Abstract
A nascent surface has high activity to catalyze the decomposition of a lubricant under boundary lubrication conditions. To reduce the decomposition of a lubricant (multialkylated cyclopentane, MAC), tricresyl phosphate (TCP) was introduced as an additive. The tribological properties and decomposition process of lubricants on the nascent surface of bearing steel 52100 were investigated by a ball-on-disk friction tester in a vacuum chamber with a quadrupole mass spectrometer (Q-MS). The addition of TCP prolonged the induction period for decomposition of the lubricant. During the friction processes, hydrogen and gaseous hydrocarbons desorbed as tribochemical reaction products. XPS analysis revealed that the tribofilm from the additive was mainly composed of iron phosphate, which decreased the probability of generating a nascent surface, resulting in the reduction of desorption rate of gaseous products. The critical load for the mechanical activation of the decomposition correspondingly doubled.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Multialkylated cyclopentanes (MACs) are one of the synthetic hydrocarbon oils which have been gaining wide acceptance in spacecraft mechanisms due to their high thermal and chemical stability, especially their super low vapor pressure [1–4]. Under boundary lubrication conditions, MACs decompose at a metal–metal contact and generate low molecular weight products, such as hydrogen, methane, ethane, etc. [2, 5]. The decomposition of lubricant brings disadvantages like contamination by volatile products, lubricant loss, and unexpected failure of the spacecraft mechanism.
It is well known that surface defects, such as step and kink sites, act as active sites for catalytic reactions. When a metal surface is activated by mechanical stimulations, it is expected to form many active sites for reactions. Many tribochemical reactions activated by the mechanochemical process have been reported [6–9]. Under boundary lubrication conditions, the surface layers of solids like organic contaminants and metal oxides are removed by mechanical stimulation. It results in the formation of nascent surfaces, on which the tribochemical reactions of the base oils and additives take place [5, 10].
One possible method to avoid the decomposition of lubricants is to reduce the activity of the surface during the friction process. Phosphorus-containing compounds are one of the typical catalyst poisons. Even at low levels, it is enough to cover the active sites and decrease the performance of a catalyst. In traditional tribology systems, tricresyl phosphate (TCP) has been used as an anti-wear (AW) and extreme pressure (EP) additive with steel parts for the past 60 years [11–16]. It is now widely accepted that the effectiveness of TCP as an anti-wear additive is due to the chemical reaction of phosphorus with iron to form an iron phosphate film [15]. The tribofilms formed from TCP can control or reduce wear under boundary lubrications and thus prolong the life of systems.
The objective of the research work was to investigate the contribution of TCP as a deactivator for the decomposition of a hydrocarbon oil through poisoning of the active sites for catalysis. In order to make clear the mechanism, the tests were carried out with different TCP concentrations and under different lubrication conditions.
2 Experimental Section
2.1 Materials
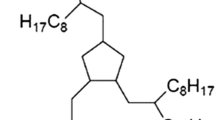
All the lubricants and additives used in this article were commercial products and were used without further purification. The base oil used in the experiments was synthetic multialkylated cyclopentane, 1, 2, 4-tris (2-octyl-dodecyl) cyclopentane (abbr. MAC) with a viscosity of 108 cSt at 40 °C, a viscosity index of 137, and a vapor pressure of 3.999 × 10−9 Pa at 25 °C. The lubricant was produced by Nye Lubricants, Inc. The additive was tricresyl phosphate (TCP), supplied by Tokyo Chemical Industry Co. Ltd., Japan. The structures of the lubricant and additive are shown in Fig. 1.
The lubricants used in this study were pure MAC as a reference, 0.1 wt% TCP, and 1 wt% TCP dissolved in MAC.
2.2 Experimental Procedure
The friction tests were carried out in a high vacuum chamber with a base pressure less than 2 × 10−4 Pa after bakeout. A ball-on-disk type sliding tester was installed in the vacuum chamber, as shown in Fig. 2. Ball and disk specimens were made of bearing steel AISI 52100. A thin lubricant film was formed on the disk surface with the average thickness of 10 μm. The experimental conditions used were as follows:
-
Ball diameter: 6.35 mm.
-
Disk diameter: 24 mm.
-
Surface roughness of the disk: Ra 0.02 μm
-
Normal load: 4, 8, 12, and 16 N.
-
Sliding velocity: 0.02, 0.03, 0.04, 0.05, and 0.06 m/s.
-
Temperature: room temperature.
During the rubbing tests, the pressure in the vacuum chamber was measured by a Bayard-Alpert ion gauge. Intensities of the molecular and fragment ions coming from the sample gas and reaction products were monitored by a quadrupole mass spectrometer (Q-MS). All the tests for a certain lubricant were carried out on the same friction track by using the same ball at room temperature. To examine the effect of mechanical conditions on the decomposition of lubricants, the load was changed in order of 4, 8, 12, and 16 N at each of the constant sliding velocities mentioned above.
After the friction tests, the disk was cleaned with hexane in order to remove the excess lubricant and then the chemical analysis of the wear scar was carried out.
The chemical composition of the wear scar was analyzed by a small area X-ray photoelectron spectroscopy (XPS). The X-ray source was a beam of monochromatic Al Kα (1486.6 eV). The diameter of the analysis area was 120 μm. The binding energy reference was taken as the main component of the C 1s peak at 285 eV for adventitious carbon.
3 Results and Discussion
3.1 Tribological Behavior
Figure 3a and b shows the effect of TCP on friction coefficients and photomicrographs of the wear scars tested in high vacuum conditions, respectively. The friction tests were conducted with a load of 8 N, a sliding velocity of 0.02 m/s, and a sliding distance of 860 m. Friction coefficients of MAC with TCP were higher than that of additive-free MAC. The wear width of additive-free MAC was about 260 μm, while that of MAC with 0.1 wt% TCP was 180 μm, and that of MAC with 1 wt% TCP was 120 μm. It can be concluded that TCP shows an excellent anti-wear property even in vacuum conditions. From the photomicrograph in Fig. 3d, it was also found that tribochemical products were formed on the wear scar when TCP was added.
Tribological properties of lubricants. a Effect of TCP on friction coefficient of lubricants. b Photomicrograph of the wear track lubricated by additive-free MAC. c Photomicrograph of the wear track lubricated by MAC with 0.1 wt% TCP. d Photomicrograph of the wear track lubricated by MAC with 1 wt% TCP (Sliding velocity: 0.02 m/s; Load: 8 N; Sliding distance: 860 m)
3.2 The Induction Period for Decomposition
Figure 4a and b shows the variation of desorption rate of hydrogen during the friction tests, which was obtained from MAC with 0.1 wt% TCP and MAC with 1 wt% TCP, respectively. The inset shows hydrogen evolution from additive-free MAC, which was reported in previous work [5]. Initially, the desorption rate of hydrogen generated from additive-free MAC decreased. Subsequently, it increased sharply after a sliding distance of 0.5 km and then became stable after about 1.5 km. Desorption rate of hydrogen generated from MAC with 0.1 wt% TCP decreased initially and then increased slightly to a constant level after a sliding distance of 11 km. In the case of MAC with 1 wt% TCP, no obvious gaseous hydrogen was observed even after a sliding distance of 40 km. It was also found that the desorption rate of hydrogen from MAC with additive in the steady state was far below that from additive-free MAC.
Desorption rate of hydrogen during surface layers removals. a MAC with 0.1 wt% TCP. b MAC with 1 wt% TCP (inset: additive-free MAC, which was recalculated from reference [5]) (Sliding velocity: 0.02 m/s; Load: 8 N)
The above results for additive-free MAC indicate that chemical reactions were initiated at sliding contacts. The surface of the steel disk was covered with a layer composed of chemisorbed water, organic contaminants, and metal oxides, which was less active than a nascent surface. During the friction process, the surface layers would be removed step by step. Hydrogen evolution at the initial stage was not from the catalytic decomposition of the lubricant, but from the thermal decomposition of surface hydroxyl groups and chemisorbed water. With the removal of the surface layers, desorption rate of hydrogen increased due to the catalytic decomposition of the lubricant on the nascent steel surface. After surface contaminants and oxide layer were removed and the formation rate of the nascent surface became stable, desorption rate of hydrogen reached a steady state. The removal process of the surface layers was named “induction period for decomposition”. From Fig. 4, it can be seen that the induction period of additive-free MAC was about 1.5 km, while that of MAC with 0.1 wt% TCP was 11 km. The induction period became much longer when TCP concentration was increased to 1 wt%. It can be concluded that TCP was an effective additive to prevent the formation of a nascent surface.
3.3 The Desorption of the Reaction Products
By the quadrupole mass spectrometer, hydrogen and gaseous hydrocarbons were observed as the main volatile products due to the decomposition of the lubricant on the nascent steel surface after the removal of surface layers. Figure 5 shows a typical chart of gas evolution which was generated from MAC with 0.1 wt% TCP. The intensity of ion fragments increased immediately when the friction started.
Desorption rate (R d, molecules/s) of the reaction products formed by mechanical activation can be estimated from the pressure change due to desorption, and it can be calculated by the following equation [6, 7]:
where C is the conductance at the gas outlet of the vacuum chamber, ΔP is the pressure change caused by the desorption in the steady state and it is proportional to the intensity change of the molecular and fragment ions, k is the Boltzmann’s constant, and T is the absolute temperature.
The dependence of the desorption rate of hydrogen generated from MAC with 0.1 wt% TCP on mechanical conditions is presented in Fig. 6. It can be seen that the desorption rate of hydrogen by mechanical activation increased proportionally with the sliding velocity at any load tested in this study. On the other hand, desorption rate of hydrogen increased linearly with the cube root of load. All the lines in Fig. 6b intersected with the load axis at the same load of about 2.2 N (W 1/3 = 1.3). In other words, no hydrogen would desorb if the load was beneath this value. Therefore, this point was named a critical load for the mechanical activation of decomposition.
Figure 7 shows the dependence of the desorption rate of methane on mechanical conditions. Similar to hydrogen, the desorption rate of methane on the nascent surface of bearing steel increased proportionally with the sliding velocity, and linearly with the cube root of load. The critical load for the activation was also observed to be about 2.2 N. For other gaseous hydrocarbons, such as C2H5 +, C3H7 +, and C4H9 +, similar results were observed. The mechanisms of the dependence of desorption rate on mechanical conditions have been discussed in our previous work [5].
The turnover number (TN) can be estimated by the ratio of the desorption rate of gaseous products to the fresh surface area (S A) [17]:
It is often used to represent the effective active sites on a nascent surface. The turnover number can be calculated by the following equation:
where a is the atomic density of Fe, m is an interaction constant, ϕ is the roughness coefficient, d is the width of the wear scar, and v is the sliding velocity.
Figure 8 presents the turnover number of hydrogen and methane. At the same mechanical conditions, the turnover number of hydrogen and methane calculated for MAC with additive was much lower than that for additive-free MAC. The results indicate that the effective active sites on the nascent surface decreased when TCP was introduced. It can be concluded that the surface was deactivated by the addition of TCP during the friction processes.
The effect of the additive on the critical load for the mechanical activation of the decomposition is described in Fig. 9. It was found that the critical load doubled compared with additive-free MAC, when even a small amount of TCP was added. The critical load barely increased with incremental rise of TCP concentration. The results indicate that the tribofilm formed from even 0.1 wt% TCP might play an important role in the critical load of activation.
3.4 XPS Analysis of the Wear Scar Surface
To further understand the effect of TCP on decomposition of MAC on a nascent steel surface, XPS analysis was carried out by exploring the chemical states of elements on the wear scar, such as phosphorus and iron. Figure 10a–d shows the XPS spectra of carbon C 1s, oxygen O 1s, iron Fe 2p, and phosphorus P 2p, respectively. The spectra were collected from the wear scar lubricated by MAC with 1 wt% TCP.
The main component in C 1s signal is from aliphatic and/or aromatic carbon, found at the binding energy of 285.0 eV. A minor contribution at 286.9 eV is assigned to the carbon covalently bonded to oxygen of the phosphate group, and the contribution at 288.7 eV to carboxyls [18, 19].
The O 1s signal consists of three contributions: the first at 530.0 eV is assigned to the metal oxide peak, the second at 531.8 eV to non-bridging oxygen bonded to phosphorus, and the third at 533.4 eV to the corresponding phosphate groups and adsorbed water [20, 21].
Curve fitting of Fe 2p 3/2 indicates five contributions: 707.2 eV, 709.6 eV, 710.9 eV, 713.5 eV, and 715.1 eV. The signal at the binding energy of 707.2 eV corresponds to the metallic iron. The main components at 709.6 eV and 710.9 eV are attributed to iron oxides, Fe(II) and Fe(III), respectively, as well as a minor peak at 715.1 eV that belongs to a Fe(II)-satellite. The signal at 713.5 eV is assigned to iron phosphate [22, 23].
The P 2p signal is a doublet with 2p 1/2 and 2p 3/2 components. The main component at 133.5 eV coincides with phosphorus in a phosphate group [14, 18].
Consequently, it can be concluded that the surface of the wear scar was mainly covered with iron phosphate and iron oxides.
3.5 Decomposition Mechanism of MAC on Bearing Steel Surface
Based on the desorption experiments and the surface analysis mentioned above, a possible process of the tribochemical reaction and decomposition mechanism of hydrocarbon oil on a nascent steel surface is described as follows: first, active sites are generated on the steel surface by the mechanical stimulation. Lubricant molecules subsequently chemisorb on the nascent surface. Followed by the rupture of C–H and C–C bonds, the molecular chain length is reduced. Hydrogen and low molecular weight hydrocarbons are simultaneously formed as decomposition products.
Photomicrography and XPS analysis of the wear scar showed that a tribofilm composed of iron phosphate was formed on the nascent steel surface when TCP was introduced as an additive. The tribochemical reaction on the nascent surface can be considered as an acid–base reaction [9, 24, 25]. According to Pearson’s HSAB (hard and soft acids and bases) principle [26], TCP is a hard base. It will react strongly with iron (Fe3+), which is a hard acid. The TCP molecule first chemisorbs on the iron oxide surface after the removal of surface contaminants. With the hard acid–hard base reaction, the TCP molecules decompose, and finally iron phosphate deposits on the wear scar as the tribochemical product. The formed tribofilm decreases the probability of generating a nascent surface, resulting in a reduction of decomposition of lubricant. Therefore, desorption rate of gaseous products decreased substantially compared with the additive-free MAC.
During the friction process, once the tribochemical reaction film deposited on the steel surface is removed, the nascent surface can be regenerated and then decomposition occurs again. The minimum load that can remove the deposited film by friction was named as the critical load for the mechanical activation. The tribofilm generated from additive-free MAC was mainly composed of organic compounds, which could be easily removed by friction. The tribofilm generated from MAC with TCP was found to be mainly composed of iron phosphate, which was more difficult to remove. Consequently, the critical load of MAC with TCP was higher than that of additive-free MAC.
4 Conclusions
In this article, the effect of tricresyl phosphate (TCP) as an additive on decomposition of hydrocarbon oil (multialkylated cyclopentane, MAC) on the nascent surface of bearing steel AISI 52100 was investigated. The gaseous products and the tribofilm were analyzed by a mass spectrometer and XPS, respectively. Main conclusions can be drawn as follows:
-
It was found that the induction period of decomposition of MAC with 0.1 wt% TCP was much longer than that of additive-free MAC. When the additive concentration increased to 1 wt%, no obvious gaseous product was observed even after 40 km of friction under mild mechanical conditions.
-
Hydrogen and gaseous hydrocarbons were found to evolve as tribochemical reaction products from MAC with TCP under severe conditions. The desorption rate of gaseous products was much lower than that of additive-free MAC at the same mechanical conditions.
-
Due to the formation of a boundary film formed by tribochemical reaction, MAC with TCP exhibited better anti-wear properties than additive-free MAC. The film was found to be mainly composed of iron phosphate. It can be concluded that the film decreased the probability of generating a nascent surface, leading to the reduction of desorption rate of gaseous products. The critical load for mechanical activation of decomposition correspondingly doubled.
References
Venier, C.G., Casserly, E.W.: Multiply-alkylated cyclopentanes (MACs): a new class of synthesized hydrocarbon fluids. Lubr. Eng. 47, 586–591 (1991)
John, P.J., Cutler, J.N., Sanders, J.H.: Tribological behavior of a multialkylated cyclopentane oil under ultrahigh vacuum conditions. Tribol Lett 9, 167–173 (2000). doi:10.1023/A:1018808921623
Cutler, J.N., Sanders, J.H., Zabinski, J.S., John, P.J., McCutchen, J.R., Kasten, L.S., Tan, K.H.: Surface chemistry of new lubrication systems for high-speed spacecraft bearings. Tribol. Lett. 8, 17–23 (2000). doi:10.1023/A:1019166630462
Chun, S.W., Talke, F.E., Kang, H.J., Kim, W.K.: Thermal characteristics of multiply alkylated cyclopentane and perfluoropolyether. Tribol. Trans. 46, 70–75 (2003). doi:10.1080/10402000308982602
Lu, R., Minami, I., Nanao, H., Mori, S.: Investigation of decomposition of hydrocarbon oil on the nascent surface of steel. Tribol. Lett. 27, 25–30 (2007). doi:10.1007/s11249-007-9203-3
Mori, S., Suginoya, M., Tamai, Y.: Chemisorption of organic compounds on a clean aluminum surface prepared by cutting under high vacuum. ASLE Trans. 25, 261–266 (1982)
Mori, S.: Adsorption of benzene on the fresh steel surface formed by cutting under high vacuum. Appl. Surf. Sci. 27, 401–410 (1987). doi:10.1016/0169-4332(87)90150-4
Wu, X., Cong, P., Nanao, H., Kobayashi, K., Mori, S.: Chemisorption and tribochemical reaction mechanisms of HFC-134a on nascent surfaces. Langmuir 18, 10122–10127 (2002). doi:10.1021/la020057u
Mori, S., Yoshida, M.: Decomposition of aromatic compounds on cut nickel surface. STLE Trans. 31, 128–132 (1988). doi:10.1080/10402008808981808
Kubo, T., Minami, I., Mori, S.: Investigation of tribochemical reactions by organic sulfides on nascent metal surfaces. Tribol. Online 2, 89–92 (2007). doi:10.2474/trol.2.89
Trivedi, H.K., Forster, N.H., Saba, C.S.: Rolling contact fatigue testing of a 3 cSt polyolester lubricant with and without TCP and DODPA/PANA at 177°C. Tribol. Lett. 16, 231–237 (2004). doi:10.1023/B:TRIL.0000009734.35530.d8
Najman, M.N., Kasrai, M., Bancroft, G.M., Miller, A.: Study of the chemistry of films generated from phosphate ester additives on 52100 steel using X-ray absorption spectroscopy. Tribol. Lett. 13, 209–218 (2002). doi:10.1023/A:1020164127000
Abdelmaksoud, M., Bender, J.W., Krim, J.: Nanotribology of a vapor-phase lubricant: a quartz crystal microbalance study of tricresylphosphate (TCP) uptake on iron and chromium. Tribol. Lett. 13, 179–186 (2002). doi:10.1023/A:1020155925183
Han, N., Shui, L., Liu, W., Xue, Q., Sun, Y.: Study of the lubrication mechanism of overbased Ca sulfonate on additives containing S or P. Tribol. Lett. 14, 269–274 (2003). doi:10.1023/A:1022637015789
Wang, L., Wood, R.J.K.: The influence of contact conditions on surface reaction layers formed between steel surfaces lubricated by an aviation oil. Tribol. Int. 40, 1655–1666 (2007). doi:10.1016/j.triboint.2007.02.014
Bertrand, P.A.: Reactions of tricresyl phosphate with bearing materials. Tribol. Lett. 3, 367–377 (1997). doi:10.1023/A:1019105811014
Somorjai, G.A.: Introduction to surface chemistry and catalysis. Wiley-Interscience, New York (1994)
Wagner, C.D., Riggs, W.M., Davis, L.E., Moulder, J.F., Mullenberg, G.E.: Handbook of X-ray photoelectron spectroscopy. Perkin Elmer Corporation, Eden Prairie, Minnesota (1979)
Piras, F.M., Rossi, A., Spencer, N.D.: Combined in situ (ATR FT-IR) and ex situ (XPS) study of the ZnDTP-iron surface interaction. Tribol. Lett. 15, 181–191 (2003). doi:10.1023/A:1024800900716
Rossi, A., Elsener, B., Hähner, G., Textor, M., Spencer, N.D.: XPS, AES and Tof-SIMS investigation of surface films and the role of inclusions on pitting corrosion in austenitic stainless steels. Surf. Interface Anal. 29, 460–467 (2000). doi:10.1002/1096-9918(200007)29:7<460::AID-SIA889>3.0.CO;2-T
Olla, M., Navarra, G., Elsener, B., Rossi, A.: Nondestructive in-depth composition profile of oxy-hydroxide nanolayers on iron surfaces from ARXPS measurement. Surf. Interface Anal. 38, 964–974 (2006). doi:10.1002/sia.2362
Rotole, J.A., Sherwood, P.M.A.: Oxide-free phosphate surface films on metals studied by core and valence band X-ray photoelectron spectroscopy. Chem. Mater. 13, 3933–3942 (2001). doi:10.1021/cm0009468
Yu, D., Wu, C., Kong, Y., Xue, N., Guo, X., Ding, W.: Structural and catalytic investigation of mesoporous iron phosphate. J. Phys. Chem. C 111, 14394–14399 (2007). doi:10.1021/jp072893o
Martin, J.M., Grossiord, C., Varlot, K., Vacher, B., Igarashi, J.: Synergistic effects in binary systems of lubricant additives: a chemical hardness approach. Tribol. Lett. 8, 193–201 (2000). doi:10.1023/A:1019147520893
Stair, P.C.: The concept of Lewis acids and bases applied to surfaces. J. Am. Chem. Soc. 104, 4044–4052 (1982). doi:10.1021/ja00379a002
Pearson, R.G.: Chemical hardness. Wiley-VCH, Weinheim (1997)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, R., Kobayashi, K., Nanao, H. et al. Deactivation Effect of Tricresyl Phosphate (TCP) on Tribochemical Decomposition of Hydrocarbon Oil on a Nascent Steel Surface. Tribol Lett 33, 1–8 (2009). https://doi.org/10.1007/s11249-008-9379-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11249-008-9379-1