Abstract
The study evaluates the effect of three hormonal protocols on ovarian dynamics and progesterone (P4) secretion of buffalo (Bubalus bubalis). Twenty-nine pluriparous Murrah buffaloes were used. The protocols were as follows: OVSYNCH (n = 10): 100 μg of gonadorelin (day 0), 500 μg of cloprostenol (day 7), and 100 μg of gonadorelin (day 9). CIDR+EB (intravaginal device (CIDR®) + estradiol benzoate; n = 10): CIDR plus 2 mg of EB (day 0), withdrew of CIDR, 500 μg of cloprostenol (day 7) and 1 mg of EB (day 8). CIDR+eCG (n = 9): CIDR plus 2 mg of EB (day 0), withdrew of CIDR, 500 μg of cloprostenol and 400 IU of eCG (day 7). Follicles were counted with an ultrasound and measured at 0, 24, and 54 h. The maximum follicle diameter and ovulation were evaluated at 70, 80, and 94 h after CIDR withdrew. Estrous was detected per 1 h three times daily. Blood samples were collected on days 0, 7, 10, 15, and 22 to determine P4 concentration. In CIDR+EB protocol, 50% of buffaloes presented estrous, at 69.6 h. All buffaloes ovulated. CIDR+eCG group had the shortest (69 h) ovulation time. No treatment differences for follicular population, maximum follicle diameter, and P4 concentration on days 7 and 10 (P > 0.05) were found. The P4 concentration in OVSYNCH and CIDR+eCG protocols were > 1 ng/ml, on days 15 and 22 (P < 0.05). There was no difference in ovarian activity; however, the P4 secretion was normal in the OVSYNCH and CIDR+eCG protocols compared to the CIDR+EB protocol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There has been a marked increase in the population of water buffalo (Bubalus bubalis) in Latin America in recent years (Zava 2013). Water buffaloes have been introduced into the hot and humid regions of Mexico, mainly in Veracruz, Tabasco, Chiapas, and Campeche states, because these entities have large wetland areas, which are the natural habitat of this species. Domesticated water buffaloes are broadly classified into 2 groups (river and swamp buffalo) based on their morphology, behavior, and karyotype (Perera 2011). The subspecies of river buffaloes are further divided into multiple breeds, such as Murrah breed, which has undergone selection for more milk production.
The water buffalo is an option to produce meat and milk under extensive management conditions, because it poses characteristics of hardiness, adaptation to humid tropical conditions, and better feed efficiency, compared to cattle (Cervantes et al. 2010; Borghese 2011). However, the productivity of buffalo is affected by the animal’s reproductive performance, which is impaired by the late maturity of females, poor estrous expression, longer calving intervals, and reduced ovarian activity, during the hot season (Madan et al. 1996; Singh et al. 2000). Artificial insemination (AI) combined with estrous synchronization is now an integral part of water buffalo production systems.
Various estrous synchronization protocols use progesterone (P4), progestogens (Carvalho et al. 2017), PGF2α, and its synthetic analogues with GnRH (Neglia et al. 2016), to facilitate the use of AI. However, due to poor estrous (30–40%) detection (Barkawi et al. 1993), heterogeneous duration of estrous (4–64 h) (Ohashi 1994), and the difficulty encountered in predicting the time of ovulation, AI in buffalo is poorly used. Therefore, the establishment of synchronization protocols is important to increase the reproductive performance in this species. Implementation of estrous synchronization protocols, which allows the control and modulation of follicular dynamics, is important to know the right time when estrous and ovulation occur, and to estimate the appropriate time for fixed time artificial insemination programs (FTAI). In addition, the knowledge of the P4 secretion of the subsequent corpus luteum (CL) will enable the use of those synchronization protocols without the need of estrous detection in buffaloes under the tropical conditions of Mexico. The objective of this study was to evaluate the effect of three synchronization hormonal protocols on the ovarian dynamics and P4 secretion in water buffalo (Bubalus bubalis) in the hot-humid tropics of Mexico.
Material and methods
Study area
The study was carried out in Coatzacoalcos, Veracruz, Mexico (17° 55′ N, 94° 06′ W) at an altitude of 19 m. The climate of the region is hot-humid with rain in summer and average annual temperature and rainfall of 25 °C and 2750 mm, respectively (INEGI 2017).
Animals and handling
During the reproductive season (November to December), 29 pluriparous Murrah buffaloes of 6.27 ± 3.62 years of age, 3.79 ± 2.04 parities, 86.52 ± 44.53 days in milk, 471.6 ± 87.2 kg of weight, and 4.1 ± 0.57 body condition score (BCS) were used. BCS was measured on a scale of 1 to 5, where 1 is emaciated and 5 is obese (Anitha et al. 2011). The buffaloes grazed freely in native pastures (Paspalum fasciculatum, Paspalum notatum, Paspalum conjugatum), and they had water ad libitum.
Hormonal treatments
To diagnose buffaloes with CL (OVSYNCH 1.09 ± 0.14, CIDR+EB 1.10 ± 0.16, CIDR+eCG 1.08 ± 0.14 mm) and without ovarian or uterine abnormalities, a real-time ultrasound (Emperor 830®, with a 6.5 MHz linear transducer) was used (Monteiro et al. 2016). Three treatment protocols were compared:
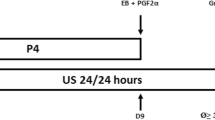
OVSYNCH (n = 10): on day 0, all buffaloes received 100 μg of gonadorelin (Gonavet® Bayer); on day 7, they were given 500 μg of cloprostenol (Ciclase®, Lab. Syntex, Argentina); and on day 9, 100 μg of gonadorelin, via intramuscular (Fig. 1a).
CIDR+EB (n = 10): on day 0, buffaloes were applied a bovine intravaginal device (CIDR® Lab. Pfizer) containing 1.9 g of natural P4, via intramuscular, plus 2 mg of EB (Estradiol Benzoate® Lab. Syntex, Argentina). The CIDR remained in situ 7 days and then it was administered 500 μg of cloprostenol, and 24 h later (day 8) 1 mg of EB, both via intramuscular (Fig. 1b).
CIDR+eCG (n = 9): similar to previous treatment, except that at the CIDR withdrawal the females received 500 μg of cloprostenol and 400 IU of eCG (Folligon®, Intervet) (Fig. 1c).
Estrous and ovarian dynamics
The estrous was detected by visual observation (the buffalo was allowed to mount and remained immobile for 3 s), 1 h, three times a day (06:00, 12:00, and 18:00 h), starting 24 h after the withdrawal of the CIDR or application of cloprostenol, finishing on day 5. The follicles of both ovaries of each buffalo of the three hormonal protocols were counted and measured with the help of an ultrasound, after the CIDR removal or cloprostenol application of PGF2α (hour 0), at 24 and 54 h. The follicles were classified as small (≤ 3 mm), medium (3.1–6 mm), and large (≥ 6.1 mm) as suggested by Vishnu et al. (2017). Subsequently, using an ultrasound, the largest follicle (70, 80, and 94 h) was followed to determine its maximum follicle diameter (MFD) and to determine the ovulation time (disappearance of the follicle), as suggested by Ali and Fahmy (2007).
Progesterone determination
Blood samples (5 ml) of the coccygeal vein were collected in heparinized tubes, on day 0 (insertion of the CIDR or application of 100 μg GnRH), day 7 (withdrawal of the CIDR or the application of 500 μg of cloprostenol), and days 10, 15, and 22 of treatment. The plasma was separated by centrifugation at 1500g by 15 min, and then transferred, in duplicate, in 2-ml vials and stored at − 20 °C until analysis. Plasma P4 concentration was measured with a solid phase radioimmunoassay kit (Coat-A-Count®, Siemens Medical Solution Diagnostics, Los Angeles, CA, USA). The sensitivity of the assay was 0.05 ng/ml, and the coefficient of variation intra assay was 5.8%.
Statistical analysis
All data were analyzed using SAS procedures (SAS 2015). Buffaloes were distributed in a completely randomized design. The data corresponding to the time (h) of CIDR withdrew to estrous and ovulation presentation were analyzed using the GLM procedure, and mean comparison was performed using the Tukey test. The estrous and ovulation data were analyzed by chi-square tests, using the PROC FREQ procedure. The statistical model that described the number of follicles and the MFD included the effects of treatment, time, and the interaction, considering time data as repeated measures. The P4 data were analyzed with the GLM procedure and treatment means compared using the Tukey test.
Results
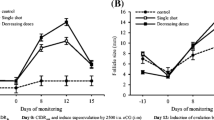
Estrous was only observed in the CIDR+EB group, in 50% of buffaloes with average of 69.6 h after the withdrawal of the CIDR (Table 1). However, 100% of the buffaloes of the three treatments ovulated. The shortest time of device withdrew to ovulation was observed in the CIDR+eCG group (69.11 h) compared to the OVSYNCH group (82 h) (P < 0.05); no difference was found with the CIDR+EB treatment. In addition, no difference was found in number of follicles (P > 0.05; Table 2). On average, 9 small (≤ 3 mm), 3 medium (3.1–6 mm), and 1 large follicle (≥ 6.1 mm) were recorded from 0 to 54 h after CIDR withdrew or the application of closprostenol. The MFD means, prior to ovulation, in the OVSYNCH, CIDR+EB, and CIDR+eCG groups were 8.25 ± 1.10 mm, 7.97 ± 0.95 mm, and 7.72 ± 0.66 mm, respectively (P > 0.05). The plasma concentration of P4 on day 7 of the CIDR withdrawal or application of 500 μg of cloprostenol was > 1 ng/ml (P > 0.05). On day 10, P4 levels were ≤ 1 ng/ml (P > 0.05) in all treatments. For days 15 and 22, the P4 concentrations in the OVSYNCH and CIDR+eCG groups were > 1 ng/ml (Fig. 2; P < 0.05).
Discussion
The fact that estrous was only observed in buffaloes of the CIDR+EB group differs from the result obtained by Khan et al. (2018), who reported 81 and 98% of buffaloes showing estrous in the CIDR+EB and the CIDR+eCG groups, respectively. Neglia et al. (2003) report 88% of buffaloes in estrous at AI (18–24 h after the second application of GnRH). Probably, estrous behavior was only observed in the CIDR+EB group because the EB was applied 24 h after CIDR withdrew, which has the pharmacological effect of inducing estrous manifestations (Sumano and Ocampo 2006). The low proportion of buffaloes with estrous in the present study, probably was due to the fact that visual observation was used as a method to determine estrous expression. Awasthi et al. (2007) reported that buffaloes show silent estrous, which may be due to the reduced diameter of the ovulatory follicle and reduced secretion of 17β-estradiol in the granulosa cell (Ginther et al. 2001). Other authors used other physiological criteria to determine estrous in buffaloes, such as vulvar edema, presence of vaginal mucus secretion, increased uterine tone, and the presence of a follicle > 9 mm (Awasthi et al. 2007; Yousuf et al. 2015; Khan et al. 2018).
In the present study, 100% of buffaloes ovulated, 69 to 78 h after the withdrawal of the CIDR. These results differ from those of Khan et al. (2018), who obtained ovulation rates of 74 and 93% (P < 0.05) at 72.2 and 68.1 h (P < 0.05) with the CIDR+EB and CIDR+eCG protocols, respectively. In the OVSYNCH protocol, 100% of the buffaloes ovulated at 82 h after the application of cloprostenol or 34 h after the second application of gonadorelin, being different from the groups synchronized with the CIDR. This result agrees with those of Paul and Prakash (2005), who reported 90% ovulation rate at 23.3 h after the second application of GnRH. The proportion of females that ovulate and the time that occurs during estrous synchronization is of great importance for the implementation of FTAI programs. The optimal AI time is estimated between 48 and 60 h after removal of the intravaginal device (Haider et al. 2015) or 16–20 h after the second application of GnRH in the OVSYNCH protocol (De Rensis et al. 2005), to get a possible fertilization.
The follicular populations recorded in this study were equal among the synchronization groups evaluated. Similar results were reported by Neglia et al. (2016), who used OVSYNCH protocols to synchronize pluriparous Italian Mediterranean river buffaloes. However, most of the research only evaluate the dominant follicle at different stages and synchronization protocols (Carvalho et al. 2017; Rathore et al. 2017; Monteiro et al. 2018). In this sense, in the present study, no effect of the MFD was found at 54 h after the withdrawal of the CIDR. Carvalho et al. (2013) found diameters of the dominant follicle of 12.06 and 13.07 mm with the CIDR+EB and CIDR+eCG protocols, respectively. The average MFD in the OVSYNCH protocol here used differs from the results of Oropeza et al. (2010), who report 15.2 mm for the dominant follicle at AI. The poor MFD here reported agree with Neglia et al. (2016) results, who found that the area of the largest follicle was smaller (P < 0.05) for the buffaloes that ovulated compared to those that did not do it (1.08 and 1.29 mm, respectively). The importance of determining the follicular population and the MFD in the synchronization protocols is that the proportion of follicles during this event must be known, and that a dominant follicle be present, which should reach its largest diameter to guarantee that the ovulated oocyte and the CL are of good quality (Vecchio et al. 2012; Monteiro et al. 2016). Probably a similar behavior occurs in cattle, where large diameter of the dominant follicle increases the probability of pregnancy in FTAI programs (Sá Filho et al. 2010, 2011).
The main difference in P4 concentration was recorded in the CIDR+EB group on days 15 and 22 of treatment. This result is similar to that of Khan et al. (2018), who report a higher concentration of P4 on days 15 (2.86 vs 1.16 ng/ml), 18 (3.91 vs 1.68 ng/ml), and 21 (5.94 vs 2.33 ng/ml) of the diestrous, in the CIDR+eCG and CIDR+EB treatments, respectively. In addition, the greater P4 concentration was related to a larger diameter of the CL. In relation to the OVSYNCH protocol, the results here found agree with those of Paul and Prakash (2005), who report concentrations ≥ 1 ng/ml of P4 from days 16 to 20 after hormonal treatment. The P4 levels of the present study corroborate the ovulations observed by ultrasonography (levels < 1 ng/ml; day 10), the formation of CL and its functionality (levels > 1 ng/ml; day 15 and 22). This indicates that the OVSYNCH and CIDR+eCG treatments had a better response forming and developing a functional CL, compared to the CIDR+EB treatment. This suggests that those CL were not of short life and this could have favored a pregnancy. In conclusion, there was no difference in ovarian activity of synchronization protocols; however, during the diestrous, the secretion of P4 was normal in the OVSYNCH and CIDR+eCG protocols compared to the CIDR+EB protocol in synchronized buffaloes in the hot-humid tropics of Mexico.
References
Ali, A., Fahmy, S., 2007. Ovarian dynamics and milk progesterone concentration in cycling and non-cycling buffalo-cows (Bubalus bubalis) during Ovsynch program, Theriogenology, 68, 23-28.
Anitha, A., Rao, K.S., Suresh, J., Moorthy, P.R.S., Reddy, Y.K., 2011. A body condition score (BCS) system in murrah buffaloes, Buffalo Bulletin, 30, 79-99.
Awasthi, M.K., Kavani, F.S., Siddiquee, G.M., Sarvaiya, N.P., Derashri, H.J., 2007. Is slow follicular growth the cause of silent estrus in water buffaloes? Animal Reproduction Science 99, 258-268.
Barkawi, A.K., Bedeir, L.H., El Wardani, M.A., 1993. Sexual behavior of Egyptian buffaloes in post-partum period, Buffalo Journal, 9, 225-236.
Borghese, A., 2011. Situation and perspectives of buffalo in the world, Europe and Macedonia, Journal of Animal Science, 1, 281-296.
Carvalho, N.A.T., Soares, J.G., Porto Filho, R.M., Gimenes, L.U., Souza, D.C., Nichi, M., Sales, J.S., Baruselli, P.S., 2013. Equine chorionic gonadotropin improves the efficacy of a timed artificial insemination protocol in buffalo during the nonbreeding season, Theriogenology, 79, 423-428.
Carvalho, N.A.T., Soares, J.G., Souza, D.C., Maio, J.R.G., Sales, J.N.S., Martins Júnior, B., Macari, R.C., D’Occhio, M.J., Baruselli, P.S., 2017. Ovulation synchronization with estradiol benzoate or GnRH in a timed artificial insemination protocol in buffalo cows and heifers during the nonbreeding season, Theriogenology, 87, 333-338.
Cervantes, A.E., Espitia, P.A., Prieto, M.E., 2010. Viabilidad de los sistemas bufalinos en Colombia, Revista Colombiana de Ciencia Animal, 2, 215-224.
De Rensis, F., Ronci, G., Guarneri, P., Nguyen, B.X., Presicce, G.A., Huszenicza, G., Scaramuzzi, R.J., 2005. Conception rate after fixed time insemination following ovsynch protocol with and without progesterone supplementation in cyclic and non-cyclic Mediterranean Italian buffaloes (Bubalus bubalis), Theriogenology, 63, 1824-1831.
Ginther, O.J., Bergfelt, D.R., Beg, M.A., Kot, K., 2001. Effect of LH on circulating estradiol and follicular fluid factor concentration during follicle deviation in cattle, Reproduction, 122, 103-110.
Haider, M.S., Hassan, M., Khan, A.S., Husnain, A., Bilal, M., Pursley, J.R., Ahmad, N., 2015. Effect of timing of insemination after CIDR removal with or without GnRH on pregnancy rates in Nili-Ravi buffalo, Animal Reproduction Science, 163, 24-29.
INEGI, 2017. Anuario estadístico y geográfico de Veracruz de Ignacio de la Llave 2017, 1-1225.
Khan, A.S., Haider, M.S., Hassan, M., Husnain, A., Yousuf, M.R., Ahmad, N., 2018. Equine chorionic gonadotropin (eCG) enhances reproductive responses in CIDR-EB treated lactating anovular Nili-Ravi buffalo during the breeding season, Animal Reproduction Science, 196, 28-34.
Madan, M.L., Das, S.K., Palta, P., 1996. Application of reproduction technology in buffalo, Animal Reproduction Science, 42, 299-306.
Monteiro, B.M., de Souza, D.C., Vasconcellos, G.S., Corrêa, T.B., Vecchio, D., de Sá Filho, M.F., de Carvalho, N.A., Baruselli, P.S., 2016. Ovarian responses of dairy buffalo cows to timed artificial insemination protocol, using new or used progesterone devices, during the breeding season (autumn-winter), Animal Science Journal, 87, 13-20.
Monteiro, B.M., Souza, D.C., Vasconcellos, G.S.F.M., Carvalho, N.A.T., Baruselli, P.S., 2018. Effect of season on dairy buffalo reproductive performance when using P4/E2/eCG-based fixed-time artificial insemination management, Theriogenology, 119, 275-281.
Neglia, G., Gasparrini, B., Di Palo, R., De Rosa, C., Zicarelli, L., Campanile, G., 2003. Comparison of pregnancy rates with two estrus synchronization protocols in Italian Mediterranean Buffalo cows, Theriogenology, 60(1), 125-133.
Neglia, G., Gasparrini, B., Salzano, A., Vecchio, D., De Carlo, E., Cimmino, R., Balestrieri, A., D’Occhio, M.J., Campanile, G., 2016. Relationship between the ovarian follicular response at the start of an Ovsynch-TAI program and pregnancy outcome in the Mediterranean river buffalo, Theriogenology, 86(9), 2328-2333.
Ohashi, O.M., 1994. Estrous detection in buffalo cow, Buffalo Journal, 10, 61-64.
Oropeza, A.J., Rojas, A.F., Velazquez, M.A., Muro, J.D., Márquez, Y.C., Vilanova, L.T., 2010. Efficiency of two timed artificial insemination protocols in Murrah buffaloes managed under a semi-intensive system in the tropics, Tropical Animal Health and Production, 42, 1149-1154.
Paul, V., Prakash, B.S., 2005. Efficacy of the Ovsynch protocol for synchronization of ovulation and fixed-time artificial insemination in Murrah buffaloes (Bubalus bubalis), Theriogenology, 64, 1049-1060.
Perera, B.M., 2011. Reproductive cycles of buffalo. Animal Reproduction Science, 124(3-4), 194-199.
Rathore, R., Sharma, R.K., Phulia, S.K., Mudgal, V., Jerome, A., Ghuman, S.P.S., Singh, I., 2017. Comparative efficacy of oestrus synchronization protocols in buffalo (Bubalus bubalis), Tropical Animal Health and Production, 49, 1377-1382.
Sá Filho, M.F., Crespilho, A.M., Santos, J.E., Perry, G.A., Baruselli, P.S., 2010. Ovarian follicle diameter at timed insemination and estrous response influence likelihood of ovulation and pregnancy after estrous synchronization with progesterone or progestin-based protocols in suckled Bos indicus cows, Animal Reproduction Science, 120, 23-30.
Sá Filho, M.F., Baldrighi, J.M., Sales, J.N.S., Crepaldi, G.A., Carvalho, J.B.P., Bó, G.A., Baruselli, P.S., 2011. Induction of ovarian follicular wave emergence and ovulation in progestin-based timed artificial insemination protocols for Bos indicus cattle, Animal Reproduction Science, 129, 132-139.
SAS, 2015. Institute Inc. SAS/STAT® User’s Guide, Version 9.4th edn. SAS Institute Inc., Cary, NC, USA.
Singh, J., Nanda, A.S., Adams, G.P., 2000. The reproductive pattern and efficiency of female buffaloes. Animal Reproduction Science, 60-61, 593-604.
Sumano, L.H., Ocampo, C.L., 2006. Farmacología Veterinaria. Tercera Edición. Editorial McGraw-Hill Interamericana. México, D.F. pp 544.
Vecchio, D., Neglia, G., Gasparrini, B., Russo, M., Pacelli, C., Prandi, A., D’Occhio, M.J., Campanile, G., 2012. Corpus luteum development and function and relationship to pregnancy during the breeding season in the Mediterranean buffalo, Theriogenology, 77, 1811-1815.
Vishnu, K., Kumar, S., Tran, L., Malla, B., Tyagi, A., 2017. Effects of dietary supplementation of different vegetable oils varying in polyunsaturated fatty acid content on reproductive performance and ovarian follicular characteristics of Murrah buffalo heifers, Buffalo Bulletin, 36(3), 505-519.
Yousuf, M.R., Martins, J.P., Husnain, A., Riaz, U., Riaz, H., Sattar, A., Javet, K., Ahmad, N., 2015. Effect of oestradiol benzoate on oestrus intensity and pregnancy rate in CIDR treated anoestrus nulliparous and multiparous buffalo, Animal Reproduction Science, 159, 104-108.
Zava, M., 2013. Developments of buffalo industry in America. Buffalo Bull, 32, 75-82.
Acknowledgments
We thank Rafael De Hombre López and Karen De Hombre Carranza who generously allowed us to sample their animals and use their facilities.
Funding
This study was supported by a grant from the Universidad Juárez Autónoma de Tabasco.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Animal rights
All of the authors declare that during the experiment, no animals or people were maltreated or injured. The procedures were performed in accordance with the Mexican Official Standard guideline 051-ZOO-1995 and the Mexican Official Standard of technical specifications for production, care, and use of experimental animals. All of the listed authors have participated in the development of this research and wrote the document.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Peralta-Torres, J.A., Torres-Chablé, O.M., Segura-Correa, J.C. et al. Ovarian dynamics of buffalo (Bubalus bubalis) synchronized with different hormonal protocols. Trop Anim Health Prod 52, 3475–3480 (2020). https://doi.org/10.1007/s11250-020-02381-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-020-02381-9