies of C. burnetii using ELISA. A multivariate logistic regression analysis was built to detect risk factors of C. burnetii infection among examined species. The prevalence of C. burnetii infection among examined animals was 9.2% (CI, 7.7–10.8)—15.6%, 9.1%, and 5.8% among goats, cattle, and sheep, respectively). The risk of getting C. burnetii infection among old animals (> 1 year old) was 23 times higher than the risk among young animals (< 1 year old) (95% CI, 10.04–53.01; P < 0.01). Goats were 2.27 (95% CI, 1.41–3.66; P < 0.01) and 3 times at higher risk than cattle and sheep, respectively, of getting C. burnetii infection. In conclusion, C. burnetii infection is widespread among different ruminant species of the eastern province of KSA which represents a high risk for environmental contamination and disseminating the infection to humans and animal species in that area. Also, our findings may reflect the disease status in other countries of the Arabian Gulf area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Q-fever is an endemic zoonotic disease in many parts of the world caused by Gram-negative bacterium, Coxiella burnetii (Eldin et al. 2017). The acute form of Q-fever in humans is mostly self-limiting and flu-like illness, while the chronic form lasts symptomless for a long time followed by endocarditis, chronic hepatitis, and pulmonary infection (Maurin and Raoult 1999). Chronic endocarditis is fatal in most cases as reported by Rolain et al. (2005). These initial unclear symptoms of human Q-fever is responsible for the underestimation the burden of such disease by health authorities who treat such case as fever of unknown origin without neither routine testing nor available diagnostic tools (Anderson et al. 2011).
The prevalence of active recent or chronic infection with C. burnetii among domestic ruminants in developing countries is estimated to be around 25% and these ruminants are considered principal sources of infection to humans in contacts (Ruiz-Fons et al. 2010; Eldin et al. 2017). The disease in ruminants is associated with late-term abortion especially in sheep and goats accompanied by secretion of a large number of C. burnetii in placentas, urine, and milk which resulted in environmental contamination and disseminating the infection to other animals and humans (Van den Brom et al. 2012). Goats were incriminated as the main source of a major epidemic of Q-fever in 4000 people in the Netherlands (Schimmer et al. 2009).
Q-fever was confirmed as an endemic disease in the Saudi population for the first time in the1960s; thereafter, in the following 50 years, studies on Q-fever in the Kingdom of Saudi Arabia (KSA) are very scarce among human and animal populations (Jarelnabi et al. 2018). Only five human cases of Q-fever endocarditis in 2007 and 2012 (Angelakis et al. 2014) and 18 cases with specific IgG antibodies to phase II of C. burnetii were detected between 2011 and 2013 by Almogren et al. (2013). In animals, Q-fever was reported in wild desert ungulates, camels, and domestic ruminants (Hussein et al. 2008; Hussein et al. 2012; Mohammed et al. 2014). Recently, Jarelnabi et al. (2018) reported the overall seroprevalence among ruminants in the central areas of KSA as 30.71% (30.67%, 34.04%, 12.38%, and 51.53% in cows, goats, sheep, and camels, respectively). This latter study concluded that domestic ruminants including camel are responsible for Q-fever endemicity in the KSA.
The scarce studies on Q-fever in KSA domestic ruminants were carried out in locally restricted areas (Jouf, Kharj, and Riyadh) of the Kingdom. There are no studies found on the Q-fever epidemiology in the eastern province of KSA; the area is very wide and has a very strong geographic connection with all or other Arabic Gulf area countries. Therefore, the understanding of Q-fever epidemiology in such an area may reflect the disease situation in all other nearby countries. So, the objectives of the current study are first to estimate the prevalence of Q-fever among different ruminant species in the eastern province of KSA for the first time up to our knowledge and second to identify risk factors for Q-fever infection among these ruminant species.
Materials and methods
Study area
The study area included the eastern province of KSA: Al-Hassa, Dammam, and Qatif (Fig. 1). Al-Hassa is the largest governorate in KSA eastern province, named after the Al-Ahssa oasis which is located about 60 km inland from the Arabs Gulf. The oasis includes the giant Empty Quarter desert that connects KSA to Gulf area countries such as UAE, Qatar, and Oman. Qatif is an urban governorate of the Eastern Province, KAS, which extends from Ras Tanura and Jubail in the north to Dammam in the south. It extends also from King Fahd International Airport in the west to the Arab Gulf in the east. The largest town in the eastern province is Dammam, which considered the fifth largest in KSA, after Riyadh, Jeddah, Mecca, and Medina. Dammam has a hot desert climate especially in summer when the temperature exceeds 40 °C. On the other hand, the winter temperatures in Dammam ranged from mild to warm and the rainfall is generally sparse usually takes place in small amounts in December.
Sample size estimation
Each animal species, cattle, sheep, and goat, is considered a separate population. The numbers of samples in each animal were calculated according to Thrusfields and Christy (2018) after the formula
where n is the required sample size; t is the confidence level at 95% (standard value of 1.96); p is the expected prevalence of Q-fever in the region, 30% following recent study in KSA (Jarelnabi et al. 2018); and m is the margin of error at 5% (standard value of 0.05). The resulted sample size for each animal species was estimated at 322 animals, 966 in total. In the current study, we increased slightly the sample size to be 1310 animals—432 for cattle, 571 for sheep, and 307 for goat.
The eastern province has organized farms mostly in the Al Qatif region mainly and Al-Hassa region which have cows and sheep and animals are breed in these closed farms without contact with other farms or free movable flocks and with adequate hygienic measures. The total numbers of cattle and sheep in these farms are 157,530 and 710,249, respectively. This eastern province also has 3 large slaughterhouses for all animal species—2 in Al-Hassa and one in Dammam—and the total numbers of animals slaughtered in 2018 were 55,089 cows, 1,153,091 sheep, and 205,701 goats. The total number of samples was stratified between farms and slaughterhouses, proportional to the total number in each source, 500 and 799, respectively. Sampled animals were equally distributed to the 3 slaughterhouses and sampled animals from farms were equally distributed between 15 farms in Al-Hassa and 30 in Al Qatif regions. The total number of samples collected from different locations, farms, and slaughterhouses is shown in Table 1. The selection of animals in farms was done randomly by passing animals from a gate and selecting an animal after a fixed number of animal pass the gate; we divide the total number of animals in the farm by the total number of samples required from this farm and the obtained figure was set as the fixed number. In the slaughterhouses, we collect the required number of samples without a specific regime.
Collection of blood serum samples and serological examination
Blood was collected from the jugular vein and serum was separated and collected by centrifugation at 4000 rpm for 20 min. Finally, the sera were stored at 20 °C with a complete data label until use. All sera samples were examined against C. burnetii using the CHEKIT Q-FEVER ELISA Test Kit which was supplied from IDEXX Europe B.V. Scorpius 60 Building F Hoofddorp, 2132 LR, Netherlands.
Epidemiological investigation
The prevalence and 95% CI were estimated following Thrusfields and Christy (2018). Two models for risk factor identification were built; the first model was built for each animal species separately, and the second model was built for all species together. In the first model, data on each animal species attributes including sex, age, breed, location, and source were collected at the time of blood sampling. The association between seropositivity and these animal attributes was identified individually for each species using a univariate logistic regression analysis model that was carried out in IBM SPSS Statistics for Windows version 21.0 (IBM SPSS Inc., Armonk, NY).
In the second model, the association between some animal attributes (age, location, source, and species) and seropositivity to C. burnetii infection to all animal species together was examined using a multivariate logistic regression model. Breed and sex were not incorporated into such a model because of lacking individual animal identification for both of these attributes. Initially, a univariate model was built to determine the association between each of the animal attributes with C. burnetii infection status. Non-significant associated attributes with C. burnetii infection status at P > 0.2 were not included in the final multivariate model. If there is a significant correlation between variables, the mostly judged probable biological variable was kept in the multivariate.
The multivariate logistic regression model equation is
where exp. (β) is the odds ratio.
Variables with P < 0.05 were kept in the final model after a manual backward selection approach was used and all two-way interactions between these retained variables were assessed. Testing for confounder was performed by checking the change of logit of variables by removing a suspected variable from the model.
Results
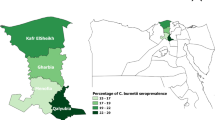
Results of prevalence estimation showed that 120 out of 1310 animals examined against Q-fever were positive (9.2% and CI 7.7–10.8). The prevalence of infection with C. burnetii among examined animals was 15.6% and CI 11.9–20.3, 9.1% and CI 6.7–12.1, and 5.8% and Cl 4.2–8.1 among goats, cattle, and sheep, respectively.
The results of risk factor identification among each animal species are shown in Table 2. The risk of getting C. burnetii infection among cows in Al-Hassa province was significantly higher than in other areas while sheep and goats in Al Qatif province had a significantly higher risk of getting the infection other than other areas. Sex was not considered a risk factor for infection with C. burnetii among goats, while female cows and male sheep were at significantly high risk of getting C. burnetii infection. Animal younger than 1 year old had a significantly lower risk of getting an infection with C. burnetii among all animal species. The prevalence of infection with C. burnetii was significantly different among different breeds of each animal species.
The results of univariate analysis for the association between different animal attributes and the seropositivity status to C. burnetii infection showed in Table 3. Age was reordered to 2 categories: < 1 year old and > 1 year old. The prevalence of infection in Al-Hassa was significantly lower than that in Al Qatif and Al Dammam regions. On the other hand, animals sampled from the slaughterhouse, older than 1 year old, and goats had a significantly higher odds ratio of getting C. burnetii infection than animals sampled from farms, younger than 1 year old, and cows and sheep, respectively.
The source variable was correlated significantly with all other variables. Also, the location variable was significantly correlated with the age variable. Therefore, both the location and the source variables were not incorporated into the final multivariable model. This final model was built with 2 variables: age and species. The results of the multivariable analysis are shown in Table 4. The results showed that the risk of getting an infection with C. burnetii among animals older than 1 year is 23 times more than that among young animals (95% CI, 10.04–53.01, and P < 0.01). Goats were at risk of getting C. burnetii infection 2.27 (95% CI, 1.41–3.66, and P < 0.01) times more than cows and almost 3 times more than sheep.
Discussion
This cross-sectional study is the first nationwide, up to our knowledge, to provide a comprehensive understanding of the epidemiology of C. burnetii infection among cattle, sheep, and goats in the eastern province of KSA. The results of the current study cleared the endemic situation of Q-fever among domestic ruminants in the eastern region of KSA at a prevalence of 9.2%. Obscure signs of Q-fever in humans and animals and its public and animal health consequences declared the importance of the current survey and its obtained prevalence data which is important for concerned authorities for applying preventive and control practices.
In the current study, Q-fever seroprevalence in sheep is 5.8%; a higher prevalence (12.38%) was recorded recently in sheep in the KSA but in regions other than the eastern region considered in this study (Jarelnabi et al. 2018). In the neighboring countries, higher prevalences of Q-fever among sheep were reported: 20% in the southern Marmara of Turkey (Kennerman et al. 2010) and 36.6%, 43.3%, and 19.6% in the western desert, Nile Valley, and eastern desert, respectively (Klemmer et al. 2018). This difference of disease prevalence in the current study from the neighboring countries’ studies may be attributed to the husbandry system (sheep in the eastern province especially Qatif region are breed in closed organized farms like cows with no exposure to free movable flocks), the specific geographical characteristics with the climatic conditions, and the density of animal population in each area (Asadi et al. 2013; Klemmer et al. 2018).
The obtained seroprevalence of infection with C. burnetii in goat was 15.6%. A similar prevalence estimate was recorded in Iran by Asadi et al. (2013). These results support the findings of Schimmer et al. (2009) for goats being incriminated as the most probable source of a major human outbreak of Q-fever. The free movement of goats’ flocks represents a major source for environmental contamination and consequently spread the infection between different areas.
The seroprevalence of C. burnetii infection in cattle recorded in our study was 9.1%; a higher seroprevalence (51%) was reported in other regions of the KSA (Jouf, Kharj, and Riyadh). In Egypt, Gwida et al. (2014) recorded 13.2% seroprevalence of C. burnetii in cattle, while Klemmer et al. (2018) reported 19.3% seroprevalence. The low prevalence obtained also may be attributed to the same reasons mentioned before especially the very high temperature in the study area and also the system of cows’ breeding in organized farms with no exposure to free movable flocks minimizes the exposure of cows to environmental pathogens. This cow’s prevalence may indicate the high risk of transmission of C. burnetii to humans who consume the milk of these cows.
The results of risk factor identification among each animal species showed that female cows and male sheep were at significantly high risk of infection with C. burnetii. Mazeri et al. (2013) showed that pooled Q-fever seroprevalence among females was higher than among males. In Cameroon, the seroprevalence of C. burnetii was higher in female cows (Cetinkaya et al. 2000). The causes of higher seroprevalence among females than males could be that C. burnetii has a high affinity for the mammary glands and uterus, and so large numbers of C. burnetii are found in these tissues (Cetinkaya et al. 2000).
Risk factor detection showed a widespread infection in slaughterhouses than farms. Animals in slaughterhouses could be reared in close farms or belonged to movable flocks and these latter animals may have more chance to be exposed to environmental pathogens. This finding also highlights the risk of zoonotic transmission of infection to humans in contact.
The results of the multivariable analysis showed that the risk of getting an infection with C. burnetii among animals animal > 1 year of age is significantly higher than that among young animals. These results are supported by Jarelnabi et al. (2018) and Barlozzari et al. (2020) findings who recorded a significantly higher seroprevalence of Q-fever among adults compared with young animals and these findings may be due to the increased likelihood of an old animal to be infected (Anastácio et al. 2013). The univariate analysis showed that sheep and goats of mid-age (1–2 years old) are at a very high significant risk of being seropositive to Q-fever more than younger or older animals and this agrees with the findings of Kennerman et al. (2010) and Mc Caughey et al. (2010). This latter finding may be attributed to the infection that occurs frequently around the time of parturition and primiparous ewes (1 year old) being at the direct chance of exposure to infection due to abortion of infected nearby animals while older animals experienced declining antibody responses after the moment of infection. Furthermore, horizontal transmission is being the predominant route of infection other than the vertical transmission of Q-fever and this may explain the low prevalence among young animals (Mc Caughey et al. 2010).
Goats were at risk of getting C. burnetii infection 2.27 times more than cows and almost 3 times more than sheep. These results agree with Akbarian et al. (2015) who recorded a seroprevalence of C. burnetii among livestock in Afghanistan as 43.4% for sheep, 52.7% for goats, and 5.2% for cattle. This could be explained by small ruminants especially that goats are kept in free-roaming village-based flocks with only limited management. During the dry seasons, most of the animals roam freely, but sheep stay closer to the villages than goats and sheep are breed mainly in organized farms in the study area. This might explain the difference in seropositivity between the two species (Kilic et al. 2005). The lower prevalence observed in cattle in this study may be due to the differences in the condition of livestock keeping where cattle are kept in farms with adequate hygienic measures and without contact with other farms. Moreover, cattle farms usually have special calving pens where pregnant animals were isolated in the late stage of pregnancy until giving birth, where other cows have no contact with placentas and discharges of such delivering animals.
The current study has certain limitations due to the lack of accurate information on the numbers of animals stratified by age and breed. This prevented us from following the stratified sampling design for both of these 2 variables. We did not expect that such a problem could affect our findings of young aged animals being at low risk of being seropositive to Q-fever. We expect that inflation of the number of sample size in our study among young animals may confirm our finding of them being at low risk to get an infection and this has been concluded by other studies as mentioned before. The limited sample size of some examined breeds in this study may affect the conclusion that some breeds are more at risk of getting Q-fever, and this point needs further and deep investigation. The limitation of our study includes the usage of ELISA which could not differentiate between the old and current infection as it does not detect the IgM. Therefore, it is required in the future to use a test which could detect the current infection to identify precisely the risk factors of Q-fever infection. Finally, we sampled more male animals than female animals and this could affect the estimated prevalence as we showed in our results that females are at high risk of being seropositive to Q-fever. This latter part is attributed mainly to the fact that most of the animals admitted to slaughterhouses are males and this point has to be considered in future work.
In conclusion, Q-fever is widely spread among domestic ruminants of the eastern province of KSA especially goats which constitute a high risk factor for contaminating the environment with C. burnetii infection within flocks and between different areas. Therefore, the public and animal and health authorities should prioritize Q-fever in their epidemiological surveys and diagnosis. The results obtained may be extrapolated to other nearby countries with close animal husbandry systems.
References
Akbarian, Z., Ziay, G., Schauwers, W., Noormal, B., Saeed, I., Qanee, A. H., Shahab, Z., Dennison, T., Dohoo, I., and Jackson, R., 2015. Brucellosis and Coxiella burnetii infection in householders and their animals in secure villages in Herat province, Afghanistan: a cross-sectional study, PloS Neglected Tropical Diseases, 9, 2-17. https://doi.org/10.1371/journal.pntd.0004112
Almogren, A., Zahid, S., and Rana, H., 2013. Q fever: a neglected zoonosis in Saudi Arabia, Annals of Saudi medicine, 33, 464-468. https://doi.org/10.5144/0256-4947.2013.464
Anastácio, S., Tavares, N., Carolino, N., Sidi-Boumedine, K., and da Silva, G. J., 2013. Serological evidence of exposure to Coxiella bur-netii in sheep and goats in central Portugal, Veterinary Microbiology, 167, 500—505. doi: https://doi.org/10.1016/j.vetmic.2013.08.004
Anderson, A. D., Baker, T.R., Littrell, A.C., Mott, R.L., Niebuhr, D. W., and Smoak, B. L., 2011. Seroepidemiologic survey for Coxiella burnetii among hospitalized U.S. troops deployed to Iraq, Zoonoses and Public Health, 58, 276–283. https://doi.org/10.1111/j.1863-2378.2010.01347.x.
Angelakis, E., Sameer, J., Azeem, A., Memish, Z., and Raoult, D., 2014. Q fever endocarditis and new Coxiella burnetii genotype, Saudi Arabia, Emerging Infectious Diseases, 20, 726-728. https://doi.org/10.3201/eid2004.131603
Asadi, J., Mojtaba, K. and Khalili, M.,2013. Seroprevalence of Q fever in sheep and goat flocks with a history of abortion in Iran between 2011 and 2012, Veterinaria Italiana, 49, 163-168. https://doi.org/10.12834/VetIt.2013.492.163.168
Barlozzari, G., Sala, M., Iacoponi, F., Volpi, C., Polinori, N., Rombolà, P., Vairo, F., Macrì, G. and Scarpulla, M., 2020. Cross-sectional serosurvey of Coxiella burnetii in healthy cattle and sheep from extensive grazing system in central Italy, Epidemiology and Infection, 148, e9, 1–8. https://doi.org/10.1017/S0950268819002115
Cetinkaya, B., Kalender, H., Ertas, H. B., Muz, A., Arslan, N., Ongor, H., and Gurçay, M., 2000. Seroprevalence of coxiellosis in cattle, sheep and people in the East of Turkey, Veterinary Record, 146, 131–136. https://doi.org/10.1136/vr.146.5.131
Eldin, C., Mélenotte, C., Mediannikov, O., Ghigo, E., Million, M., Edouard, S., Mege, L., Maurin, M., and Raoult, D., 2017. From Q fever to Coxiella burnetii infection: a paradigm change, Clinical Microbiology Reviews, 30, 115–90. https://doi.org/10.1128/CMR.00045-16
Gwida, M., El-Ashker, M., El-Diasty, M., Engelhardt, C., Khan, I., and Neubauer, H., 2014. Q fever in cattle in some Egyptian Governorates: a preliminary study, BMC Research Notes, 7, 881. https://doi.org/10.1186/1756-0500-7-881
Hussein, M. F., Alshaikh, M., Gad El-Rab, M. O., Aljumaah, R. S., Gar El Nabi, A., and Abdel Baki, A.M., 2008. A Serological prevalence of Q fever and chlamydiosis in camels in Saudi Arabia, Journal of Animal and Veterinary Advances, 7, 685-688. http://medwelljournals.com/abstract/?doi=javaa.2008.685.688. Accessed June 2019.
Hussein, M.F., Al-Khalifa, I.M., Aljumaah, R.S., Gar Elnabi, A., Mohammed, O. B., Omer, S. A., and Macasero, W. V., 2012. Serological prevalence of Coxiella burnetii in captive wild ruminants in Saudi Arabia, Comparative Clinical Pathology, 21, 33-38. https://doi.org/10.1007/s00580-010-1061-y
Jarelnabi, A. A., Mohammed, A. A., Bakhiet, A. O., Omer, S. A., Aljumaah, R. S., Harkiss, G. D., Mohammed, O. B. and Hussein, M. F.,2018. Seroprevalence of Q fever in farm animals in Saudi Arabia, Biomedical Research, 29, 895-900. https://doi.org/10.4066/biomedicalresearch.29-17-770
Kennerman, E., Rousset, E., Gölcü, E., and Dufour, P., 2010. Seroprevalence of Q fever (coxiellosis) in sheep from the Southern Marmara Region, Turkey, Comparative Immunology, Microbiology and Infectious Diseases, 33, 37-45. https://doi.org/10.1016/j.cimid.2008.07.007
Kilic, S., Pasa, S., Babur, C., and Ozlem, M.B., 2005. Investigation of Coxiella burnetii antibodies in sheep in Aydin region, Turkey. Revue de Medecine Veterinaire 156, 336-340.
Klemmer, J., Njeru, J., Emam, A., El-Sayed, Ma., Moawad, A. A., Henning, K., Elbeskawy, M. A., Sauter-Louis, C., Straubinger, R. K., Neubauer, H., and El-Diasty, M. M., 2018. Q fever in Egypt: Epidemiological survey of Coxiella burnetii specific antibodies in cattle, buffaloes, sheep, goats and camels, PLOS ONE, 13, 1-12. https://doi.org/10.1371/journal.pone.0192188
Maurin, M. and Raoult, D., 1999. Q fever, Clinical Microbiology Reviews, 12, 518–553.
Mazeri, S., Scolamacchia, F., Handel, I. G., Morgan, K.L., Tanya, V.N., and Bronsvoort, B.M., 2013. Risk factor analysis for antibodies to brucella, leptospira and C. Burnetii among cattle in the adamawa region of cameroon: A cross-sectional study, Tropical Animal Health and Production, 45, 617–623. https://doi.org/10.1007/s11250-012-0268-0
Mc Caughey, C., Murray, J., Mckenna, J., Menzies, F., Mccullough, S., O’neill, H., Wyatt, D., Cardwell, C. and Coyle, P., 2010. Coxiella burnetii (Q fever) seroprevalence in cattle, Epidemiology and Infection, 138, 21 -27.
Mohammed, O. A., Jarelnabi, A. A., Aljumaah, R. S., Alshaikh, M. A., Bakhiet, A. O., Omer, S. A.,Alagaili, A. N., and Hussein, M. F., 2014. Coxiella burnetii, the causative agent of Q fever in Saudi Arabia: molecular detection from camel and other domestic livestock, Asian Pacific Journal of Tropical Medicine, 7,715-719. https://doi.org/10.1016/S1995-7645(14)60122-X
Rolain, J. M., Boulos, A., Mallet, M. N., and Raoult, D., 2005. Correlation between ratio of serum doxycycline concentration to MIC and rapid decline of antibody levels during treatment of Q fever endocarditis, Antimicrobial Agents and Chemotherapy, 49, 2673–2676. https://doi.org/10.1128/AAC.49.7.2673-2676.2005
Ruiz-Fons, F., Astobiza, I., Barandika, J. F., Hurtado, A., Atxaerandio, R., Juste, R. A. and García-Pérez, A. L., 2010. Seroepidemiological study of Q fever in domestic ruminants in semi-extensive grazing systems, BMC Veterinary Research, 6, 3. http://www.biomedcentral.com/1746-6148/6/3. Accessed Mar 2020.
Schimmer, B., Dijkstra, F., Vellema, P., Schneeberger, P.M., Hackert, V., ter Schegget, R., Wijkmans, C., van Duynhoven, Y., and van der Hoek, W., 2009. Sustained intensive transmission of Q fever in the south of the Netherlands, Euro Surveillance, 14, 1-3. https://doi.org/10.2807/ese.14.19.19210-en
Thrusfields, M. and Christy, R., 2018. Veterinary epidemiology, 4th edition, Wily-Blackwell
Van den Brom, R., Van Engelen, E., Luttikholt, S., Moll, L., Van Maanen, K., and Vellema, P., 2012. Coxiella burnetii in bulk tank milk samples from dairy goat and dairy sheep farms in The Netherlands in 2008, Veterinary Record, 170, 310. https://doi.org/10.1136/vr.100304
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics statement
The fieldwork described herein was performed by the staff of the Department of Clinical Studies and King Faisal University Veterinary Teaching Hospital, College of Veterinary Medicine, King Faisal University, Kingdom of Saudi Arabia. Authors confirm that the ethical policies of the journal, as noted in the journal’s author guidelines page, have been adhered to with no experimentation, inoculation, or treatment of live animals.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aljafar, A., Salem, M., Housawi, F. et al. Seroprevalence and risk factors of Q-fever (C. burnetii infection) among ruminants reared in the eastern region of the Kingdom of Saudi Arabia. Trop Anim Health Prod 52, 2631–2638 (2020). https://doi.org/10.1007/s11250-020-02295-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-020-02295-6