Abstract
Forty Dorper × Pelibuey sheep females were used to evaluate the effects of physiological state on physiological variables and serum concentrations of metabolites, thyroid hormones, and electrolytes under outdoor heat stress conditions. Females were selected as follows (n = 10 per group): weaning ewe lambs (WEL; 3 months old), replacement nulliparous ewes (RNE; 8 months old), non-pregnant and non-lactating multiparous ewes (NME; 3–4 years old) and lactating multiparous ewes (LME; 3–4 years old). While physiological variables were measured both morning and afternoon, blood samples were collected before feeding in the morning to determine all blood components. Three contrasts were constructed: (1) WEL vs. older ewes, (2) RNE vs. multiparous ewes, and (3) NME vs. LME. Compared with older ewes, WEL had higher (P < 0.01) rectal temperature (RT) and hair coat temperatures through the day, and also higher (P < 0.01) respiratory rate (RR) only in the afternoon. Serum levels of glucose and cholesterol were lower (P ≤ 0.02) in WEL than in older ewes. Nulliparous ewes compared with multiparous had always similar RT but higher (P ≤ 0.05) hair coat temperatures in most of the body regions by the morning and higher (P < 0.01) RR, without difference for hair coat temperatures in the afternoon. Only serum glucose (P = 0.07) and urea nitrogen (P < 0.01) levels were affected by parturition number, being lower in multiparous ewes. Regarding the effect of lactation, while RR was unaffected, afternoon RT and hair coat temperatures in most of the body regions through the day were higher (P ≤ 0.03) in lactating ewes. In addition, LME had lower (P < 0.01) serum levels of glucose, cholesterol, and urea nitrogen, but higher (P = 0.02) triiodothyronine levels than NME. In conclusion, ewe lambs and lactating ewes were less tolerant to heat stress, while nulliparous and multiparous ewes showed similar thermoregulatory ability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heat stress is a global problem limiting meat production of the sheep industry located in warm regions. Heat-stressed sheep activate evaporative thermoregulation mechanisms that lead to higher maintenance nutritional requirements, while decreasing feed intake and increasing water intake (Marai et al. 2007). Contrasting with other ruminants where sweating plays a key role to avoid hyperthermia, sheep may dissipate between 60 and 90% of the total heat load by increasing respiratory rate (RR), and less than 10% by sweating (Marai et al. 2007; Fonseca et al. 2017). The increase in RR has an elevated energy cost, and since is crucial to avoid hyperthermia, it is preferred to use the body energy for the correct operation of this thermoregulatory mechanism instead of maintaining other functions such as growth and reproduction (Al-Dawood 2017). Consequently, energy metabolism, and in extreme case the protein metabolism, is altered to direct the energetic substrate towards the tissues related with the process of respiration in sheep subjected to heat stress (Sejian et al. 2010). Additionally, it has been observed that heat-stressed sheep tend to reduce their thyroid hormone levels in blood as a metabolic thermoregulation mechanism to prevent excessive production of metabolic heat. Attainment of high amount of metabolic heat can lead to hyperthermia conditions in this specie (Al-Dawood 2017). However, there are differences among sheep breeds in relation to its tolerance level to environmental heat stress, and within breeds, differences in the thermoregulation ability and tolerance to high temperatures can exist depending on age and physiological state.
Hair sheep are breeds used to produce meat, adapted to warm climates, able to take advantage of poor quality forage and with few negative effects from heat stress on their feedlot fattening and reproductive performance (Macías-Cruz et al. 2013, 2016a). These hair sheep are less sensitive to severe heat stress than wool breeds (Titto et al. 2016), which has been attributed to hair characteristics, higher number of sweat glands, and synthesis of HSP-70 in cells, as well as better efficiency in non-evaporative and evaporative heat losses (McManus et al. 2011; Macías-Cruz et al. 2016b; Fonseca et al. 2017). Also, it has been documented the daytime strategic activation of physiological mechanisms in heat-stressed hair sheep to dissipate heat more efficiently; for example, Macías-Cruz et al. (2016b; in press) reported that, while summer heat stress promoted circadian changes in hair coat temperature according to environmental temperature, circadian fluctuations in RR depended on changes in the thermal gradient between temperatures of hair coat and environment. However, the impacts of age and physiological state on the thermoregulatory capacity of hair sheep females have not been studied under heat stress conditions, while results using wool sheep females are scarce.
At birth, the thermoregulation center in lambs is completely immature so that they exhibit core temperatures above the normal range. As weeks and months goes by, there is a decrease in the core temperature as a result of a gradual maturation of the thermoregulation center (Thwaites 1967). Under thermoneutral conditions (17 °C), Piccione et al. (2002) reported that lambs reach a circadian rhythm of body temperature similar to the adult sheep at 30 days of age. MacFarlane (1958) in sheep and Arfuso et al. (2016) in goats found that these small ruminants archived stability in their body temperatures approximately 1 year after birth. Symonds et al. (1995) also observed that metabolic rate and triiodothyronine hormone concentrations decreased in wool lambs reared in warm environment during the first days of life. On the other hand, lactation is a physiological process that involves a significant increase in metabolic activity, which is reflected in greater metabolic heat production (Polsky and von Keyserlingk 2017). Abdalla et al. (1993) detected higher rectal temperature (RT), RR, and blood thyroid hormone levels in lactating ewes, but lower milk production without effects on blood glucose levels by effect of the heat stress. Thus, given these alterations due to age and lactation, some background indicates that young (Thwaites 1967) and lactating (Abdalla et al. 1993) sheep are more sensitive to heat stress and, therefore, less tolerant to warm climates.
It is necessary to expand our knowledge in relation to the physiologic and metabolic adjustments that are used by sheep adapted to heat stress, according to their age and physiological state, to maintain normothermia conditions in high temperature environments. With this information, heat stress mitigation strategies may be established more appropriately for hair sheep production systems. In this sense, the present study aimed to evaluate the effects of physiological state on physiological variables and serum concentrations of metabolites, thyroid hormones, and electrolytes in hair sheep females under outdoor heat stress conditions.
Materials and methods
All procedures involving sheep were conducted within the guidelines of approved Mexican Official Standards for the production, care, and use of laboratory animals (NOM-062-ZOO-1999). This study was conducted during the summer season (August 23 to September 22, 2014) at the Instituto de Ciencias Agrícolas (ICA), which belongs to the Universidad Autónoma de Baja California (UABC). The ICA is located in a desert region known as the Mexicali Valley, Baja California, Mexico (32°24′ N and 115°22′W); this region has a warm desert climate (Bwh), with extreme average temperatures in summer (> 40 °C) and low rainfall (85 mm per year) concentrated mainly in the winter months (INEGI 2014).
Animals and managements
The study was carried on with 40 Dorper × Pelibuey crossbred hair sheep, all females, and phenotypically white, which were selected (n = 10 each group) as follows: (1) weaning ewe lambs (WEL) with body weight (BW) = 24.8 ± 1.3 kg and age = 3.0 months; (2) replacement nulliparous ewes (RNE) with BW = 37.3 ± 1.9 kg and age = 8.0 months; (3) non-pregnant and non-lactating multiparous ewes (NMEs) with BW = 49.6 ± 2.1 kg, age = 3 to 4 years and around 5 months after the last lambing; and (4) lactating multiparous ewes (LMEs) with BW = 38.0 ± 1.7 kg, age = 3 to 4 years and around 30 days post-lambing. Sheep females within each group were selected according to age, BW, no visual signs of disease, and phenotype (completely white). The availability of animals to select was as follows: 34 for WEL, 30 for RNE, 39 for NME, and 28 for LME. All animals were born in the Sheep Experimental Unit of the ICA, where the production system is intensive, and consequently, feeding consists in whole-mixed diets and formulated according to the nutritional requirements of each fattening stage and physiological state of the breeding ewes (NRC 2007). The feeding hours are 0800 and 1800 h, and the feed offered daily is ad libitum as well as the water availability. Table 1 shows the different diets utilized and their chemical composition.
Corrals where sheep females were housed had sheep mesh walls, galvanized sheet shade at the center, and feed and drinking troughs. The dimensions of the four corrals were 3 × 6 m (1.8 m2/animal), one per each type of sheep female group. The experiment lasted 30 days; the first 10 days were used for adaptation to the group and the remaining 20 days for taking measurements and blood sampling.
Body weight and climatic and physiological variables
Individual BW was recorded the first day when physiological variables were measured. Additionally, data on temperature (Te, °C), relative humidity (RH, %), and wind speed recorded during experimental period were collected from a meteorological station located near the study site, which was programmed to measure climatic conditions every 15 min. The temperature-humidity index (THI) was calculated as: THI = Te-[(0.31–0.31*RH)*(Te-14.4)] (Marai et al. 2007). Daily maximum, minimum, and average of the climatic variables were obtained overall and for two different periods of the day: morning (0600 to 0800 h) and afternoon (1700 to 1900 h).
The physiologic variables RT, RR, and skin and hair coat temperatures in different body regions were measured before feeding (7 samplings at 3-d interval each) both in the morning (0600 h) and afternoon (1700 h). A digital thermometer (DeltaTrak, Pleasanton, CA, USA) was introduced rectally during 1 min to determine RT; RR was measured by counting the number of breaths per minute (bpm). Temperatures of eye, nostril, and hair coat in different body regions (i.e., ear, loin, right flank, belly, rump, shoulder, and leg) were obtained by taking individual thermal images with an infrared thermographic camera (Fluke Ti10, Everett, WA, USA). Ewes were in the shade for at least 15 min before taking the images at a 2.5-m distance. All images were downloaded to a computer to be analyzed using the Fluke SmartView® software.
Blood components
On the same days that physiological variables were taken, a blood sample per female was collected from the jugular vein into 10-mL vacutainer tube, just before the morning feeding. These samples were transported to the Animal Physiology Laboratory of the ICA-UABC, located 500 m from the sampling site, to be centrifuged at 3500×g for 15 min at 10 °C. Vials of 2 mL were used to store duplicate aliquots of serum from each sample at − 20 °C. Concentrations of glucose, cholesterol, triglyceride, urea nitrogen (BUN), and total protein were determined using a blood auto-analyzer of liquid phase (EasyVet, KONTROLab, Morelia, Mich., México); concentrations of sodium, potassium, and chlorine were obtained with an electrolyte analyzer (LW E60A, LandWind, Shenzhen, China); and finally, thyroid hormone concentrations were measured using validated commercial kits (Monobind Inc., Lake Forest, CA, USA) in a fully automated ELISA analyzer (Thunderbolt, Gold Standar Diagnostics, CA, USA). The kit for triiodothyronine hormone (T3) had the following precision parameters: sensitivity = 0.04 ng/mL, intra-assay CV = 5.4%, and inter-assay CV = 6.7%, while the kit for thyroxine hormone (T4) had sensitivity = 0.1 ng/mL, intra-assay CV = 1.6%, and inter-assay CV = 6.1%. All blood compounds were analyzed in duplicate to record averages across all sampling times for each female.
Statistical analysis
Descriptive statistics were used to summarize climatic variables. Data of BW, physiological variables in each sampling time (morning or afternoon), and blood components were subjected to analysis of variance under a completely randomized design with the PROC GLM of the SAS program (SAS 2004). Means were analyzed through the following orthogonal contrasts: (1) ewe lambs (WEL) vs. older ewes (RNE + NME + LME), (2) nulliparous ewes (RNE) vs. multiparous ewes (NME + LME), and (3) non-lactating ewes (NMEs) vs. lactating ewes (LMEs). Statistical differences were declared at P ≤ 0.05 and tendencies between 0.05 < P ≤ 0.10.
Results
Climatic variables
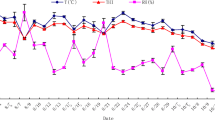
The daily averages for Te, RH, THI, and wind speed recorded during the study period were 31.6 °C, 43.3%, 28.5 units, and 5.4 m/s, respectively. Whereas Te was ≥ 30 °C only between 0900 and 2200 h, the THI remained above 22.2 units throughout the day (Fig. 1). Thus, Te and THI during the morning were 25.0 ± 2.4 °C and 24 ± 2.1 units, respectively, and both climatic variables drastically increased during the afternoon (Te = 35.6 ± 3.3 °C and THI = 31.1 ± 1.9 units; Table 2). The wind speed was 5.1 m/s greater in the afternoon than in the morning.
Ewe lambs versus older ewes
As was expected, ewe lambs weighed approximately 16.8 kg less (P < 0.01) than older ewes (Table 3). Rectal temperatures as well as temperatures from nostril, eye, and different regions of hair coat (i.e., ear, loin, right flank, belly, rump, shoulder, and leg) were higher (P < 0.01) in ewe lambs than in older ewes during both morning and afternoon periods. However, RR was only different (P < 0.01) in the afternoon, being higher in ewe lambs. Also, ewe lambs had lower (P ≤ 0.02) serum glucose and cholesterol concentrations, but higher (P < 0.01) BUN concentrations than older ewes (Table 4). No other blood component varied between these female groups.
Nulliparous ewes versus multiparous
Nulliparous ewes had 14% less BW (P < 0.01) than multiparous ewes (Table 3). In the morning, there was no difference between these ewes for RT and RR, whereas in the afternoon, RR was higher (P < 0.01) for nulliparous ewes. Eye temperature, as well as hair coat temperatures in the majority of the body regions (i.e,. loin, right flank, belly, rump, shoulder, and leg) were higher (P < 0.01) in nulliparous ewes than in multiparous during the morning but not in the afternoon, when no differences were detected for those variables. Temperatures from nostril and ear did not differ between these ewes in any time. In the case of blood components, nulliparous ewes had higher (P < 0.01) BUN concentrations and also a trend to have higher (P = 0.07) serum glucose concentrations compared to multiparous ewes (Table 4). The serum chlorine level was higher (P = 0.03) in multiparous ewes than in nulliparous. The remaining blood components were similar between these female groups.
Non-lactating ewes versus lactating
Non-lactating ewes showed higher initial BW (P < 0.01; 38 vs. 49.6 ± 1.8 kg) in relation to lactating ewes (Table 3). Both RT and RR were similar between these ewes in the morning, but in the afternoon, RT was higher (P < 0.01) in lactating ewes than in non-lactating, whereas RR was unaffected by lactation. With exception of temperatures from ear and loin recorded in the morning which did not differ between these ewes, hair surface temperatures obtained with the infrared thermographic camera were higher (P ≤ 0.03) in lactating ewes than in those non-lactating in any sampling time. Lactating ewes had lower (P < 0.01) serum levels of glucose, cholesterol, and BUN, but higher (P = 0.02) serum T3 levels than non-lactating (Table 4). The remaining blood components were unaffected by lactation.
Discussion
Conditions of heat stress for hair sheep breeds have been documented at Te ≥ 30 °C (Neves et al. 2009; Macías-Cruz et al. 2016a) and THI ≥ 22.2 units (Marai et al. 2007). While a THI between 22.2 and < 23.3 units is classified as moderate heat stress, THI from 23.3 to < 25.6 units is classified as severe heat stress, and a THI ≥ 25.6 unit is considered extreme severe heat stress (Marai et al. 2007). In this sense, sheep females of the present study were maintained under heat stress during the 24 h of the day (THI ≥ 23.3 units), being severe heat stress during the morning (THI = 24 ± 2.1 units) and extreme severe (THI = 31.1 ± 1.9 units) during the afternoon. However, despite the heat stress recorded in the morning, RR did not drastically increase (~ 43 rpm) compared with the thermoneutrality normal range of RR (16–30 rpm) indicated by Al-Dawood (2017).
Ewe lambs versus older ewes
Results suggest that age is a predisposing factor of the thermoregulatory capacity that hair breed sheep female exhibit under heat stress conditions. Ewe lambs were less able to dissipate body heat load through the day given that they exhibited higher RT than older ewes. There are reports indicating an increase in RT of lambs due to a greater metabolic heat production in thermoneutral conditions; this is a consequence of the higher basal metabolism showed by young animals (Mortola 2001; Todini 2007). However, we did not find differences in serum concentrations of thyroid hormones between ewe lambs and older ewes under heat stress conditions. Symonds et al. (1995) reported a reduction in blood T3 levels of lambs exposed to 28 °C compared with lambs exposed to < 14 °C. Therefore, the absence of the effect of age on thyroid hormone concentrations in this study could be attributed to a metabolic adjustment experienced by ewe lambs to reduce metabolic heat production and body heat load. Thus, ewe lambs reduced the activity of their thyroid gland to a similar status to that exhibited by older ewes. Meanwhile, the greater RT in ewe lambs was related to the lack of maturation of their thermoregulatory system (Thwaites 1967).
In sheep and other mammals, the homeostasis of body temperature is often incipient at birth and matures during the course of the first year of age (Piccione et al. 2002); so core temperature fluctuates with changes on Te in young animals and can reach stability until the second year of life in small ruminants (MacFarlane 1958; Piccione et al. 2007; Arfuso et al. 2016). Interestingly, ewe lambs had a difference in RT of 1.0 °C between morning and afternoon, but in older ewes was only 0.5 °C, confirming that hair breed sheep also achieve stability of core temperature at age older than 3 months. Therefore, weaning ewe lambs are expected to be less tolerant to hyperthermia conditions compared with older ewes.
Temperatures obtained by thermography suggest that ewe lambs dissipate a higher amount of body heat through the skin than older ewes during the morning and afternoon, since the temperature gradient of hair coat and Te was markedly higher in ewe lambs than in older ewes. This can be due to a greater surface area to dissipate heat load in ewe lambs, since they had around 40% less live weight than older ewes. Previous studies have demonstrated the importance of the BW/surface area ratio in the loss of body heat in animals, being more efficient in those smaller because they have higher surface area per each kilogram of weight (Lenis et al. 2015; Al-Dawood 2017). But when heat stress changed from severe (morning) to extreme severe (afternoon), independently of age, evaporative mechanisms were activated to make more efficient the body heat loss, being the main mechanism to increase RR; however, RR was 43% higher in ewe lambs during the afternoon. These findings demonstrate that, although lambs do not have complete control of their thermoregulation system, they can make great physiological efforts to avoid a hyperthermia to the point that may lead to death. In congruence with our results, Thwaites (1967) reported greater RT and RR in younger (16 months old) females compared to older (52 months old) females using Dorset Horn × Merino crossbred; however, no study was found comparing temperatures from nostril, eye, and hair surface of different body regions between lambs and older sheep under thermoneutral and heat stress conditions. Recently, higher RT and lower rumen temperature was observed in 3 to 5-month-old kids than older male goats in a thermoneutral environment (Arfuso et al. 2016).
Ewe lambs also showed lower serum glucose and cholesterol levels than older ewes, which does not coincide with previous results in relation to the effect of age on blood concentrations of these metabolites in sheep maintained under thermoneutral conditions. In Morada Nova hair breed sheep, Carlos et al. (2015) reported that lambs younger than 6 months old had greater serum concentrations of cholesterol without difference in glucose compared to sheep older than 12 months old. It should be noted that no study was found regarding serum metabolite concentrations in young and old sheep female subjected to heat stress environmental conditions. However, some studies carried out with crossbred hair sheep (Macías-Cruz et al. 2016b) and Malpura breed sheep (Indu et al. 2015), both genotypes adapted to high temperatures, indicate that outdoor heat stress in arid regions promotes a decrease in blood metabolites related to the energetic metabolism (i.e., glucose, cholesterol, triglycerides) as a consequence of the rise in RR. The functioning of muscles associated to the rise in breathing requires high energy amounts (Sejian et al. 2010). Thus, considering that ewe lambs had 45% more RR than older ewes in the afternoon, this RR increased explains the low levels of these metabolites in ewe lambs. Other possible explanation for results on glucose and lipid metabolites could be an elevation of blood insulin levels in ewe lambs due to heat stress (Baumgard and Rhoads 2013). In growing Afshari lambs subjected to severe heat stress conditions, Mahjoubi et al. (2015) reported an increase in blood insulin levels which promoted lipogenesis and prevented lipolysis in adipose tissue. So heat stress in ewe lambs decreased the blood levels of those metabolites because insulin incorporates glucose into cells, as well as cholesterol and triglycerides into fatty tissue.
Moreover, increased BUN levels in ewe lambs could be an indicative of low distribution of blood flow to the kidneys, which in turn decreases the elimination of this metabolite through urine (Srikandakumar et al. 2003). Higher coat temperatures in ewe lambs than in older ewes suggest a preferential redistribution of blood flow towards peripheral tissues rather than internal organs for heat-stressed lambs. Also, it should be considering that under thermoneutral conditions, a similar effect of age on BUN concentration for Dorper (Madureira et al. 2013) and Santa Ines (Lima et al. 2015) hair sheep female has been observed, and this was attributed to the fact that the diets of weaned lambs were formulated with a greater amount of protein. Although, none of those studies reported differences in serum total protein levels when compared ewe lambs versus older ewes, situation that matches with our results. Consequently, more research is required to clarify if higher BUN levels in heat-stressed lambs are due to environmental temperature, dietary protein intake, or the combination of both.
Finally, body water balance was unaffected by age in this sheep breed, given that serum electrolyte concentrations did not vary between young and older sheep females, and mean values of each electrolyte were within the reference range (Blood 2002). Previous studies were not found relative to the effect of age on electrolyte levels in heat-stressed sheep.
Nulliparous ewes versus multiparous
It seems that this is the first study comparing tolerance to heat stress between nulliparous and multiparous ewes of any breed. Both nulliparous and multiparous ewes showed similar ability to tolerate outdoor heat stress conditions, given that their RT did not differ across days. We expected a slightly lower tolerance to high Te by nulliparous ewes as their age is less than 1 year (8 months old), and consequently, their thermoregulatory system can be considered still immature (Piccione et al. 2002). However, overall results suggest that, at 8 months of age, hair breed nulliparous ewes have almost reached the correct functioning of their thermoregulatory system.
Nulliparous ewes reached similar RT than multiparous ewes throughout the day as product of the activation of different thermoregulatory mechanisms of physiological type, being heat loss by the skin the most important. In the morning, higher hair surface temperatures in most of the body regions of nulliparous ewes suggest greater thermogenesis, but this also indicates that greater body heat production in nulliparous ewes was mainly dissipated across the skin as RR did not vary with parturition number and Te was below hair temperatures. It is widely known that an initial response to heat stress in hair breed sheep is vasodilatation, resulting in a peripheral redistribution of the blood flow (Macías-Cruz et al. 2016b). Thus, skin temperature increases to activate non-evaporative heat loss mechanisms, mainly radiation and convection (Al-Dawood 2017). Results of physiological variables in the morning can be explained by the simultaneous action of two facts: (1) nulliparous ewes had higher body surface area to dissipate heat (Lenis et al. 2015), and (2) half of the multiparous ewes were lactating and preferred to use the skin of the mammary gland and the milk secretion as main pathways of heat loss rather than other body regions (Smith et al. 1977). On the other hand, in the afternoon, when the environment was of greater heat stress (extreme), RR showed to be an important evaporative mechanism to dissipate body heat load in both types of ewes (169 and 154% higher for nulliparous and multiparous ewes, respectively, regarding to the morning RR). Although afternoon RR was statistically higher in nulliparous ewes than in multiparous, the difference of 9 rpm between groups was marginal; in consequence, we do not believe that, in this daytime, nulliparous ewes had similar RT than multiparous ewes due to a greater RR. Even though these results are contradictory with those of hair coat temperatures (i.e., no statistical difference), the authors hypothesized that nulliparous ewes reached similar RT than multiparous ewes because they were more efficient to dissipate heat load across the skin regions. While hair coat temperatures were numerically higher in nulliparous ewes compared to non-lactating multiparous ewes, the contrast of non-lactating vs. lactating multiparous ewes showed that lactation is a factor to increase hair coat temperatures in the afternoon. Consequently, in the afternoon, the effect of lactation could mask the true effect of the parturition number on heat loss through the skin in the present study.
The metabolic response to outdoor heat stress conditions was slightly affected by the parturition number in the hair ewes used. Serum glucose levels tended to be lower in multiparous ewes, which was not attributed to the activation of the same thermoregulatory mechanism, but to the fact of having lactating ewes in the multiparous group. Thus, while serum glucose concentrations were 50.1 ± 1.5 and 50.4 ± 1.5 ng/dL for nulliparous and non-lactating multiparous ewes, respectively, for lactating multiparous ewes were of 42.1 ± 1.5 ng/dL. In agreement with these findings, previous studies in hair (Carlos et al. 2015) and wool (Durak et al. 2015) breed sheep did not report changes in serum glucose concentrations between nulliparous and non-lactating multiparous ewes kept under thermoneutral conditions. Therefore, the energy metabolism of nulliparous and non-lactating multiparous ewes is similar under heat stress conditions.
Moreover, BUN concentrations varied between ewe groups, being higher in nulliparous. This result does not agree with other reports indicating that BUN concentrations did not change with the number of deliveries in ewes without hyperthermia (Carlos et al. 2015; Durak et al. 2015). Possibly, heat stress increased BUN levels in nulliparous ewes because it provoked a reduction in the urea waste at kidney level. The reduction in the kidney function observed in nulliparous ewes could be due to the activation of two thermoregulatory mechanisms that helped these ewes to maintain an internal temperature similar to that of multiparous ewes: (1) redistribution of blood flow to peripheral tissue to dissipate body heat through the skin (Srikandakumar et al. 2003) and (2) reduction of water loss through urine to avoid dehydration (Piccione et al. 2012). The presence of the first mechanism in the nulliparous ewes can be inferred with thermography results while the second mechanism with electrolyte results (no difference between groups). The activation of these thermoregulatory mechanisms in Dorper × Pelibuey nulliparous ewe as a result of heat stress had already been reported by our research group (Macías-Cruz et al. 2016b), so these findings confirm them.
Non-lactating ewes versus lactating
Results of serum T3 concentrations and RT suggest that lactation increases the metabolic heat production and decreases the tolerance to heat stress in heat-stressed hair breed ewes. These findings are in line with previous results reported in wool breed ewes (Abdalla et al. 1993) and dairy cattle (Das et al. 2016) subjected to high Te conditions. Milk production is a process demanding high availability of nutrients and cellular activity within the mammary gland during the post-lambing period. So, the release of thyroid hormones increases and favors a rise in the metabolic activity of lactating mammals (Polsky and von Keyserlingk 2017). However, this metabolic scenario promotes intensification of the metabolic heat production that, in combination with a natural heat stress environment, can compromise the ability of lactating ewes to maintain normothermia, the situation observed in the current study. Therefore, lactation negatively affects the thermoregulatory capacity of multiparous hair breed ewes in hot environments.
Lactation did not affect RR during the morning and afternoon in our ewes, which disagrees with a study conducted by Abdalla et al. (1993). It has been extensively documented that increased RR is the main evaporative thermoregulation mechanism used by hair breed sheep to dissipate excess of body heat load when non-evaporative mechanisms fail to prevent hyperthermia (Macías-Cruz et al. 2016b). Recently, Fonseca et al. (2017) estimated that 90% of the total body heat loss was through the respiratory evaporation in Mora Nova hair sheep subjected to 35 °C or more. Based on the above, we do not have a clear response to this RR result, especially to the fact that afternoon RR did not increase despite the higher RT observed in lactating ewes. Although, it is important to note that losses of body heat though the respiratory tract played a key role during the afternoon in both ewe types, since afternoon RR increased more than the double (153%) compared to the morning. Therefore, the lack of lactation effect on RR suggests that, in combination with increased RR, there are other mechanisms helping to maintain the body heat balance in heat-stressed hair ewes during lactation.
Heat losses through the skin, milk secretion, and sweating could represent thermoregulatory mechanisms that maintain the thermal balance in lactating ewes under heat stress. Higher sweating rate and water intake, but lower feed intake, has been observed in heat-stressed wool ewes during early lactation (Abdalla et al. 1993). Meanwhile, Grebremedhin and Wu (2016) pointed out that dairy cows could be cooled by cooling the udder only, since large amounts of blood circulate constantly in the mammary gland; this situation allows loss of sensible and latent heat in a more efficient way. It has also been reported that the udder plays an important role in the body heat dissipation of lactating ewes (Smith et al. 1977). During summer season of an arid region, higher surface temperatures of body, udder, teat, and mammary veins were observed in lactating camels compared with those non-lactating (Samara et al. 2013). We did not measure temperature of udder skin and milk in the current study; however, belly temperature was higher in lactating ewes with a favorable thermal gradient for belly regarding environment Te. Overall hair coat temperatures were increased by lactation. Interestingly, the afternoon thermal gradients calculated between Te and thermography temperatures were always favorable to thermography temperatures in lactating ewes (1.1 °C in average), while for non-lactating ewes were only favorable in ear (0.9 °C) and belly (0.4 °C). Possibly, lactating ewes dissipated more heat through the skin because they had lower live weight than their counterpart, which led to a greater availability of surface area per kilogram of live weight in these females (Sevi and Caroprese 2012). Additionally, perhaps the body reserve mobilization in lactating ewes resulted in a reduction of the external fat thickness and this is facilitated by the dissipation of excess of body heat across the skin, since the body becomes less insulated by decreasing fat tissue (McManus et al. 2011; Al-Dawood 2017). Therefore, under extreme severe heat stress conditions, lactating hair breed ewes regulate their body temperature by combining heat loss through evaporative and non-evaporative mechanisms, while non-lactating ewes used mainly evaporative mechanisms.
Finally, lactation negatively affected serum levels of glucose, cholesterol, and BUN in hair breed ewes of the present study. Comparing high and low yielding dairy cows in a moderate heat stress environment, Alameen et al. (2014) found lower blood concentrations of glucose, cholesterol, urea, and cortisol in cows with high milk production. Abdalla et al. (1993) reported a drop in milk production and a rise in serum-free fatty acids levels, with no effect on serum glucose, due to heat stress in lactating wool breed ewes. Additionally, it is known that the synthesis of lactose in milk is very dependent on glucose uptake in the mammary gland because mammary tissue cannot produce glucose from glucogenic precursors in ruminants (Lérias et al. 2014). Thus, lactose production uses between 50 and 60% of glucose that reaches the mammary gland (Brockman 2005). Fatty acids mobilized from lipid tissue are also necessary for fat synthesis in milk. In consequence, the metabolic adjustments observed in lactating ewes could be related to the ability of this breed to maintain their milk yield despite high temperatures. Future studies on hair sheep should be directed to evaluate the effect of heat stress on milk production to support this hypothesis, since recent research has indicated that heat stress conditions promote low availability of glucose and fatty acids for milk synthesis in lactating cows (Baumgard and Rhoads 2013) and goats (Al-Dawood 2017). Hair sheep breeds are characterized by their high adaptation to warm climates, without any drastic effect on their productive and reproductive capacity (Macías-Cruz et al. 2013, 2016a).
Conclusions
Thermoregulatory capacity, and consequently tolerance to warm climates, varies with age and physiologic state in hair breed sheep females. Weaning ewe lambs and lactating multiparous ewes are more sensitive to heat stress than non-lactating nulliparous and multiparous ewes. While ewe lambs tolerate less heat stress by the lack of maturation of the thermoregulation center, lactating ewes are less tolerant to high temperatures because they have higher metabolic rate and metabolic heat production. Hair breed ewes at 8 months of age have adequate functionality of their thermoregulation center to tolerate heat stress condition in a similar way than dry and non-pregnant multiparous ewes.
References
Abdalla EB, Kotby EA, Johnson HD (1993) Physiological responses to heat-induced hyperthermia of pregnant and lactating ewes. Small Rumin Res 11:125–134
Alameen AO, Abdelatif AM, Elnageeb ME (2014) Circadian variations of thermoregulation, blood constituents and hormones in crossbred dairy cows in relation to level of milk production. J Vet Adv 4:466–480
Al-Dawood A (2017) Towards heat stress management in small ruminants—a review. Ann Anim Sci 17:59–88
Arfuso F, Rizzo M, Giannetto C, Giudice E, Fazio F, Piccione G (2016) Age-related changes of serum mitocondrial uncoupling 1, rumen and rectal temperature in goats. J Therm Biol 59:47–51
Baumgard LH, Rhoads P (2013) Effects of heat stress on postabsorptive metabolism and energetics. Annu Rev Anim Biosci 1:7.1–7.27
Blood DC (2002) Manual de Medicina Veterinaria (9 Ed.). Editorial McGraw-Hill/Interamericana de España. Pp. 1–790
Brockman RP (2005) Glucose and short chain fatty acid metabolism. In: Dijkstra J, Forbes JM, France J (eds) Quantitative aspects of ruminant digestion and metabolism. CAB International, Wallingford, pp 291–309
Carlos MML, Leite JHGM, Chaves DF, Vale AM, Facanha DAE, Melo MM, Soto-Blanco B (2015) Blood parameters in the Morada Nova sheep: influence of age, sex and body condition score. J Anim Plant Sci 25:950–955
Das R, Sailo L, Verma N, Bharti P, Saikia J, Imtiwati KR (2016) Impact of heat stress on health and performance of dairy animals: a review. Vet World 9:260–268
Durak MH, Erkan REC, Ḉelik R, Yokus B, Kurt D, Gürgöze S (2015) The effects of age and gender on some biochemical serum parameters in Zom sheep raised in the vicinity of Karacadağ. Isr J Vet Med 70:33–39
Fonseca VCF, Saraiva EP, Maia ASC, Nascimiento CCN, da Silva JA, Pereira WE, Filho ECP, Almeida MEV (2017) Models to predict both sensible and latent heat transfer in the respiratory Trat of Morada Nova sheep semiarid tropical environment. Int J Biometeorol 61:777–784
Grebremedhin KG, Wu B (2016) Modeling heat loss from the udder of a dairy cow. J Therm Biol 59:34–38
Indu S, Sejian V, Naqvi SMK (2015) Impact of simulated heat stress on growth, physiological adaptability, blood metabolites and endocrine responses in Malpura ewes under semiarid tropical environment. Anim Prod Sci 55:766–776
INEGI (2014) Anuario estadístico y geográfico de Baja California. Available in: http://www.datatur.sectur.gob.mx/ITxEF_Docs/BCN_ANUARIO_PDF.pdf
Lenis SY, Zuluaga CAM, Tarazona MAM (2015) Adaptive response to thermal stress in mammals. Rev Med Vet 31:121–135
Lérias J, Hernández-Castellano L, Suárez-Trujillo A, Castro N, Pourlis A, Almeida A (2014) The mammary gland in small ruminants: major morphological and functional events underlying milk production—a review. J Dairy Res 81:304–318
Lima MB, Monteiro MVB, Jorge EM, Campello CC, Rodrigues LFS, Viana RB, Monteiro FOB, Costa CTC (2015) Blood reference intervals and the influence of age and gender on hematologic and biochemical parameters of Santa Ines sheep in the eastern Amazon. Acta Amazon 45:317–322
MacFarlane WV (1958) Experimental approaches to the functions of tropical livestock. Arid Zone Res (UNESCO) 11:227–234
Macías-Cruz U, Avendaño-Reyes L, Álvarez-Valenzuela FD, Torrentera-Olivera NG, Meza-Herrera CA, Mella-Bosque M, Correa-Calderón A (2013) Growth and carcass characteristics of ewe lambs treated with zilpaterol hydrochloride during spring and summer. Rev Mex Cienc Pecu 4:1–12
Macías-Cruz U, Gastelum MA, Álvarez-Valenzuela FD, Correa-Calderón A, Díaz R, Meza-Herrera CA, Mellado M, Avendaño-Reyes L (2016a) Effects of summer heat stress on physiologic variables, ovulation and progesterone secretion in Pelibuey ewes under natural outdoor conditions in an arid region. Anim Sci J 87:354–360
Macías-Cruz U, López-Baca MA, Vicente R, Mejía A, Álvarez FD, Correa-Calderón A, Meza-Herrera CA, Mellado M, Guerra-Liera JE, Avendaño-Reyes L (2016b) Effects of seasonal ambient heat stress (spring vs. summer) on physiological and metabolic variables in hair sheep located in an arid region. Int J Biometeorol 60:1279–1286
Madureira KM, Gomes V, Barcelos B, Zani BH, Shecaira CL, Baccili CC, Benesi FS (2013) Hematological and biochemical parameters of Dorper ewes. Semina Ciências Agárias 34:811–816
Mahjoubi E, Hossein YM, Aghaziarati N, Noori GR, Afsarian O, Baumgard LH (2015) The effect of cyclical and severe heat stress on growth performance and metabolism in Afshari lambs. J Anim Sci 93:1632–1640
Marai IFM, El-Darawany AA, Fadiel A, Abdel-Hafez MAM (2007) Physiological traits as affected by heat stress in sheep- a review. Small Rumin Res 71:1–12
McManus C, Louvandini H, Gugel R, Sasaki LCB, Bianchini E, Bernal FEM, Paiva SR, Paim TP (2011) Skin and coat traits in sheep in Brazil and their relation with heat tolerance. Trop Anim Health Prod 43:121–126
Mortola JP (2001) Respiratory physiology of newborn mammals: a comparative perspective. Johns Hopkins University Press, Baltimore
Neves MLMW, de Azecedo M, da Costa LAB, Guim A, Leite AM, Chagas JC (2009) Critical levels of the thermal comfort index for Santa Ines sheep under grazing at the agreste region of Pernambuco state. Acta Sci Anim Sci 31:169–175
NRC (2007) Nutrient requirements of small ruminants: sheep, goat, cervids, and new world camelids. Natl Acad. Press, Washington, D.C.
Piccione G, Caola G, Ferinetti R (2002) Maturation of the daily body temperature rhythm in sheep and horse. J Therm Biol 27:333–336
Piccione G, Caola G, Refinetti R (2007) Annual rhythmicity and maturation of physiological parameter in goats. Res Vet Sci 83:239–243
Polsky L, von Keyserlingk MAG (2017) Invited review: effects of heat stress on dairy cattle welfare. J Dairy Sci 100:8645–8657
Samara EM, Ayadi M, Al-Haidary AA, Aljumaah RS (2013) Thermophysiological study in lactating and dry camels (Camelus dromedarius) under summer conditions. Emir J Food Agric 25:308–313
SAS (ed) (2004) SAS/STAT: User’s guide statistics released 9.1, 2nd edn. SAS Institute, Inc., Cary
Sejian V, Maurya VP, Naqvi SMK (2010) Adaptability and growth of Malpura ewes subjected to thermal and nutritional stress. Trop Anim Health Prod 42:1763–1770
Sevi A, Caroprese M (2012) Impact of heat stress on milk production, immunity and udder health in sheep: a critical review. Small Rumin Res 107:1–7
Smith RE, Heath ME, Ingram DL (1977) Role of the udder in heat loss from the sheep. J Therm Biol 3:125–128
Srikandakumar A, Johnson EH, Mahgoub O (2003) Effect of heat stress on respiratory rate, rectal temperature and blood chemistry in Omani and Australian merino sheep. Small Rumin Res 49:193–198
Symonds ME, Andrews DC, Buss DS, Clarke L, Darby CJ, Johnson P, Lomax MA (1995) Environmental effects on thermoregulation and breathing patterns during early postnatal development in hand-reared lambs. Exp Physiol 80:779–792
Thwaites CJ (1967) Age and heat tolerance in sheep. Int J Biometeorol 11:209–212
Titto CG, Veríssimo CJ, Pereira AMF, Geraldo AM, Katiki LM, Titto EAL (2016) Thermoregulatory response in hair sheep and shorn wool sheep. Small Rumin Res 144:341–345
Todini L (2007) Thyroid hormones in small ruminants: effects of endogenous, environmental and nutritional factors. Animal 1:997–1008
Piccione G, Messina V, Vazzana I, Dara S, Giannetto C, Assenza A (2012) Seasonal variations of some serum electrolyte concentrations in sheep and goats. Comparative Clinical Pathology 21 (5):911–915.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Macías-Cruz, U., Correa-Calderón, A., Mellado, M. et al. Thermoregulatory response to outdoor heat stress of hair sheep females at different physiological state. Int J Biometeorol 62, 2151–2160 (2018). https://doi.org/10.1007/s00484-018-1615-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-018-1615-2