Abstract
To optimize the superovulation protocol in Thai native cattle, the present research was designed to (1) compare three different protocols designed to induce superstimulation and (2) study the effect of gonadotropin-releasing hormone (GnRH) administration at insemination time (to induce ovulation) on ovarian follicular activities in terms of the number of large follicles, corpora lutea (CLs) and unovulated follicles, and the number and quality of ova/embryos recovered in Thai native heifers. Initially, the estrous cycles of animals (n = 36) at unknown stages were synchronized by two prostaglandin F2α (PGF2α) injections at an interval of 12 days. Follicular development of heifers was randomly superstimulated with one of three different treatment protocols: treatment A—a total of 100 mg of pituitary-derived FSH (pFSH; Folltropin®-V) administered in eight decreasing doses; treatment B—a single dose of 100 mg pFSH dissolved in 30% (w/v) polyvinylpyrrolidone; or treatment C—ablation of all follicles ≥5 mm with a single dose of pFSH. All heifers received PGF2α 48 h after the initiation of FSH treatment to induce luteolysis from the previous cycle, and they were twice inseminated at 12 and 24 h after the onset of estrus. Heifers in each treatment were assigned to be injected or not with GnRH at the time of first insemination with frozen/thawed semen to induce ovulation. About 7 days after artificial insemination (AI), ova/embryos were collected and classified. The numbers of large follicles at the onset of estrus were not statistically significantly different; meanwhile, the maximum diameters of follicles at the time of first insemination in treatment C were smaller compared with the other treatment groups (p < 0.001). The administration of GnRH at the first insemination time resulted in a greater number of CLs and fewer unovulated follicles at the time of ova/embryo collection (p = 0.001), which subsequently resulted in a higher number of total ova/embryos recovered (p = 0.030). Among heifers treated with different superstimulation protocols, the ablation of all follicles ≥5 mm in diameter before superstimulation (treatment C) resulted in significantly higher quality of fertilized ova and transferable embryos (p = 0.001). In summary, it could be inferred that GnRH treatment improved ovarian function rather than embryo quality. Dominant follicle ablation prior to superstimulation is preferable for collecting a greater number of transferable embryos.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For several centuries, Thai native cattle (Bos indicus) have been closely related with the Thai farmers’ lifestyle and have played an important role in rice cultivation. The dominant features of this breed are higher heat tolerance and resistance to regional ectoparasitic infections; they are also ideally suited to utilize low-quality forages as a source of nutrients (FAO 2006). However, over the past several decades, the population of native cattle in Thailand has decreased dramatically due to a combination of factors, including the influx of commercial breeds and the modernization of agriculture (Department of Livestock Development of Thailand 2011), as well as increased meat consumption as the human population has grown. This reduction in the number of Thai native cattle may lead to a decrease in genetic diversity and, consequently, to a greater risk of extinction of the pure breed.
It is well accepted that assisted reproductive technologies (ARTs) are effective concerns in controlled breeding. In this context, superovulation (SOV) has considerable advantages because it induces a greater number of ovulations; consequently, more gametes and embryos can be cryopreserved to prevent genetic loss and to increase prolificacy of the remaining animals. However, only few studies on SOV in Thai native cattle have been reported (Chasombat et al. 2013a, b).
Conventional protocols for SOV are initially conducted between 8 and 12 days after estrus, which is coincident with the emergence of the second follicular wave (Lindsell et al. 1986; Ginther et al. 1989; Mapletoft et al. 2002). Hormone treatment traditionally has involved twice-daily intramuscular (i.m.) injections of a pituitary extract containing pituitary-derived follicle-stimulating hormone (pFSH) (Mapletoft et al. 2002), as the biological half-life of pFSH in cows has been reported to be less than 5 h (Laster 1972; Demoustier et al. 1988). Therefore, the administration of this hormone requires constant attention by farm personnel, increasing the possibility of failure due to mishandling or errors in administration. In addition, twice-daily treatments could cause undue stress in donor cows, with a subsequent decreased superovulatory response (Bó et al. 2010). Various studies in cattle have reported successfully combining FSH with a biodegradable polymer which resulted in sustained, slow release of the hormone over several days (Tríbulo et al. 2011). Our recent study was the first to determine the optimal dose of a single injection—100 mg FSH dissolved in 30% (w/v) polyvinylpyrrolidone (PVP)—that would induce follicular growth in Thai native heifers comparable to that obtained by using multiple injections (Chasombat et al. 2013a).
The initiation of gonadotropin treatment near the time of follicular wave emergence has a positive effect on the maximal superovulatory response (Adams et al. 1994; Lima et al. 2007). When a large or functional dominant follicle is present at the initiation of gonadotropin treatment, the superovulatory response is apparently reduced (Guilbault et al. 1991; Adams et al. 1994). Follicle ablation is used for cauterization of those follicles, resulting in the emergence of a new follicular wave about 1 to 2 days later (Bergfelt et al. 1997; Baracaldo et al. 2000). To optimize the superovulatory response in Thai native cattle, a single i.m. injection of FSH given 36 h after ablation of all follicles more than 5 mm in diameter was found to be an effective protocol for superstimulation of follicular growth (Chasombat et al. 2013a).
Although SOV has been successfully induced in Thai native cattle, there have been no reports on in vivo embryo production after stimulation. Moreover, the methods used have resulted in a high number of unovulated follicles. Increasing ovulation while decreasing the number of unovulated follicles presents a challenge; improvement of SOV techniques in this species could be achieved principally by optimizing superstimulation protocols. Administration of gonadotropin-releasing hormone (GnRH) during estrus synchronization stimulates the release of luteinizing hormone (LH), resulting in ovulation of large follicles (Chenault et al. 1990). The use of GnRH at insemination has been successfully applied in fixed-time artificial insemination synchronization protocols in beef and dairy cattle (Morgan and Lean 1993; Pursley et al. 1997). However, the use of GnRH at the time of insemination to induce ovulation in superovulation protocols has been limited.
The present research was therefore designed to (1) compare three different protocols designed to induce superstimulation and (2) study the effect of GnRH administration at the time of insemination on ovarian follicular activity in terms of the number of large follicles, corpora lutea and unovulated follicles, and the number and quality of ova/embryos recovered in Thai native heifers.
Materials and methods
Animals
Thirty-six cyclic Thai native heifers were used in the experiment. All were nulliparous, 2 to 3 years of age, weighing 180 to 205 kg, and with body condition scores ranging from 3.5 to 4.0 on a 0- to 5-point scale (Lowman et al. 1976). Animals were raised under standard management conditions at the Khon Kaen University beef farm; they were fed approximately 1 kg/cow/day of concentrate feed and maintained in a pasture comprised primarily of “buffalo grass” (Panicum maximum). Water was provided ad libitum.
Estrus synchronization and superovulation treatment
Initially, the estrous cycles of animals at unknown stages were synchronized as in previous report (Chasombat et al. 2013b) by i.m. injection of 500 μg prostaglandin F2α (PGF2α) (Estrumate® [cloprostenol]; Coopers, Berkhamsted, UK) twice at an interval of 12 days. Heifers were checked for estrus by visual observation every 12 h after the last PGF2α injection. The onset of standing heat was designated as day 0 (initial day of treatment). Heifers were randomly assigned into one of three treatment groups with different superstimulation protocols (multiple doses of FSH injection, single dose of FSH injection, and dominant follicle ablation with single dose of FSH injection), and also the administration of GnRH (with or without GnRH administration at the first time of insemination to induce ovulation), as follows. Treatment A heifers (multiple doses of FSH injection) were given a total dose of 100 mg of pFSH (Folltropin®-V; Bioniche Animal Health, Belleville, ON, Canada) by 8 i.m. injections, one every 12 h at decreasing doses (Chasombat et al. 2013b), beginning on the morning of day 9. Treatment B (single dose of pFSH injection) heifers were treated by i.m. injection of a single dose of 100 mg pFSH dissolved in PVP (molecular weight 40,000; 30% (w/v) solution) (PVP-40; Sigma-Aldrich, St. Louis, MO, USA) at the same moment as the first FSH injection of treatment A. Treatment C heifers (dominant follicle ablation with single dose of FSH injection) were subjected to ultrasound-guided transvaginal ablation of all follicles ≥5 mm in diameter at 36 h post-onset of estrus but before ovulation to avoid corpus luteum development; superstimulation was conducted 36 h later using a single dose of pFSH, as described in treatment B.
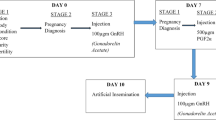
All heifers received a single injection of 500 μg PGF2α 48 h after the initiation of pFSH treatment to induce luteolysis from the previous cycle. Visual observation of estrous behavior was carried out twice daily. Animals were artificially inseminated at 12 and 24 h after the onset of standing heat using frozen/thawed Thai native bull semen. To examine the effect of GnRH, heifers in each treatment (A, B, and C) were randomly divided into two groups: (1) control (artificial insemination [AI] without GnRH treatment), or (2) GnRH (AI with GnRH treatment). Heifers in the GnRH group received GnRH administration (Receptal®, 2.5 mL i.m.) at first insemination. Treatment protocols are shown in Fig. 1.
Treatment protocols. All heifers received PGF2α at an interval of 12 days for estrus synchronization. The onset of estrus was designated as day 0. Heifers in treatment A: 100 mg of pituitary-derived FSH (Folltropin®-V) administered in eight decreasing doses; treatment B: a single dose of 100 mg pFSH dissolved in 30% (w/v) PVP; treatment C: ablation of all follicles ≥ 5 mm with a single dose of pFSH. All heifers received PGF2α 48 h after the initiation of FSH treatment to induce luteolysis. Estrus signs were observed. Heifers in each treatment were assigned to be injected or not with GnRH at the first inseminated time with frozen/thawed semen to induce ovulation. About 7 days after AI, ova/embryo was collected
Assessment of ovarian follicular response
All heifers were subjected to transrectal ultrasonography (HS-2000 ultrasound scanner; Honda Electronics Co., Toyohashi, Japan), as described in our previous study (Chasombat et al. 2013b), beginning the day before pFSH treatment to determine the ovarian follicular activity in terms of the number of large follicles (≥7 mm) and the diameter of follicles. The total follicular stimulation was evaluated by counting the number of corpus lutea (CLs) and unovulated follicles (over 5 mm) at the time of ova/embryo collection on day 7 after insemination. The ovulation rate was assessed by CL count.
Embryo recovery
Embryos were collected from heifers non-surgically by whole uterine flushing 7 days after AI. Before the embryo flushing procedure, feces were removed from the rectum. The perineal area of the vulva was cleaned with tap water and 70% ethanol. Then, each cow received caudal epidural anesthesia, using 5 mL of 2% lidocaine, to decrease peristalsis and discomfort. To perform uterine flushing, a dilator with a diameter of 7 mm was inserted through the heifer cervix in order to dilate the cervix. Then, a two-way catheter was passed through the cervix, and the tip of catheter was placed in the caudal uterine body about 4 cm from the bifurcation. The balloon was inflated with approximately 12 mL of air. Due to the small size of the uterine horn of Thai native heifers, the uterine horn was flushed the first time with 50 mL of Dulbecco’s phosphate-buffered saline (DPBS). Thereafter, 25 mL of DPBS was used each time until the total volume of flushing medium was 500 mL for each uterine horn. All flushing procedures were performed by the same experienced researcher under the inspection of a veterinarian. Collected embryos were counted and evaluated following the criteria of Lindner and Wright (1983). The numbers of ova and embryos were recorded. The recovery rate was calculated from the number of ova and embryos recovered divided by the number of CLs for each cow. The percentage of fertilized ova was calculated from the number of fertilized ova per total number of ova/embryos recovered. Grades A to D correspond to excellent, good, fair, and poor quality, respectively. Embryos graded A and B were considered to be transferable embryos. The percentage of transferable embryos was calculated by transferable embryos per number of fertilized embryos.
Statistical analysis
The data were analyzed using SAS statistical software version 9.0. Data were first tested for normality and homogeneity of variance, and then analyzed by ANOVA using PROC GLM procedure. Two levels of GnRH (use and not use) and three levels of different treatment protocols (A, B, and C) were analyzed by 2 × 3 factorial experiments in completely randomized design. The statistical model used was as follows:
where
- y ijk :
-
observation of treatment combination between GnRH (i) and treatment protocol (j) factors at replication k when k = 1,…,r (r = 6)
- μ :
-
overall mean
- ∝ i :
-
effect of GnRH factor at i when i = 1 to 2
- β j :
-
effect of treatment protocol factor at j when j = 1 to 2
- αβ ij :
-
effect of treatment combination between GnRH (i) and protocol factor (j) at ij
- ε ijk :
-
residual error
Results
Estrus and ovarian follicular response
The results of estrus and efficacy of three superstimulation protocols either with or without GnRH administration on ovarian follicular response are presented in Table 1. All heifers (n = 36) showed signs of estrous behavior; they therefore received AI. As regards ovarian responses, all heifers were superstimulated (more than two ovulations in each heifer). The numbers of large follicles at the onset of estrus were not statistically significantly different; meanwhile, the maximum diameters of follicles at the time of first insemination in treatment C were smaller than in the other treatment groups (p = 0.001). GnRH administration had no effect on follicular development (p > 0.05).
Ova and embryo collection
The results of ova/embryo collection from three superstimulation protocols either with or without GnRH administration are summarized in Table 2. Although the number of large follicles (according to the results in Table 1) did not differ among treatments (p > 0.05), the administration of GnRH at first insemination resulted in a greater number of CLs and fewer unovulated follicles at the time of ova/embryo collection (p = 0.001). The recovery rates of ova/embryos were approximately 82 to 95%. Also, the number of total ova/embryos recovered was higher when GnRH was administered compared with the control (p = 0.030). Thus, there was a significant difference between the control and the GnRH groups in the mean percentage of ovulation and the mean numbers of ova/embryos recovered. However, GnRH treatment did not affect the number of fertilized ova and transferable embryos (p > 0.05). Among heifers treated with different superstimulation protocols, ablation of all follicles ≥ 5 mm in diameter before superstimulation (treatment C) resulted in significantly higher quality of fertilized ova and transferable embryos (p = 0.001).
Discussion
This study compared the efficacy of three different protocols designed to induce superstimulation, as well as the effect of GnRH administration at insemination time, on ovarian follicular activity in terms of the number of large follicles, corpus lutea and unovulated follicles, and the number and quality of ova/embryos recovered in Thai native heifers. We hypothesized that the superovulatory response of heifers treated with a single injection of FSH (either with ablation or not) would be similar to those treated with a conventional superovulation protocol using multiple injections of FSH. In addition, GnRH administration at the time of insemination should induce greater ovulation, which would subsequently result in more ova/embryos recovered compared with non-GnRH administration. Overall, the results demonstrated that the ovarian response, in terms of the number of large follicles and total follicular stimulation, was comparable among the three superstimulation treatments. However, when GnRH was given at the time of insemination in the superovulation protocols, the number of CLs (representing the ovulation rate) was increased, while the number of unovulated follicles was decreased at the time of ova/embryo collection. Nevertheless, GnRH treatment did not affect the number of fertilized ova and transferable embryos. It could be inferred that GnRH treatment improved ovarian function rather than embryo quality. Interestingly, follicular ablation apparently resulted in significantly higher percentages of fertilized ova and transferable embryos.
Although there was no difference in the mean total ovarian follicular stimulation after treatment by three different protocols, the mean maximum diameter of follicles at the time of first insemination was significantly larger in the non-ablation groups (treatments A and B) compared with the ablation group (treatment C). It is well known that superovulation by ultrasound-guided transvaginal follicle ablation generally offers the advantage of initiating superstimulatory treatment at any stage of the estrous cycle (Bergfelt et al. 1997; Baracaldo et al. 2000). However, the ablation of all follicles ≥5 mm in diameter in this study was conducted precisely during the onset of estrus, before ovulation. Therefore, the designated cycle of the new follicular wave in treatment C was initiated at the expected time of the first follicular wave, compared with treatments A and B which could be the second or third follicular wave as the treatment was initiated at the mid-diestrus stage. In accordance with recent reports involving the characteristics of follicular waves and dominant follicles in Thai native heifers (Sakhong et al. 2011; Chasombat et al. 2014), the maximum diameter of the first dominant follicle was smaller than those of the others. Therefore, we inferred that in the present study, the smaller diameter of follicles in the ablation group resulted from superstimulation during the first follicular wave.
Numerous studies have developed successful superovulation protocols; however, the number of unovulated follicles was rarely mentioned. We considered whether more ova/embryos would be recovered if the capacity for ovulation was improved. To achieve this, superovulation protocols should be combined with synchronous ovulation. Therefore, in this study, GnRH was administered at the time of insemination to induce ovulation. GnRH treatment during estrus is well documented for use either to prevent ovulation failure or to reduce any variation in the ovulation interval by inducing an LH peak (Kaim et al. 2003; López-Gatius et al. 2006). In agreement with those reports, heifers in the GnRH group had a higher number of CLs compared with those in the non-GnRH group. These findings were similar to those in an experiment on Holstein cows, where the ovulation time was controlled by administering GnRH or pLH at a set time; this confirmed that an ovulatory stimulus resulted in an increased superstimulatory response and embryo production (Martins et al. 2012). However, in the latter research, it should be noted that neither superovulation protocols nor fixed-time artificial insemination were similar to our conditions. In our study, the fertilization rate and the percentage of transferable embryos were not affected by GnRH administration. Interestingly, those responses were increased for heifers treated with dominant follicle ablation and a single dose of FSH injection (treatment C).
Although GnRH promoted ovulation in this study, the number of fertilized ova was not affected by GnRH treatment. This outcome is not in accordance with previous studies, in which GnRH treatment during estrus either in synchronization (Chenault et al. 1990; Morgan and Lean 1993; Pursley et al. 1997; López-Gatius et al. 2006) or superstimulation protocols (Martins et al. 2012) could promote the fertilization rate. This can be explained by the estrus detection and insemination programs in this study, in which visual observation of estrous behaviors was carried out twice daily and each animal was first inseminated approximately 12 h after standing heat detection, either with or without GnRH administration. Moreover, the second insemination was performed 12 h later to ensure that all ovulated oocytes were inseminated. Therefore, we speculate that daily heat detection together with the twice artificial insemination could increase the chance for fertilization.
As mentioned above, GnRH treatment did not improve the rate of fertilized ova or the embryo quality. However, we found significant differences in the percentages of fertilized ova and transferable embryos between the follicular ablation group and the others (p = 0.001). The higher percentages of fertilized ova and transferable embryos indicate that follicular ablation prior to superstimulation improved the total embryo yield and the embryo quality, which was similar to previous reports in cattle (Amiridis et al. 2006; Lima et al. 2007). However, the timing of superstimulatory treatments (first vs. second follicular waves) and the number of FSH injections (single vs. multiple injections) were different from those reports. The ablation of either all visible follicles (Bergfelt et al. 1997) or even only of the dominant follicle (Amiridis et al. 1999; Baracaldo et al. 2000) causes a subsequent decrease of estradiol concentration together with an increase of FSH, which induces the emergence of a new follicular wave (Amiridis et al. 2006). Adams et al. (1994) reported that there were equivalent responses to superstimulation treatment between the first and second follicular wave if gonadotropin treatment was initiated at the precise time of follicular wave emergence. Our previous study documented that administration of FSH at 36 h after follicular aspiration was the best SOV protocol for ovarian follicular growth (Chasombat et al. 2013b). Besides the timing of superstimulatory treatments, a single injection of FSH, which is generally applied successfully to reduce personnel attention requirements, had a comparable response to the conventional program. Therefore, we inferred that dominant follicle ablation with a single dose of FSH injection (treatment C) offers a higher rate of transferable embryos than the other treatment methods.
In summary, this study is the first paper to compare the efficacy of superstimulation protocols and the effect of GnRH administration at the time of insemination on ovarian follicular activities in terms of the number of large follicles, corpus lutea and unovulated follicles, and the number and quality of ova/embryos recovered in Thai native heifers. The study demonstrates that the ovarian response, in terms of the number of large follicles and total follicular stimulation, was comparable among the three superstimulation treatments. GnRH treatment at the time of insemination effectively increased ovulation but did not affect the number of fertilized ova and transferable embryos. Follicular ablation significantly improved the superovulatory response in Thai native heifers in terms of the number of fertilized ova and transferable embryos.
References
Adams, G. P., Nasser, L. F., Bó, G. A., Garcia, A., Del Campo, M. R., & Mapletoft., R. J., 1994. Superovulatory response of ovarian follicles of wave 1 versus wave 2 in heifers, Theriogenology, 42, 1103–1113
Amiridis, G. S., Robertson, L., Reid, S., Boyd, J. S., O’Shaughnessy, P. J., & Jeffcoate., I. A., 1999. Plasma estradiol FSH and LH concentration after dominant follicle aspiration in the cow, Theriogenology, 52, 995–1003
Amiridis, G. S., Tsiligianni, T., & Vainas., E., 2006. Follicle ablation improves the ovarian response and the number of collected embryos in superovulated cows during the early stages of lactation, Reproduction in Domestic Animals, 41, 402–407
Baracaldo, M. I., Martinez, M. F., Adams, G. P., & Mapletoft. R. J., 2000. Superovulatory response following transvaginal follicle ablation in cattle, Theriogenology, 53, 1239–1250
Bergfelt, D. R., Bó, G. A., Mapletoft, R. J., & Adams. G. P., 1997. Superovulatory response following ablation-induced follicular wave emergence at random stages of the oestrous cycle in cattle, Animal Reproduction Science, 49, 1–12
Bó, G. A., Guerrero, D. C., Tríbulo, A., Tríbulo, H., Tríbulo, R., Rogan, D., & Mapletoft. R. J., 2010. New approaches to superovulation in the cow. Reproduction Fertility and Development, 22, 106–112
Chasombat, J., Sakhong, D., Nagai, T., Parnpai, R., & Vongpralub, T., 2013a. Superstimulation of follicular growth in Thai native heifers by a single administration of follicle stimulating hormone dissolved in polyvinylpyrrolidone, Journal of Reproduction and Development, 59, 214–218
Chasombat, J., Nagai, T., Parnpai, R., & Vongpralub, T., 2013b. Ovarian follicular dynamics, ovarian follicular growth, oocyte yield, in vitro embryo production and repeated oocyte pick up in Thai Native heifers undergoing superstimulation, Asian-Australasian Journal of Animal Science, 26, 488–500
Chasombat, J., Nagai, T., Parnpai, R., & Vongpralub, T., 2014. Ovarian follicular dynamics and hormones throughout the estrous cycle in Thai native (Bos indicus) heifers. Animal Science Journal, 85, 15–24
Chenault, J. R., Kratzer, D. D., Rzepkowski, R. A., & Goodwin, M. C., 1990. LH and FSH response of Holstein heifers to fertirelin acetate, gonadorelin and buserelin, Theriogenology, 34, 81–98
Demoustier, J. M., Beckers, J. F., Van Der Zwalmen, P., Closset, J., Gillard, J., & Ectors, F. R., 1988. Determination of porcine plasma Folltropin-V levels during superovulation treatment in cows, Theriogenology, 30, 379–386
Department of Livestock Development of Thailand, 2011. Number of livestock and farmers, annual report, pp. 1–4
FAO, 2006. Livestock impacts on the environment. Food and Agriculture Organization of the United Nations, Rome, Italy
Ginther, O. J., Knopf, L., & Kastelic., J. P., 1989. Temporal associations among ovarian events in cattle during oestrous cycles with two and three follicular waves, Journal of Reproduction and Fertility, 87, 223–230
Guilbault, L. A., Grasso, F., Lussier, J. G., Rouillier, P., & Matton, P., 1991. Decreased superovulatory responses in heifers superovulated in the presence of a dominant follicle, Journal of Reproduction and Fertility, 91, 81–89
Kaim, M., Bloch, A., Wolfenson, D., Braw-Tal, R., Rosenberg, M., Voet, H., & Folman., Y, 2003. Effects of GnRH administered to cows at the onset of estrus on timing of ovulation, endocrine responses, and conception, Journal of Dairy Science, 86, 2012–2021
Laster, D. B., 1972. Disappearance and uptake of [125I]FSH in the rat, rabbit, ewe and cow, Journal of Reproduction and Fertility, 30, 407–415
Lima, W. M., Vieira, A. D., Thaller Neto, A., Mezzalira, A., Matos, R. C., & Gregory, R. M., 2007. Improved superovulatory response in beef cattle following ovarian follicular ablation using a simplified transvaginal device, Animal Reproduction Science, 100, 364–370
Lindner, G. M. & Wright, R. W., 1983. Bovine embryo morphology and evaluation, Theriogenology, 20, 407–416
Lindsell, C. E., Murphy, B. D., & Mapletoft., R. J., 1986. Superovulatory and endocrine responses in heifers treated with FSH-P at different stages of the estrous cycle, Theriogenology, 26, 209–219
López-Gatius, F., Santolaria, P., Martino, A., Delétang, F., & De Rensis, F., 2006. The effects of GnRH treatment at the time of AI and 12 days later on reproductive performance of high producing dairy cows during the warm season in northeastern Spain, Theriogenology, 65, 820–830
Lowman, B. G., Scott, N. A., & Somerville, S. H., 1976. Condition scoring of cattle, East of Scotland College of Agriculture, Edinburgh, pp. 1–31
Mapletoft, R. J., Bennett-Steward, K., & Adams, G. P., 2002. Recent advances in the superovulation of cattle, Reproduction Nutrition Development, 42, 601–611
Martins, C. M., Rodrigues, C. A., Vieira, L. M., Mapletoft, R. J., Bó, G. A., Sá Filho, M. F., & Baruselli, P. S., 2012. The effect of timing of the induction of ovulation on embryo production in superstimulated lactating Holstein cows undergoing fixed-time artificial insemination, Theriogenology, 78, 974–980
Morgan, W. F. & Lean, I. J. 1993. Gonadotrophin-releasing hormone treatment in cattle: a meta-analysis of the effects on conception at the time of insemination, Australian Veterinary Journal, 70, 205–209
Pursley, J. R., Wiltbank, M. C., Stevenson, J. S., Ottobre, J. S., Garverick, H. A., & Anderson, L. L., 1997. Pregnancy rates per artificial insemination for cows and heifers inseminated at a synchronized ovulation or synchronized estrus, Journal of Dairy Science, 80, 295–300
Sakhong, D., Vongpralub, T., Katawatin, S., & Sirisathien., S., 2011. Ovarian follicular patterns and hormone profile in Thai native cattle (Bos indicus), The Thai Journal of Veterinary Medicine, 41, 439–447
Tríbulo, A, Rogan, D., Tríbulo, H., Tríbulo, R., Alasino, R. V., Beltramo, D., Bianco, I., Mapletoft, R. J., & Bó, G. A., 2011. Superstimulation of ovarian follicular development in beef cattle with a single intramuscular injection of Folltropin-V, Animal Reproduction Science, 129, 7–13
Acknowledgements
The authors would like to thank the National Research University Project of Thailand, Office of the Higher Education Commission, through the Food and Functional Food Research Cluster of Khon Kaen University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
The study was approved by the Animal Ethics Committee of Khon Kaen University, Thailand.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Chankitisakul, V., Pitchayapipatkul, J., Chuawongboon, P. et al. Comparison of three superovulation protocols with or without GnRH treatment at the time of artificial insemination on ovarian response and embryo quality in Thai native heifers. Trop Anim Health Prod 49, 633–639 (2017). https://doi.org/10.1007/s11250-017-1243-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-017-1243-6