Abstract
Twenty Dorper × Pelibuey primiparous ewes were used to evaluate effects of seasonal ambient heat stress (i.e., spring vs. summer) on physiological and metabolic responses under production conditions in an arid region. Ten ewes experiencing summer heat stress (i.e., temperature = 34.8 ± 4.6 °C; THI = 81.6 ± 3.2 units) and 10 under spring thermoneutral conditions (temperature = 24.2 ± 5.4 °C; THI = 68.0 ± 4.8 units) were corralled together to measure rectal temperature, respiratory frequency, and skin temperatures at 0600, 1200, 1800, and 2400 h on four occasions over 40 days. Blood metabolite and electrolyte concentrations were also measured at 0600 and 1800 hours. Data were analyzed with a completely randomized design using repeated measurements in time. Rectal and skin temperatures, as well as respiratory frequency, were higher (P < 0.01) in summer than spring at all measured days. Blood serum glucose, cholesterol, triglycerides, and chlorine concentrations were lower (P < 0.01) in summer than spring at 0800 and 1800 hours. In contrast, summer heat stress increased (P < 0.01) blood urea and potassium concentrations at 0800 and 1800 hours. Compared with spring thermoneutral conditions, summer heat stress affected the physiological and metabolic status of hair breed ewes in an arid region, which included blood metabolite and electrolyte adjustments to efficiently cope with summer heat stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sheep production is a livestock activity widely practiced in semiarid and arid ecological regions, which covers one third of the world surface (González-Bulnes et al. 2011). However, meat production in those regions is seasonal due to reproductive and growth seasonality because of high variability in climatic conditions (Macías-Cruz et al. 2013a). While summer is warm and dry with temperatures ≥40 °C with little rainfall, the remaining seasons are generally thermoneutral (i.e., autumn, spring) or slightly cold (i.e., winter). Thus, the heat stress conditions prevailing during summer becomes a key factor that usually leads to low productivity of sheep flocks. However, there are sheep breeds more adapted than others to hot conditions that have demonstrated ability to grow and reproduce in any season in arid climates (Silanikove 2000; Marai et al. 2007).
Hair sheep breeds such as Pelibuey, Dorper, Katahdin, and their crosses have adapted to the environmental conditions of arid regions, and they exhibit rectal temperatures (Ross et al. 1985; Macías-Cruz et al. 2015) and growth rates within normal ranges (Macías-Cruz et al. 2013b). Gastelum-Delgado et al. (2015) showed that summer heat stress of an arid region does not compromise the reproductive activity of Pelibuey ewes. However, available information is insufficient to understand how hair sheep face the thermal insult without affecting their production under summer conditions in arid regions.
Hair sheep breeds easily adapt to high environmental temperatures because they produce low concentrations of thyroid hormones, so they tend to reduce their metabolic heat production, and their breathing is slower and deeper than wool breeds (Ross et al. 1985; Correa et al. 2012; Romero et al. 2013). McManus et al. (2011) mentioned that hair sheep breeds have characteristics of their skin and coat which allow adaptation to heat stress including low coat thickness, absence of wool, short hair, reduced numbers of hair per square centimeter of skin, large sweat glands, and large proportion of skin with sweat glands. However, no study has examined the role of blood metabolites and electrolytes in the ability of hair sheep breeds to efficiently tolerate heat stress. It is well known that heat stress-adapted ruminants have metabolic alterations in response to activation of the physiological mechanisms of thermoregulation, although results concerning effects of heat stress on metabolic status in sheep are contradictory, even within the same breed.
In Australian Merino sheep, Alhidary et al. (2012) reported no effect of induced heat stress on serum concentrations of glucose, cholesterol, total protein, sodium, and potassium. In Malpura sheep, a breed adapted to arid regions, Sejian et al. (2013) observed increased blood glucose and cholesterol concentrations, but decreased blood total protein and albumin concentrations due to induced heat stress (i.e., 42 °C). In a more recent study using the same breed, Indu et al. (2015) reported lower blood glucose and cholesterol levels, but higher blood urea N in heat-stressed Malpura sheep compared to controls. Regarding this discrepancy among results, Alhidary et al. (2012) and Sejian et al. (2013) applied a short-term heat stress (7 days), and Indu et al. (2015) long-term heat stress (35 days). Apparently in sheep, hyperthermia conditions during prolonged periods reduce blood metabolite levels related to the energetic metabolism and increase metabolite concentrations linked to protein metabolism. Meanwhile, under short-term heat stress, the metabolites associated with energy metabolism may increase to ensure high availability of energy substrates required at the time of physiological adjustments (Niyas et al. 2015).
Therefore, considering the high fluctuations in results of blood metabolite and electrolyte concentrations by effect of heat stress in sheep and the lack of information in heat-stressed hair breeds, it is very relevant to investigate physiological and metabolic adjustments made by hair breed sheep to adapt to high summer temperatures registered in arid regions. The objective of this study was to evaluate effects of the season of the year (i.e., spring vs. summer) on physiological and metabolic variables of hair sheep subjected to intensive production conditions in an arid region.
Materials and methods
Experimental location
This study was completed at the Sheep Experimental Unit of the Instituto de Ciencias Agrícolas, Universidad Autónoma de Baja California, located in the Mexicali Valley, northwestern México (32° 24′ N and 115° 22′ W). The climate in the region is arid and dry, with environmental conditions of Sonoran Desert. All procedures involving sheep were conducted within the guidelines of approved local official techniques of animal care in Mexico (NOM-051-ZOO-1995: humanitarian care of animals during mobilization).
Animals, treatments, and managements
Ten Dorper × Pelibuey primiparous ewes (young, not pregnant, never lambed) with initial body weights (BW) of 46.7 ± 3.1 kg and BCS of 3.0 ± 0.2 units (5-point scale; 1 = emaciated and 5 = obese; Russel et al. 1969) were used on each season. All ewes were ∼10 months of age at the start of each experimental period and phenotypically were completely white. Daily weather conditions during the experimental periods were obtained from a meteorology station located near the study site. Combining the environmental temperature (Te) and relative humidity (RH), the temperature humidity index (THI) was estimated using the formula proposed by Hahn (1999) as THI = 0.81 × Te + RH (Te − 14.40) + 46.40. According to the measured THI values, climatic conditions during summer were considered as heat stress (Te = 34.8 ± 4.6 °C, RH = 32.1 ± 17.3 %, and THI = 81.6 ± 3.2 units), while spring conditions were considered to be thermoneutral (Te = 24.2 ± 5.4 °C, RH = 24.8 ± 14.8 %, and THI = 68.0 ± 4.8 units).
The group of ewes used in each season was confined in one 5 × 6 m pen (3 m2/animal) during the evaluation period. The pen was equipped with feed and drinking troughs; also, with galvanized sheet shade located in the center of the pen at 2.5 m above grade. Walls of the corral were sheep mesh to ensure an adequate air flow. The diet consisted of chopped forage (50 % wheat straw and 50 % alfalfa hay [90 % of DM, 10 % of CP, and 1.8 Mcal of metabolizable energy (ME) per kilogram of dry matter (DM)]) and was offered ad libitum in the morning (0700 hours) and afternoon (1700 hours). Fresh water was available at all the times.
Sampling and measurements
The study variables were recorded four times in 40 days at 10 days interval in summer and spring. Physiological variables measured four times per sampling day (i.e., 600, 1200, 1800, 2400 hours) were rectal temperature (RT), respiration rate (RR), and skin temperature (i.e., head, shoulder, right flank, rump, leg). The RT was measured by introducing a transrectal digital thermometer (DeltaTrak, Pleasanton, CA, USA), RR was measured by counting the number of breaths per minute (bpm), and all skin temperatures were measured through infrared thermography using a thermal imaging camera with infrared fusion (Fluke Ti10, Everett, WA, USA).
Blood samples were collected at 0600 and 1800 hours from the jugular vein into 6-ml vacutainer tubes, which were then centrifuged at 3500×g for 15 min at 10 °C. The serum was stored in duplicate 2-ml vials at −20 °C until analysis for blood metabolites and electrolytes. A blood auto-analyzer of liquid phase (EasyVet, KONTROLab, Morelia, Mich., México) was utilized to determine metabolite concentrations (i.e., glucose, cholesterol, triglyceride, urea N [BUN], total protein), while blood electrolytes (i.e., sodium, potassium, chlorine) were obtained with an electrolyte analyzer (LW E60A, LandWind, Shenzhen, China).
Experimental ewes were not adapted to sampling management (i.e., blood sampling). However, from birth to the time of the experiment, females were accustomed to the presence of people and to restrain activities since they received monthly management; likewise, students and employees entered daily to the pens to provide food both morning and afternoon.
Statistical analysis
Data were analyzed under a completely randomized design with repeated measures in time using the MIXED procedure of SAS (2004). The model included fixed effects of season, hour of the day and the interaction season × hour of the day. Animal was nested within season as a random effect. Several variance-covariance structures were verified to fit the model, and the structure compound symmetry showed the best fit according to BIC and AIC criteria. Means were separated using the option LSMEANS/PDIFF, accepting significant differences if P ≤ 0.05.
Results
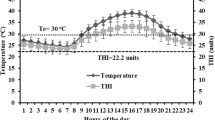
Climatic conditions of both seasons are in Table 1 and Fig. 1. In spring, the average temperature, RH, THI, and wind speed were 24.2 ± 3.8 °C, 24.8 ± 14.8 %, 68.0 ± 3.5 units, and 2.5 ± 1.5 m/s, respectively, but in summer, they were 34.8 ± 2.4 °C, 32.1 ± 17.3 %, 81.6 ± 1.8 units, and 2.8 ± 1.3 m/s, respectively. The highest temperature, THI, and wind speed were at 1200 and 1800 hours in both seasons. The highest RH in spring and summer was at 0600 and 2400 hours (Table 1). In general, THI values across hours of the day ranged from 77.0 (0500 hours) to 83.9 (1500–1600 hours) units in summer, and from 62.3 (0500 hours) to 72.6 (1500 to 1600 hours) units in spring (Fig. 1).
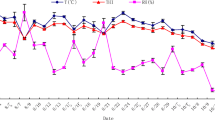
The RT and RR season × hour of the day interaction are in Fig. 2. Rectal temperature and RR were higher (P ≤ 0.03) in summer than in spring at all sampling hours. Compared with spring, RT and RR in summer were higher by 0.25 °C and 56 bpm, respectively. The largest difference (P < 0.01) between seasons for RT was at 1200 hours (i.e., 39.2 vs. 39.5 ± 0.09 °C) and for RR at 1800 hours (75.1 vs. 172.0 ± 4.3 bpm).
The skin temperature season × hour of the day interaction is in Table 2. Overall, skin temperatures (i.e., head, shoulder, right flank, leg, rump) in summer were higher (P ≤ 0.01) than spring at all sampling hours. The highest (P ≤ 0.01) skin temperature in the different regions of the body was found at 1200 hours in both seasons. However, the difference between seasons for each skin temperature at 1200 (∼8.2 °C) was lower (P ≤ 0.01) compared with the times of the day 0600 (∼15.7 °C), 1800 (∼11.4 °C), and 2400 (∼15.7 °C) hours.
The blood metabolite concentrations season × hour of the day interaction is in Table 3. Blood concentrations of glucose, cholesterol, and triglyceride were higher (P ≤ 0.04) in spring than summer at 0600 and 1800 hours, although opposite results occurred for total protein concentration at 0600 hours and BUN at 0600 and 1800 hours. Blood levels of total protein at 1800 hours were not affected by season.
The blood electrolyte concentrations season × hour of the day interaction is in Table 4. Blood K+ concentrations were higher (P = 0.04) in summer at 0600 and 1800 hours, while blood Cl− levels were higher (P < 0.01) in spring at the same times of the day. Season did not affect blood Na+ concentrations in any sampling hour.
Discussion
The THI is an important indicator to categorize the degree of environmental heat stress because its calculation combines Te and RH. According to Neves et al. (2009), hair breed sheep tends to exhibit heat stress when the mean temperature is above 30 °C and the THI is ≥78 units; consequently, our ewes were subject to heat stress during summer (∼35 °C and 82 units) and to thermoneutral conditions in spring (∼24 °C and 68 units). Marai et al. (2007) observed that moderate and severe heat stress in sheep occurs when THI is ≥82 and <84 units and ≥84 and <86 units, respectively. Thus, summer heat stress among hours of the day was classified as moderate in this study. Although, recently, López et al. (2015) found that hair sheep as the West African breed can start to experience heat stress at THI ≥72 units, such as in dairy cattle that has been reported (Avendaño-Reyes 2012). Moreover, the average wind speed in both seasons was ≥2 m/s during daylight hours, which would be helpful to dissipate body heat (Mader et al. 1999). Furthermore, a reduction of Te at night during spring was favorable to loss of accumulated heat during daytime and, therefore, to avoid negative effects on the development of these ewe lambs.
The RT was within the normal range in spring and summer, which is from 38.3 to 39.9 °C (Marai et al. 2007), suggesting a high adaptation of hair breed sheep to arid climates. In general, ewes had 0.25 °C more of RT in summer than in spring. In this regard, a review of Wildeus (1997) indicated that hair breed sheep such as Black Belly, Dorper, Kathadin as well as their crosses with wool breeds are highly adaptable to heat stress, since their thermoregulation mechanisms very efficiently maintain homeothermy. Similarly, Romero et al. (2013) reported the same result (i.e., RT within normal range) when comparing Pelibuey hair sheep with Suffolk wool sheep. Using West African ewe lambs grazing with and without artificial shade, Pinto-Santini et al. (2014) found also averages of RT between 39.0 and 39.7 °C. Moreover, compared with spring, environment conditions of summer provoked higher RT variation during the day, although the RT circadian rhythm in both seasons showed some variation as the environment temperature changed. Marai et al. (2007) and Kalyan et al. (2014) mention that increased body temperature in sheep is an expected physiological response to the rise in environmental temperature through the day or, in general, during the hot season of the year. Consistent with our results, studies in Pelibuey (Tabarez-Rojas et al. 2009; Romero et al. 2013), West African (Pinto-Santini et al. 2014), and St. Croix (Monty et al. 1991) sheep also found higher RT due to heat stress, but no study in hair sheep has reported diurnal variations for RT. However, some wool breeds such as Kheri (Kalyan et al. 2014) and Najdi (Al-Haidary et al. 2012) which are adapted to heat stress have exhibited similar circadian rhythm of RT during summer and spring to those in our Dorper × Pelibuey ewe lambs.
However, the activation of thermoregulation mechanisms in sheep is an expected compensatory response to dissipate body heat load and to avoid compromising their homoeothermic conditions. Thus, in seasons where climatic conditions are thermoneutral, sensible heat losses combined with diurnal variations in environmental temperature are adequate to regulate body temperature, although exposure to heat stress during the warm season may result in activation of heat loss mechanisms that take place in a sequential manner, starting with non-evaporative loss and then evaporative loss, or a combination of both processes (Cain et al. 2006; Marai et al. 2007). Initially, blood flow is redistributed to peripheral organs from body core, which promotes increased skin temperature and more heat loss via radiation and convection by a differential in the temperature gradient between skin and environment. If this mechanism alone is ineffective to maintain homeothermy in sheep, evaporative heat losses are implemented, such as increased respiration frequency or sweating (Robinson 2002). Indeed, it is estimated that ∼65 % of body heat losses in sheep is through the respiratory tract during hyperthermia (Hales and Brown 1974). This explains why ewe lambs in summer had higher coat surface temperature in different body areas and RR through the day, as well as variations in circadian rhythms in each season for RR. Consistent with these results, Romero et al. (2013) reported an increase in RR of Pelibuey and Suffolk ewes as the temperature increased in a climatic chamber from 18.0 to 39.5 °C during a 6-h period. Similarly, using Santa Ines lambs or their crosses with Texcel or Ile de France, Correa et al. (2012) observed lower RR, skin temperature, and sweating rate in the morning (THI < 70 units) than the afternoon (THI = 71 to 78 units).
In general, our results suggest that variations across the day in coat surface temperature and RR from white hair sheep are directly related to the environmental temperature in spring (thermoneutral); however, in summer (hyperthermia), circadian fluctuations in coat surface temperatures depends on the environment temperature, while changes in RR depends on the presence of a minimum difference in the thermal gradient between temperatures of surface and environmental. Thus, interestingly, the circadian rhythm of RR varied by season but the circadian rhythm of coat surface temperatures (i.e., head, shoulder, leg, right flank, rump) was not impacted. In spring, the highest surface temperatures and RR were at midday, while in summer, they were at midday and afternoon, respectively. This variation in circadian rhythm of RR could be a physiological adaptative mechanism implemented by hair ewes to maintain homeothermy and to avoid dehydration in warm natural environments.
Congruent with this finding, some Desert-adapted ungulates have developed an ability to tolerate high accumulated heat load during sunlight hours, which by the afternoon or night is unloaded by evaporative means, mainly increasing RR (Cain et al. 2006). Using induced heat stress (28 to 38 °C) to Australian Merino wethers during a week (24 h, 7 days), Alhidary et al. (2012) found the highest RR at 1800 hours, even when the exposition between 1200 and 1800 hours was constant at 38 °C, which agrees with our results. However, Kalyan et al. (2014) reported similar circadian rhythm in Kheri sheep when they were exposed to spring conditions of 9.6 to 14.8 °C, summer (30.7 to 44.3 °C), and autumn (17.6 to 36.74 °C) in a semiarid region. Thus, our results show that circadian rhythm of RR in hair sheep genotypes is altered in warm season, possibly as a strategy to lose body heat more effectively.
The impact of heat stress on the metabolic status of Pelibuey breed sheep or its crosses has not been studied. Marai et al. (2008) indicated that the metabolic response to hyperthermia conditions depends on several factors including genetics (i.e., breed, age, hair color, physiological status) and environmental (i.e., diet, water availability, temperature, time of exposure to hyperthermia), so that research results can appear inconsistent. Alhidary et al. (2012) reported that heat stress for 7 days did not affect blood metabolite concentrations (i.e., glucose, cholesterol, total protein) in Merino wethers, and Srikandakumar et al. (2003) found lower glucose concentration and higher BUN concentration in Omani wethers (a heat-adapted breed) during the warm season (THI = 93 units) compared with thermoneutral season (THI = 72 units). However, results were the opposite when used Merino wool breed. Heat stress conditions also lead to a decrease in blood levels of glucose, cholesterol, and total protein (Sejian et al. 2010; Indu et al. 2015) and to an increase in BUN concentrations in Malpura ewes (Indu et al. 2015). Consistent with most studies previously described, Dorper × Pelibuey ewe lambs showed, both during morning and afternoon, a reduction in blood concentrations of glucose, cholesterol, and triglyceride associated with summer temperatures. Also, summer hyperthermia conditions elicited a rise in BUN concentrations at 0600 and 1800 hours. It is possible that ewe lambs had decreased blood glucose in summer because of the elevated energy cost that occurs with the rise in RR as a thermoregulatory mechanism (Srikandakumar et al. 2003; Sejian et al. 2010). At the same time, the reduction in glucose levels may have caused the drop in blood cholesterol and triglyceride levels due to lipolysis (Rasooli et al. 2004; Indu et al. 2015). Moreover, BUN results are attributed to the low blood flow directed to kidneys when heat stress is experimented in this breed, since most blood is redirected to skin and muscles associated with breathing in order to dissipate heat (Srikandakumar et al. 2003). Thus, urea release from the body in the urine was low. Other studies in sheep adapted to heat stress are consistent with this hypothesis (Srikandakumar et al. 2003; Indu et al. 2015).
In our study, heat stress increased K+ and decreased Cl− in blood without affecting Na+. Other studies also found an effect of season of the year on electrolyte levels (Al-Haidary et al. 2012; Piccione et al. 2012; Ramana et al. 2013; Rashid et al. 2013). For example, using Najdi sheep, Al-Haidary et al. (2012) reported higher serum Na+ and Cl− levels in summer than winter; while Rashid et al. (2013), using indigenous sheep, indicated no effect of warm season on these electrolytes. However, Ramana et al. (2013) observed decreased serum electrolyte concentration with heat stress in Deccani and Nellore sheep. Additionally, no study observed changes between seasons in serum K+ concentration. Discrepancies among studies can be due to genetic variations among breeds, feeding, heat stress level, or other factors. In general, Dorper × Pelibuey ewes were not dehydrated in the high environmental temperatures, given that serum Na+ concentrations were not affected by season of the year and that the measured electrolytes had mean values within normal ranges for sheep (Blood 2002). Sodium is the primary ion involved in metabolic process and holds a central position in fluid and electrolyte balance (Piccione et al. 2012). Also, we observed that serum Cl− and K+ of ewes decreased and increased, respectively, during summer season, which in combination with the result of BUN, can be an indicative that ewes maintained their water balance through reduction of fecal and urinary water losses (Piccione et al. 2012).
Conclusions
In arid regions, season of the year modified the physiological and metabolic status of hair breed ewes housed in corrals. The ewe lambs had normal values of physiological variables and blood levels of metabolites and electrolytes under spring thermoneutral conditions, while in summer, the respiratory frequency, skin temperatures, and blood components were altered as physiological adjustment to cope with high ambient temperatures. In general, Dorper × Pelibuey ewes had a high adaptive capacity to summer heat stress because they maintained rectal temperatures in a normal range and they were very efficient in losing body heat load through the day by increasing RR and skin temperature and variations in the RR circadian rhythm. Likewise, the reduction in kidney function in summer may indicate activation of mechanisms to prevent dehydration, which is favorable to maintain normothermia.
References
Al-Haidary AA, Aljumaah RS, Alshaikh MA, Abdoun KA, Samara EM, Okab AB, Alfuraiji MM (2012) Thermoregulatory and physiological responses of Najdi sheep to environmental heat load prevailing in Saudi Arabia. Pak Vet J 32:515–519
Alhidary IA, Shini S, Al Jassim RAM, Gaughan JB (2012) Physiological responses of Australian Merino wethers exposed to high heat load. J Anim Sci 90:212–220
Avendaño-Reyes L (2012). Heat stress management for milk production in arid zones. Milk production—an up-to-date overview of animal nutrition, management and health. Prof. Narongsak Chaiyabutr (Ed.), ISBN: 978–953-51-0765-1, InTech, DOI: 10.5772/51299.
Blood DC (2002) Manual de Medicina Veterinaria (9 Ed.). Editorial McGraw-Hill/Interamericana de España. Pp. 1–790.
Cain IIIJW, Krausman PR, Rosenstock S, Turner JC (2006) Mechanisms of thermoregulation and water balance in desert ungulates. Wildlife Soc B 34:570–581
Correa MPC, Cardoso MT, Castanheria M, Landim AV, Dallago BSL, Louvandini H, McManus C (2012) Heat tolerance in three genetic groups of lambs in central Brazil. Small Ruminant Res 104:70–77
Gastelum-Delgado MA, Avendaño-Reyes L, Álvarez-Valenzuela FD, Correa-Calderón A, Meza-Herrera CA, Mellado M, Macías-Cruz U (2015) Conducta estral circanual en ovejas Pelibuey bajo condiciones áridas del noroeste de México. Rev Mex Cienc Pecu 6:109–118
González-Bulnes A, Meza-Herrera CA, Rekik M, Ben Salem H, Kridli RT (2011) Limiting factors and strategies for improving reproductive outputs of small ruminants reared in semi-arid environments. In: Degenovine KM (ed) Semi-arid environments: agriculture, water supply and vegetation. Nova Science Publishers, Inc., New York, pp. 41–62
Hahn GL (1999) Dynamic responses of cattle to thermal heat loads. J Dairy Sci 82(Suppl. 2):10–20
Hales JR, Brown GD (1974) Net energetic and thermoregulatory efficiency during panting in the sheep. Comp Biochem Physiol a Comp Physiol 49:135–190
Indu S, Sejian V, Naqvi SMK (2015) Impact of simulated heat stress on growth, physiological adaptability, blood metabolites and endocrine responses in Malpura ewes under semiarid tropical environment. Anim Prod Sci 55:766–776
Kalyan De, Kumar D, Singh AK, Sahoo A, Naqvi SMK (2014) Seasonal variation of physiological response in ewes of farmers’ flocks under semi-arid tropical environment. Biol Rhythm Res 45:397–405
López R, Pinto-Santini L, Perozo D, Pineda J, Oliveros I, Chacón T, Rossini M, Ríos AL (2015) Thermal comfort and growth of West African lambs grazing with and without access to artificial shade. Arch Zootec 64:139–146
Macías-Cruz U, Álvarez-Valenzuela FD, Correa-Calderón A, Díaz-Molina R, Mellado M, Avendaño-Reyes L (2013a) Thermoregulation of nutrient-restricted hair ewes subjected to heat stress during late pregnancy. J Therm Biol 38:1–9
Macías-Cruz U, Avendaño-Reyes L, Álvarez-Valenzuela FD, Torrentera-Olivera NG, Meza-Herrera CA, Mella-Bosque M, Correa-Calderón A (2013b) Growth and carcass characteristics of ewe lambs treated with zilpaterol hydrochloride during spring and summer. Rev Mex Cienc Pecu 4:1–12
Macías-Cruz U, Gastelum MA, Álvarez-Valenzuela FD, Correa-Calderón A, Díaz R, Meza-Herrera CA, Mellado M, Avendaño-Reyes L (2015) Effects of summer heat stress on physiologic variables, ovulation and progesterone secretion in Pelibuey ewes under natural outdoor conditions in an arid region. Anim Sci J. doi:10.1111/asj.12430
Mader TL, Dahlquist JM, Hahn GL, Gaughan JB (1999) Shade and wind barrier effects on summer-time feedlot cattle performance. J Anim Sci 77:2065–2072
Marai IFM, El-Darawany AA, Fadiel A, Abdel-Hafez MAM (2007) Physiological traits as affected by heat stress in sheep—a review. Small Ruminant Res 71:1–12
Marai IFM, El-Darawany AA, Fadiel A, Abdel-Hafez MAM (2008) Reproductive performance traits as affected by heat stress and its alleviation in sheep. Trop Subtrop Agroecosys 8:209–234
McManus C, Louvandini H, Gugel R, Sasaki LCB, Bianchini E, Bernal FEM, Paiva SR, Paim TP (2011) Skin and coat traits in sheep in Brazil and their relation with heat tolerance. Trop Anim Health Prod 43:121–126
Monty DE, Kelly LM, Rice WR (1991) Acclimatization of St. Croix, Karakul and Rambouillet sheep to intense and dry summer heat. Small Ruminant Res 4:379–392
Neves MLMW, de Azecedo M, da Costa LAB, Guim A, Leite AM, Chagas JC (2009) Critical levels of the thermal comfort index for Santa Ines sheep under grazing at the agreste region of Pernambuco state. Acta Sci Anim Sci 31:169–175
Niyas AAA, Chaidayna K, Shaji S, Sejian V, Bhatta R, Bagath M, Rao GHSLVP, Kurien EK, Girish V (2015) Adaptation of livestock to environmental challenges. J Vet Sci Med Diagn 4:3
Piccione G, Messina V, Vazzana I, Dara S, Giannetto C, Assenza A (2012) Seasonal variations of some serum electrolyte concentrations in sheep and goats. Comp Clin Pathol 21:911–915
Pinto-Santini L, Ríos AL, Oliveros I, Pigliacampo A, Chacón T (2014) Shadow effect at grazing on some physiological variables of West African lambs under emergency conditions of mild heat. Livest Res Rural Develop 26:1–11
Ramana DBV, Pankaj PK, Nikhila M, Rani R, Sudheer D (2013) Productivity and physiological responses of sheep exposed to heat stress. J Agrometeorol (Special issue):71–76
Rashid MM, Hossain MM, Azad MAK, Hashem MA (2013) Long term cyclic heat stress influences physiological responses and blood characteristics in indigenous sheep. Bangladesh J Anim Sci 42:96–100
Rasooli A, Nouri M, Khadjeh GH, Rasekh A (2004) The influence of seasonal variations on thyroid activity and some biochemical parameters of cattle. Iran J Vet Res 5:1383–1391
Robinson NE (2002) Hemostasis. In: Textbook of vet physiology, second edition, editors, J. G. Cunningham. St Louis: W. B. Saunders Company, pp 516–544
Romero RD, Pardo AM, Montaldo HH, Rodríguez AD, Cerón JH (2013) Differences in body temperature, cell viability, and HSP-70 concentrations between Pelibuey and Suffolk sheep under heat stress. Trop Anim Health Pro 45:1691–1696
Ross TT, Goode L, Linnerud AC (1985) Effects of high ambient temperature on respiration rate, rectal temperature, fetal development and thyroid gland activity in tropical and temperate breeds of sheep. Theriogenology 24:259–269
Russel AJF, Doney JM, Gunn RJ (1969) Subjective assessment of body fat in live sheep. J Agric Sci 72:451–454
SAS (2004) SAS/STAT: User’s guide statistics released 9.1, 2nd Ed. SAS Institute, Inc. Cary, NC, USA.
Sejian V, Indu S, Naqvi SMK (2013) Impact of short term exposure to different environmental temperature on the blood biochemical and endocrine responses of Malpura ewes under semi-arid tropical environment. Indian J Anim Sci 83:1155–1160
Sejian V, Maurya VP, Naqvi SMK (2010) Adaptability and growth of Malpura ewes subjected to thermal and nutritional stress. Trop Anim Health Prod 42:1763–1770
Silanikove N (2000) Effects of heat stress on the welfare of extensively managed domestic ruminants: a review. Livest Prod Sci 67:1–18
Srikandakumar A, Johnson EH, Mahgoub O (2003) Effect of heat stress on respiratory rate, rectal temperature and blood chemistry in Omani and Australian Merino sheep. Small Ruminant Res 49:193–198
Tabarez-Rojas A, Porras-Almeraya A, Vaquera-Huerta H, Hernández-Ignacio J, Valencia J, Rojas-Maya S, Hernández-Cerón J (2009) Embryonic development in Pelibuey and Suffolk ewes under heat stress conditions. Agrociencia 43:671–680
Wildeus S (1997) Hair sheep genetic resources and their contribution to diversified small ruminant production in the United States. J Anim Sci 75:630–640
Acknowledgments
This study was supported through the first author by the PROMEP-SEP program in the 2011, likewise by the Autonomous University of Baja California, Mexico. The authors thank Yolanda Osorio and Samantha Perard for their technical assistance in the development of the current study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures involving sheep were conducted within the guidelines of approved local official techniques of animal care in Mexico (NOM-051-ZOO-1995: humanitarian care of animals during mobilization).
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Macías-Cruz, U., López-Baca, M.A., Vicente, R. et al. Effects of seasonal ambient heat stress (spring vs. summer) on physiological and metabolic variables in hair sheep located in an arid region. Int J Biometeorol 60, 1279–1286 (2016). https://doi.org/10.1007/s00484-015-1123-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-015-1123-6