Abstract
This study was conducted to test the efficacy of gonadotropic hormone (GnRH)-based synchronization protocols (Ovsynch, Heatsynch, and Ovsynch Plus) in buffaloes under field condition. Based on anamnesis and transrectal palpation twice at 10-day interval and serum progesterone (P4) concentration, 150 anoestrous buffaloes and delayed pubertal heifers were selected to induce oestrus using GnRH-based protocols. These selected animals were randomly divided into three groups: group I: Ovsynch (n = 50), group II: Heatsynch (n = 50), and group III: Ovsynch Plus (n = 50) regimen. Before treatment initiation, blood samples were collected for P4, beta-hydroxy butyric acid (β-OHB), and mineral estimation, in addition to the monitoring of oestrus signs. In this investigation, no significant difference (P > 0.05) in oestrus signs was deduced among three groups. Oestrus induction rate (OIR) was comparable (P > 0.05) among the groups (Ovsynch 82%, Heatsynch 86%, and Ovsynch Plus 88%). Conception rate (CR) following fixed time artificial insemination (FTAI) was slightly higher with Ovsynch Plus group (28%) as compared to Ovsynch (24%) and Heatsynch (18%) groups, though non-significant. Furthermore, serum glucose, β-OHB, macrominerals (calcium, potassium, and magnesium), and trace minerals (copper, zinc, and iron) remained comparable (P > 0.05) among the groups. In conclusion, all the protocols (Ovsynch, Heatsynch, and Ovsynch Plus) are efficient in oestrus induction in anoestrous buffaloes under field condition with Ovsynch Plus protocol resulting in higher CR as compared to other protocols.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The major constraint to full exploitation of the productive potential of buffalo is its inherent low reproductive efficiency due to delayed puberty and sexual maturity, seasonality of breeding, prolonged postpartum anoestrus, silent oestrus, and postpartum uterine disorders (Das and Khan 2010). In India, incidence of anoestrus has been reported between 9.18 and 82.50% (Thakor and Patel 2013), whereas suboestrus varies from 3 to 73% in postpartum buffaloes (Kumar et al. 2013). Under field conditions, negative energy balance and aberrant mineral profile leads to delayed postpartum restoration of luteinizing hormone (LH) pulsatility, resulting into prolonged postpartum anoestrus (Kumar et al. 2010).
Use of gonadotrophic hormone (GnRH)-based hormone protocols for oestrus synchronization in cyclic animals has been tried in cattle and buffalo (Pursley et al. 1995; Paul and Prakash 2005). These protocols initiate ovarian cyclicity through endogenous production of progesterone (P4) by inducing corpus luteum (CL) formation by the ovulation of dominant follicle. Ovsynch protocol has been used effectively in cyclic buffaloes with conception rate (CR) ranging from 33 to 64% after fixed time artificial insemination (FTAI) (Paul and Prakash 2005). However, CR following Ovsynch in acyclic buffaloes varied from 0 to 50% (Mohd et al. 2013). At the time of first GnRH injection, presence of large follicle diameter >8.5–9 mm has been reported to be a prerequisite for the success of Ovsynch program in anoestrous buffaloes (Karuppanasamy et al. 2017).
Hence, in an attempt to ensure similar ovarian follicular picture of all anoestrous buffaloes at the time of first GnRH injection, a new protocol “Ovsynch Plus” has been developed (Sharma et al. 2004). Another protocol viz. Heatsynch is similar to Ovsynch, except for the replacement of GnRH on day 9 by estrogen on day 8, resulting in 100% oestrus response (Ali et al. 2012). Therefore, the present study was designed to test the efficacy of these synchronization protocols (Ovsynch, Heatsynch, and Ovsynch Plus) in buffaloes under field conditions.
Materials and methods
Study location
The present study was conducted at Haryana state of India when the humidity was 75–85% and the ambient temperature ranges between 25 and 39 °C during summer and 1.1–15 °C during winter. The average annual rainfall is 459 mm and climate subtype according Köppen climate classification is hot semi-arid climate (BSh).
Selection of animals and their management system
The study comprised of 150 anoestrous buffaloes (1–2 parity) and heifers aged between 4 and 5 years, with body condition score <3 maintained under field conditions. Anoestrous buffaloes averaged 173.80 ± 9.20 days (range 93–720 days) postpartum and delayed pubertal heifers averaged 42.32 ± 1.02 months (range 36.5–60 month) age at the beginning of the hormonal treatment. Anoestrum was diagnosed in the animals on the basis of anamnesis and conducting gynaeco-clinical examinations twice at 10-day interval. Further, the cyclicity confirmed either by transrectal ultrasonography or P4 estimation at the start of treatment. The selected animals (n = 150) were randomly divided into three groups and treated with hormonal regimen as group I (Ovsynch; n = 50), II (Heatsynch; n = 50), and III (Ovsynch Plus; n = 50). Day of initiation of treatment was considered as day 0.
Blood sampling
Blood sample was collected by jugular venipuncture from each buffalo, in a serum activator polystyrene tube on day 0 of treatment in each group. All aseptic precautions were taken prior to collection. Blood glucose was estimated immediately after collection of blood samples. Blood samples were chilled on ice, transported to the laboratory, and centrifuged at 3000 rpm for 15 min to separate the serum. Serum was kept in sterilized vials and stored in a deep freezer at −20 °C until estimation of serum P4, β-OHB, and mineral profile.
Hormone treatment protocols
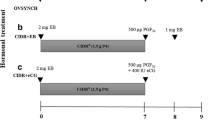
Ovsynch regimen
In this protocol, 10 μg (2.5 ml) of GnRH analogue i.m. (Buserelin-Acetate, Receptal®, Intervet International GmbH, Germany) was administered on day 0 followed by an i.m. injection of 25-mg prostaglandin F2α (PGF2α, dinoprost tromethamine, Lutalyse™, Pfizer Animal Health, Belgium) on day 7. A second injection of 10 μg of GnRH was administered on day 9. Then two FTAIs were carried out on day 9 and 10 of the treatment.
Heatsynch regimen
Under this treatment, buffaloes were administered an i.m. injection of 10 μg of GnRH on day 0 and PGF2α on day 7. An injection of 1 mg of β-estradiol i.m. (Sigma Aldrich, USA) was administered on day 8. Then two FTAIs were carried out on days 10 and 11 of the experiment.
Ovsynch Plus regimen
Buffaloes were administered pregnant mare serum gonadotropin (PMSG) 400 IU (Folligon®, Intervet) followed by GnRH 10 μg (i.m.) on day 3. An injection of PGF2α was administered on day 10 followed by 10 μg GnRH 48 h later (on day 12). FTAI will be done on days 12 and 13 of treatment.
Oestrus induction, FTAI, and pregnancy diagnosis
Oestrus was assessed by behavioral signs, viz. excitement, bellowing, frequent urination, swollen vulva, chin resting on other animal, tail-raising, and mucus discharge. Ease of cervical passage during artificial insemination was also considered to judge the quality of oestrus. Oestrus induction rate (OIR) was calculated following induced oestrus and within 1 month of posttreatment. FTAI was performed in buffaloes using frozen/thawed semen from bulls of known fertility as per standard protocol. Pregnancy diagnosis was performed 60 days postartificial insemination (AI) in animals through per-rectal palpation. CR was deduced during FTAI and subsequent two AIs following spontaneous oestrus.
Hormone, metabolite, and mineral estimation
Serum P4 was estimated in serum samples by liquid-phase radioimmunoassay (RIA) procedure using Iodine-125-based kits (code: RIAK-15V; Board of Radiation and Isotope Technology, BRIT, Navi Mumbai, India). Blood glucose estimation was done by using glucometer (ACCU-CHEK® Active). Beta-hydroxy butyric acid concentration was estimated by enzyme-linked immunosorbent assay kit (Sincere Biotech Co., Ltd., Beijing, China) as per instructions. Sensitivity of kit was <1.0 ng/ml. Calcium, magnesium, potassium, zinc, copper, and iron contents in serum samples were estimated by Atomic Absorption Spectrophotometer (Electronics Corporation of India Ltd. (ECIL), Hyderabad, India, Model No. 4141).
Statistical analyses
Data was analyzed for using Chi-square test and one-way ANOVA with statistical software package (SPSS, Version 16). Results were considered significant at P < 0.05.
Results
Follicular profile
Transrectal ultrasound scanning was performed in 82 treated buffaloes (Ovsynch group (40); Heatsynch (15); Ovsynch Plus group (27)) on day 0 of treatment. Mean diameter (mm) of the largest follicle on day 0 in groups I, II, and III was 9.62 ± 0.55, 9.71 ± 0.69, and 8.50 ± 0.48, respectively (Table 1). No visible CL was found in anoestrous buffaloes treated with different hormonal regimens.
Hormonal profile
Mean P4 concentration (ng/ml) was 0.59 ± 0.12, 0.45 ± 0.09, and 0.46 ± 0.10 in Ovsynch, Heatsynch, and Ovsynch Plus groups, respectively. There was no significant different (P > 0.05) in mean serum P4 concentration among three groups (Table 1).
Oestrus signs
Oestrus was assessed by behavioral signs, viz. excitement, bellowing, frequent urination, swollen vulva, and vaginal discharge as observed by buffalo owner and results remained comparable between the three groups (Table 2).
Ease of AI gun passage during FTAI
Non-significant (P > 0.05) difference was found between the three groups with respect to ease of AI gun passage during FTAI (group I 70% (35/50); group II 72% (36/50); group III 64% (32/50)) (Table 3).
Oestrus induction rate
OIR in groups I, II, and III was 74 (37/50), 80 (40/50), and 76% (38/50), respectively. There was no significant difference (P > 0.05) in OIR during induced oestrus among three protocols. In anoestrous buffaloes, OIR remained comparable between the three groups (Ovsynch 71.79% (28/39); Heatsynch 80% (32/40); Ovsynch Plus 78.05% (32/41)). In heifers, 81.82 (9/11), 80 (8/10), and 66.67% (6/9) exhibited oestrus signs following Ovsynch, Heatsynch, and Ovsynch Plus treatments, respectively (P > 0.05). It was also observed that buffaloes and heifers retuning to oestrus after 1 month posttreatment remained comparable between the three groups. Overall, OIR following treatment was 82 (41/50), 86 (43/50), and 88% (44/50) in Ovsynch, Heatsynch, and Ovsynch Plus treatment, respectively (P > 0.05) among the groups (Table 4).
Conception rate
Overall, CR following FTAI was comparable between the three groups (Ovsynch 24% (12/50); Heatsynch 18% (9/50); Ovsynch Plus 28% (14/50)). In buffaloes, CR following FTAI was 20.51 (8/39), 20 (8/40), and 29.27% (12/41) in Ovsynch, Heatsynch, and Ovsynch Plus treatment, respectively (P > 0.05). In heifers, CR remained comparable (P > 0.05) between the three groups (Ovsynch 36.36% (4/11); Heatsynch 10% (1/10); Ovsynch Plus 22.22% (2/9)).
Serum glucose and beta-hydroxy butyrate (β-OHB) profile
Mean blood glucose and β-OHB level of the study animals on day 0 of treatment was 66.39 ± 0.92 mg/dl and 0.66 ± 0.04 mmol L−1 with no significant difference (P > 0.05) among three groups (Table 5).
Mineral profile
Mean level of macrominerals calcium, magnesium, and potassium was 11.04 ± 0.23, 2.77 ± 0.15, and 23.66 ± 0.49 mg/dl, respectively. Non-significance difference (P > 0.05) in peripheral levels of magnesium and potassium among three groups was observed, but a significant difference (P < 0.05) was in serum calcium level found between the three groups. Likewise, serum zinc, iron, and copper concentration were 1.34 ± 0.10, 3.40 ± 0.10, and 75.77 ± 1.53 μg/ml, respectively, without significant difference (P > 0.05) among three groups (Table 6). It is evident that all study animals had normal metabolic and mineral profile at the start of experiment.
Discussion
Behavioural oestrus signs, oestrus discharge of secretions, and ease of passing AI gun observed in buffaloes treated with three protocols remained comparable. Yotov et al. (2012) reported similar findings in postpartum buffaloes treated with Ovsynch regimen. In this study, OIR of 74% using Ovsynch protocol was higher than Gupta et al. (2015) in postpartum buffaloes. Moreover, OIR during FTAI in Heatsynch regimen was found to be 80% in our study, which was discordant with Bhoraniya et al. (2012) and Ali et al. (2012) in buffaloes. Lower response might be due to overlook of oestrus signs by farmers, individual variation, seasonal effect, and hormonal preparation used. Furthermore, OIR was slightly higher (78.05%) in anoestrous buffaloes as compared to those in heifers (66.67%), but it was comparable between buffaloes and heifers in other protocols. The lower response of these protocols in this study might be due to the fact that these GnRH-based protocols were primarily designed for oestrus synchronization in cyclic animals. Ovsynch protocol has been developed for synchronizing oestrus in cyclic animals, as several studies scrutinizes the effect of Ovsynch protocol in anoestrous buffaloes. The size and functionality of dominant follicle are important factors at the time of GnRH treatment, which determine ovulation in cow and buffalo. The dominant follicle should possess a diameter of >8.5–9 mm for GnRH action in Ovsynch protocol in anoestrous buffaloes (Karuppanasamy et al. 2017). Thus, prior to GnRH treatment, ovarian follicular size needs to be assessed by ultrasonography in order to achieve optimal ovulatory response following Ovsynch protocol in anestrous buffaloes. Since, accurate follicular size assessment is difficult under field conditions with routine per-rectal palpation; enhancement of follicular growth prior to exogenous administration of GnRH is an important determinant to achieve an optimum fertility in Ovsynch protocol. Administration of PMSG 2–3 days prior to Ovsynch protocol enhances the dominant follicle size and ensuring the presence of one dominant follicle at the time of first GnRH injection (Sharma et al. 2004). These results indicate that Ovsynch Plus protocol is preferred choice as compared to Ovsynch and Heatsynch protocols for inducing oestrus in anoestrous buffaloes.
Overall, CR following FTAI in Ovsynch, Heatsynch, and Ovsynch Plus protocols was 24 (12/50), 18 (9/50), and 28% (14/50), respectively. CR following Ovsynch protocol was higher than acyclic cows (9%) and buffaloes (0–7%) following Ovsynch treatment (Azawi et al. 2012). However, higher rates (33 to 50%) following Ovsynch in anoestrous buffaloes have been reported (Mohd et al. 2013). Ovsynch protocol has been used effectively in cyclic buffaloes with CR ranging from 33 to 64% after TAI (Paul and Prakash 2005). In addition, CR of Heatsynch protocol was in contrast with study of Ali et al. (2012) and Mirmahmoudi et al. (2013) in anoestrous buffaloes (50–64%). But, our findings were comparable with Bhoraniya et al. (2012) in cattle (16%). It was expected that buffaloes following estradiol-17β administration in Heatsynch group would respond to its positive feedback effect. It is possible that first GnRH in Heatsynch protocol may not be able to induce ovulation and form CL leading to absence of P4 priming and poor oestrus induction. CR using Ovsynch Plus protocol was 28%, which is agreed with the findings of Sharma et al. (2004) in postpartum anoestrous buffaloes (CR 28.6%) during non-breeding season. Overall, OIR in these three protocols ranged between 74 and 80%, and CR was slightly higher with Ovsynch Plus protocol (28%) as compared to Ovsynch (24%) and Heatsynch (18%) protocols. This can be supported by the fact that for successful conception at FTAI, treated anoestrous buffalo must respond to both GnRHs which is evident in Ovsynch Plus protocol (Sharma et al. 2017). In addition, the role of season on the outcome of these three protocols should not be underplayed and needs to be investigated in future.
With respect to peripheral mineral and metabolic profile, serum calcium was similar to Abd Ellah et al. (2014) in buffaloes, but higher than Jayachandran et al. (2013). This disparity in serum calcium needs to be investigated further. Likewise, comparable serum magnesium among groups was reported by Abd Ellah et al. (2014) in buffalo. Further, peripheral potassium was in accordance with Chaurasia et al. (2010) in buffaloes. Mean copper concentration was within normal range among the three groups as reported by Akhtar et al. (2009). Also, peripheral zinc concentration showed no significant difference among three treatment groups which was concurrent with Jayachandran et al. (2013) in anoestrous buffaloes. Moreover, peripheral glucose and β-OHB showed no significant difference among three treatment groups being in agreement with Monteiro et al. (2012) in buffaloes. It is evident that blood metabolites including macro- and microminerals play an important role in fertility of buffalo (Khan et al. 2014; Kalasariya et al. 2016), but the exact mechanism of action and their interaction needs to be further investigated.
In summary, this study reports that GnRH-based protocols, viz. Ovsynch, Heatsynch, and Ovsynch Plus show comparable oestrus induction and CR in buffaloes under field conditions. Furthermore, the mineral and metabolic profile of study animals was within normal physiological range.
References
Abd Ellah, M. R., Hamed, M. I., Ibrahim, D. R. and Rateb, H. Z., 2014. Serum biochemical and haematological reference intervals for water buffalo (Bubalus bubalis) heifers, Journal of the South African Veterinary Association, 85 (1), 962.
Akhtar, M. S., Farooq, A. A. and Mushtaq, M., 2009. Serum concentrations of copper, iron, zinc and selenium in cyclic and anoestrous Nili-Ravi buffaloes kept under farm conditions, Pakistan Veterinary Journal, 29(1), 47–48.
Ali, R., Shukla, S. P. and Nema, S. P., 2012. Hormonal induction of ovarian cyclicity and conception rate in postpartum anoestrous buffaloes, Indian Journal of Field Veterinarians, 7, 44–46.
Azawi, O. I., Ali, M. D., Oday, S. A., Al-Hadad, A. S., Mouayad, S. J. and Abdul Hussien, A. S., 2012. Treatment of anoestrous in Iraqi buffaloes using Ovsynch alone or in combination with CIDR, Journal of Advanced Veterinary Research, 2, 68–72.
Bhoraniya, H. L., Arjunbhai, J., Dhami, A. J., Naikoo, M., Parmar, B. C. and Sarvaiya, N. P., 2012. Effect of estrus synchronization protocols on plasma progesterone profile and fertility in postpartum anoestrous Kankrej cows, Tropical Animal Health and Production, 44, 1191–1197.
Chaurasia, R., Kushwaha, H. S., Chaurasia, D., Gendley, M. K. and Santra, A. K., 2010. Comparative studies of certain macro minerals during various reproductive states in buffaloes, Buffalo Bulletin, 29, 291–298.
Das, G. K. and Khan, F. A., 2010. Summer anoestrous in buffalo—a review, Reproduction in Domestic Animals, 45, 483–494.
Gupta, K. K., Shukla, S. N., Inwati, P. and Shrivastava, O. P., 2015. Fertility response in postpartum anoestrus buffaloes (Bubalus bubalis) using modified Ovsynch based timed insemination protocols, Veterinary World, 8 (3), 316–319.
Jayachandran, S., Nanjappan, K., Muralidharan, J., Selvaraj, P. and Manoharan, A., 2013. Blood biochemical and mineral status in cyclic and postpartum anoestrous buffaloes, International Journal of Food, Agriculture and Veterinary Sciences, 3 (1), 93–97.
Kalasariya, R.M., Dhami A.J., Hadiya K.K., Mungad K.S., Ramani V.P. and Parmar S.C., 2016. Impact of peripartum nutritional supplementation on plasma minerals profile and postpartum fertility in buffaloes, International Journal of Science, Environment and Technology, 5(6), 3749–3759.
Karuppanasamy, K., Sharma, R.K., Phulia, S.K., Jerome, A., Kavya, K.M., Ghuman, S.P.S., Kumar, H., Singh, I. and Krishnaswamy, N., 2017. Ovulatory and fertility response using modified Heatsynch and Ovsynch protocol in the anovular buffalo, Theriogenology, 95:83–88.
Khan, H.M., Bhakat, M., Mohanty, T.K. and Pathbanda, T.K., 2014. Influence of vitamin E, macro and micro minerals on reproductive performance of cattle and buffalo—a review, Agricultural Review, 35 (2), 113–121.
Kumar, S., Saxena, A. and Ramsagar., 2010. Comparative studies on metabolic profile of anoestrous and normal cyclic Murrah buffaloes, Buffalo Bulletin, 29, 1.
Kumar, P. R., Shukla, S. N., Shrivastava, O. P. and Purkayastha, R. D., 2013. Incidence of postpartum anoestrous among buffaloes in and around Jabalpur, Veterinary World, 6(9), 716–719.
Mirmahmoudi, R., Souri, M. and Prakash, B. S., 2013. Endocrine changes, timing of ovulation, ovarian follicular growth and efficacy of a novel protocol (Estradoublesynch) for synchronization of ovulation and timed artificial insemination in Murrah buffaloes (Bubalus bubalis), Theriogenology, 30, 1–6.
Mohd, Alyas, Razzaque, W. A. A., Rameez Ali., Mutha Rao, M., Sudhir Kumar., Bharadwaj, H. R. and Kafil Hussain., 2013. Supplementation of progesterone in Ovsynch to improve fertility in post-partum anestrous buffaloes, International Journal of Advanced Research, 1, 79–82.
Monteiro, B. M., Yasuoka, M. M., Pogliani, F. C., Ayres, H., Viana, R. B. and Junior, E. H. B., 2012. Lipid and glucose profiles of dairy buffaloes during lactation and dry period, Amazonian Journal of Agriculture and Environmental Science, 55 (1), 33–39.
Paul, V. and Prakash, B. S., 2005. Efficacy of the Ovsynch protocol for synchronization of ovulation and fixed-time artificial insemination in Murrah buffaloes, Theriogenology, 64, 1049–1060.
Pursley, J. R., Mee, M. O. and Wiltbank, M. C., 1995. Synchronization of ovulation in dairy cows using PGF2α and GnRH, Theriogenology, 44, 915–923.
Sharma, R. K., Singh, I., Singh, J. K. and Sethi, R. K., 2004. Ovsynch-Plus—a new protocol for synchronized estrus induction and fixed time insemination in peripubertal Murrah buffalo heifers, In, Proc. National Symposium on Livestock Biodiversity vis-à-vis Resource Exploitation, An Introspection. NBAGR Karnal, Feb 11–12, pp. 199.
Sharma, R. K., Phulia, S. K., Jerome, A. and Singh, I., 2017. Ovsynch Plus protocol improves ovarian response in anovular Murrah buffaloes in low-breeding season, Reproduction in Domestic Animals, 10.1111/rda.13020.
Thakor, D. and Patel, D., 2013. Incidence of infertility problems in cattle and buffaloes, dairy cattle. http,//en.engormix.com.
Yotov, S., Atanasov, A. and Ilieva, Y., 2012. Therapy of ovarian inactivity in postpartum Bulgarian Murrah buffaloes by PRID and Ovsynch oestrus synchronization protocols, Asian Pacific Journal of Reproduction, 1, 293–299.
Acknowledgments
The authors thank the Director, ICAR-CIRB, and Indian Council of Agricultural Research, New Delhi for supporting this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Rathore, R., Sharma, R., Phulia, S. et al. Comparative efficacy of oestrus synchronization protocols in buffalo (Bubalus bubalis). Trop Anim Health Prod 49, 1377–1382 (2017). https://doi.org/10.1007/s11250-017-1337-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-017-1337-1