Abstract
Combined effect of additives, over-based calcium sulfonate (OBCS) and phosphate ester were investigated on the decomposition of multi-alkylated cyclopentane (MAC). The decomposition processes of the lubricants on the nascent surface of bearing steel AISI52100 were investigated using a ball-on-disk friction tester in a vacuum chamber with a quadrupole mass spectrometer. According to our previous report, the decomposition reaction can be deactivated by the effect of lubricant additives such as organic sulfides and organic phosphates. In this report, the order of efficiency in decreasing the decomposition rate was: OBCS < phosphate ester < phosphate ester + OBCS. The mixed additive was more effective and stable for suppression of the tribochemical decomposition under severe contact conditions of high load and high sliding speed. The critical load for the decomposition which is the lowest load to detect the gas evolution due to the decomposition increased with the mixed additive. XPS and TOF–SIMS analysis revealed that additive molecules competitively chemisorbed on the steel surface and reaction occurred by the formation of calcium phosphate, which covered the steel surface and deactivated the catalytic activity of active sites leading to deactivation. As a result, decomposition of MAC decreased significantly by the combined effect of the additives.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Multi-alkylated cyclopentane (MAC), a synthetic hydrocarbon oil, is widely in use from the space technologies to the semiconductor industries [1–3]. MAC is superb in heat resistance and chemical stability, as well as in viscosity characteristics. Specifically, due to its low vapor pressure, the oil prevents lubricant loss and maintains high lubricity for an extended period of time under vacuum conditions [4]. However, direct contact between sliding metals causes MAC to decompose into hydrogen and low molecular hydrocarbons such as methane and ethane [5, 6]. As a consequence, the decomposition of MAC invites hydrogen embrittlement of materials, surface contamination and lubrication loss leading to multiple failures [7, 8]. Three factors are involved in the decomposition of MAC where sliding is present: (1) bond scission of the lubricant molecule by shearing, (2) a temperature rise on sliding surfaces caused by shearing, and (3) the catalytic action of the steel surfaces [9]. The decomposition of MAC can be initiated by bond scission and accelerated by the temperature rise and catalytic action of steel surfaces. These factors have complex effects on the decomposition of MAC. Specifically, under severe lubrication conditions, the removal of metal oxide films and surface contaminants by shearing results in the exposure of nascent surfaces that are active due to many lattice defects [10, 11]. The nascent surface exhibits high catalytic activity and facilitates the decomposition of organic substances on steel surfaces during friction [12–14].
Significant factors involved in tribological phenomena under boundary conditions include surface reaction as well as the chemisorption of additives. The chemical conditions of metal surfaces depend on their contact conditions. Under mild conditions, metal oxide films remain present. In contrast, under severe conditions, the surface layers are removed and nascent surfaces are exposed. Consequently, when selecting additives, attention must be focused on contact conditions or the chemical properties of surfaces. According to Mori et al., metal oxides and polar compounds, such as fatty acids and phosphates, are highly effective on oxide-covered surfaces under mild conditions. Under severe conditions, sulfides and other nonpolar compounds are expected to be effective on nascent metal surfaces. It has been reported that these effects can be explained by Pearson’s theory of hard and soft acids and bases (HSAB) [9, 15]. Meanwhile, it has also been reported that a single additive is effective in retarding the tribochemical decomposition of MAC oil [16]. This paper examines the effects of combined dosage of a phosphorous additive and over-based calcium sulfonate (OBCS) against the decomposition of MAC on nascent steel surfaces (Table 1).
2 Experimental Details
2.1 Samples
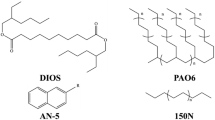
Figure 1 shows the structure of MAC. The sample oil is a synthetic hydrocarbon oil 1,2,4-tris(2-octyldodecyl) cyclopentane [multi-alkylated cyclopentane (MAC)]. Its viscosity at 40 °C is 108 mm2/s, viscosity index is 137, and vapor pressure is 4.0 × 10−9 Pa. Figure 2 shows the structures of the additives. Phosphate ester was used as a phosphorous additive. The TBN (total base number) of over-based calcium sulfonate (OBCS) was 400, and it contains colloidal CaCO3 particles in the detergent. The concentration of each of these additives was set to 1 wt% (see Table 2).
2.2 Experimental Procedure
Figure 3 shows a diagrammatic sketch of the test apparatus. After bake-out, a friction test started when the vacuum chamber had reached 2 × 10−4 Pa. A ball-on-disk test apparatus was used. The material of both ball and disk test specimens was AISI52100 (see Table 2). The ball was 6.35 mm in diameter, and the disk was 24 mm in diameter and 7 mm in thickness. The disk was polished first with emery paper, then with an alumina powder. The surface roughness factor, Ra, of the disk was 0.02 μm. Before testing, each test specimen was ultrasonically cleaned in petroleum ether for 10 min and in hexane for 10 min. A sample oil amount of 5 mg was applied. The average oil film thickness which was estimated from the oil density, the amount of coating and the coated area was 10 μm. During the testing, the vacuum level was measured with a Bayard–Alpert ion gauge, and the gas components of the decomposition product were monitored with a quadrupole mass spectrometer (QMS). All tests were conducted on the same friction track at room temperature.
Table 3 shows test conditions. First, before reaction testing, the test specimens were rubbed with MAC for 2000 m at a load of 8 N and a speed of 0.02 m/s to remove organic contaminants and metal oxide films from the steel surfaces. A friction test was conducted at loads of 2, 4, 6, 8, 12 and 16 N, and speeds of 0.02, 0.03, 0.04, 0.05 and 0.06 m/s. After testing, the test specimens were ultrasonically cleaned for 10 min in hexane to remove the excess amount of the lubricant. The surface of the friction track was analyzed using XPS and TOF–SIMS. An area of 120 μm2 was analyzed. In addition to a qualitative analysis, TOF–SIMS mapping was used to analyze the components of the lubricant film, and their distributions present on the sliding surface were analyzed.
3 Results and Discussion
3.1 Gas Formation and Formation Rate
Figure 4 shows how different gaseous products were formed during the friction test. The decomposition behaviors shown in the figure were observed after continuing more than 2 km of friction testing and the exposure of active surfaces on the friction track. Hydrogen and hydrocarbons such as methane and ethane increased in intensity of their fragment ions indicating the formation of these gases during friction. The intensity increased following the start of rubbing due to gas formation and reached a steady state. The ion intensity returned to the initial value when the rubbing came to a stop. By converting changes in ionic intensity (ΔI) under steady-state conditions to pressure changes (ΔP) of gas products and using Eq. 1, the gas formation rate (R d ) was determined.
where C is outlet gas conductance (m3/s), k Boltzmann’s constant (1.38 × 10−23 J/K), and T absolute temperature (K).
3.2 Induction Period
Figure 5 shows changes over time in the hydrogen formation rate due to MAC decomposition during friction testing. In the initial phase of the test, the main component of the formed gas was hydrogen, which gradually decreased with rubbing distance. This indicates to be a result of the decomposition of organic contaminants and absorbed water on the test specimen surfaces. At rubbing distances of 0.5–1.6 km, the gas formation rate of hydrogen gradually increased, while it became stable at distances over 1.6 km. This result suggests that organic contaminants and metal oxide films were removed from test specimen surfaces due to friction and rubbed surfaces became gradually activated. In this paper, the term “induction period” refers to the period from the beginning of the test to the 1.6-km point at which MAC decomposition reaction became stable. In this experiment, no change in the coefficient of friction was observed, exhibiting almost the same value before and after the induction period. This implies that the heat generated by the friction remained at the same level before and after the induction period. Hence, it is suggested that frictional heating was not the sole factor having an effect on the decomposition; but rather, the activity of the rubbed surfaces increased with rubbing duration. More specifically, it can be concluded that synergistic effects between nascent steel surfaces exposed due to friction and frictional heating caused the MAC oil to decompose.
Figure 6 shows changes over time in the formation rate of hydrogen using phosphate ester, OBCS and phosphate ester + OBCS (Fig. 2). Each of the additives suppressed the decomposition reaction, with the hydrogen gas formation rate being lower than the sample with no additive (Fig. 5) by an order of magnitude or more. Phosphate ester exhibited a large scatter, although the hydrogen formation gas rate was low. In the case of the dosage of OBCS alone, the formation rate increased gradually over a distance of 4 km. In the case of combined dosage of phosphate ester and OBCS, the hydrogen gas formation rate was higher than in the case of the dosage of phosphate ester alone. However, the combined dosage exhibited a relatively stable suppression effect. More specifically, combined dosage turned out to provide a more stable decomposition suppression effect than the dosage of a single additive.
3.3 Critical Load
Figure 7 shows the load dependence of gas formation rate of hydrogen of MAC oil decomposition caused by friction. The hydrogen gas formation rate tended to increase with increasing load and sliding speed. Meanwhile, the hydrogen gas formation rate increased linearly with the cube root of the load. Moreover, all lines intercept the load axis at 1.2 N (W 1/3 = 1.1). This implies that no hydrogen gas was generated at a load below 1.2 N. Since similar results were exhibited with CH3 +, C3H5 +, C3H7 + and C4H9 + ions, this load was defined as the critical load for starting decomposition [6].
Figure 8 shows critical loads observed with the use of different additives. Each of these additives was effective in raising the critical load. Although OBCS exhibited a higher critical load than phosphate ester, its drawback is a substantial variation. In contrast, combined dosage of phosphate ester and OBCS exhibited a higher critical load than dosage of phosphate ester alone. This combined dosage characteristically exhibited a smaller scatter than the dosage of OBCS alone. Consequently, combined dosage provides a stable and high critical load, proving itself to be useful in suppressing the decomposition.
3.4 Decomposition Suppression Effects of Additives in Severe Condition
The decomposition of MAC on rubbed surfaces depends strongly on rubbing speed and load, as revealed above. This section examines the decomposition suppression effects of the additives at a higher-than-critical load of 16 N.
Figure 9 shows hydrogen gas formation rates of different additives under a severe condition. The y-axis is the ratio of hydrogen gas formation rate observed with the use of additive(s) to the formation rate of hydrogen observed without additive, representing effects of additives on the hydrogen gas formation reduction. Each of the additives provided a lower hydrogen gas formation rate than in without additive by an order of magnitude or more, proving the effects of the additives. Under low-load and low-speed conditions (8 N, 2 cm/s), phosphate ester demonstrated the highest suppression effect. However, under high-load and high-speed conditions (16 N, 6 cm/s), phosphate ester exhibited a degraded effect. Meanwhile, OBCS exhibited a low suppression effect. Its suppression effect was lowest under high-load and high-speed conditions. By mixing the two additives, a higher suppression effect than the dosage of a single additive was obtained. This suggests to have resulted from surface protection provided by boundary films formed by phosphate ester and OBCS, which retarded the creation of nascent surfaces.
3.5 Surface Analysis of Wear Tracks
Since additives were found to be effective against the decomposition of MAC oil, wear tracks were analyzed using XPS and TOF–SIMS to analyze the chemical structure of the boundary films formed on rubbed surfaces. Samples were wear tracks resulting from the use of a mixture of phosphate ester and OBCS, which was most effective in suppressing MAC decomposition.
Figure 10 shows XPS spectra of wear tracks from friction tests using a mixed additive. Judging from the C1s spectra, a several-nanometer carbonate layer is present on the outermost surface, as well as organic adsorbates. The carbonate layer is believed to have come from OBCS adsorbates. The P2p spectra reveal the formation of phosphates, which are present even in relatively deep regions. The Ca2p peaks show detection of Ca in relatively deep regions. The presence of Ca and P indicates that boundary films contain calcium phosphate mainly.
Figure 11 shows cation spectra obtained with TOF–SIMS. The subject of the analysis was a 120-μm2 area within a wear track. In addition to Ca+ derived from OBCS, Fe+ from the test specimen material was detected. Na+ was from contaminants. Anion spectra indicated the detection of fragment ions including Ca and P. Although PO2 − and PO3 − anions had been detected from friction tracks lubricated with a TCP-added oil [17], none of these anions were detected in the 120-μm2 area. One possible reason for this is the presence of P in the form of salt, calcium phosphate, rather than in phosphate ester. Figure 12 shows the chemical images obtained with TOF–SIMS. These chemical images were obtained after removing surface contaminants by etching the test specimen surfaces by a depth of approximately 1 nm. The chemical image for Na+ shows a high-intensity region at the right edge, which is an unetched surface and indicates the presence of remaining contaminants. The total ion image for cations reveals the location of the track. The image for Ca+ ions suggests the condensation of Ca in the friction track. Meanwhile, the intensity of Fe+ ions, a component of the substrate, is low in the track and presents an inverted image of the image for Ca+. This is indicative of condensation of calcium compounds in the track.
The chemical analysis of wear tracks using XPS and TOF–SIMS reveals that a boundary film was formed on wear tracks, with the film’s principal constituent being calcium phosphate.
3.6 Mechanism of the Deactivation Effects of Additives for Nascent Surface
An MAC oil, a saturated hydrocarbon, was applied onto steel and the steel was rubbed under vacuum conditions. The MAC oil was tribochemically decomposed into hydrogen and hydrocarbons such as methane. The causes of the decomposition include temperature increases due to friction and the activity of the rubbed surfaces. Decomposition and gas formation increased gradually with rubbing time. The activity of rubbed surfaces remained at an approximately constant level beyond the induction period. Since the coefficient of friction remained almost the same between the start and the end of the friction test, it can be assumed that no change over time in friction heating occurred. This implies that the temperature rise caused by friction heating is not the sole factor involved in the decomposition. It is clear that the gas formation rate increased with the rubbing time due to the exposure of chemically active nascent surfaces after the surface layers were removed by friction. Moreover, in a test of nascent surfaces exposed not by friction but by cutting, breakdown products were detected immediately following the cutting, without any induction period. This proves that nascent surfaces play a significant part in decomposition reaction.
Now the roles of nascent surfaces are examined below. It has been reported that in an experiment of chemisorption of gas by nascent steel surfaces, chemisorption of benzene and olefin that have π electrons occurs on nascent steel surfaces, while hexane, a saturated hydrocarbon, is not absorbed [9]. This implies that no chemical interaction occurs between nascent steel surfaces and saturated hydrocarbons at room temperature. Consequently, one possible cause of the decomposition of MAC, a saturated hydrocarbon, is synergistic action brought about by nascent steel surfaces and a temperature rise in the contact area. It has been observed that additives suppress MAC oil decomposition reaction and the rate of hydrogen gas formation by decomposition decreases by an order of magnitude. This is an effect of surface layers formed by additives. It is generally known that elements high in electronegativity play as a catalyst poison to reduce catalytic activity. Sulfur exhibits a stronger catalyst poison effect than phosphorous [18]. However, to suppress tribochemical decomposition, phosphorous compounds are more effective than high-electronegativity sulfur. Consequently, it is necessary to take into consideration not only the poisoning effect of phosphorous and sulfur, but also additives’ effectiveness in reducing the exposure of nascent surfaces (i.e., surface protection) as contributing to the deactivation effects of additives.
The results of a surface analysis included the detection of phosphate films in phosphate ester and carbonate films in OBCS. It was revealed that the dosage of additives led to the covering of rubbed surfaces with calcium phosphate and other salts. It suggests that the covering raises the critical load and enhances the effect on hydrogen formation. More specifically, it is believed that the exposure of nascent surfaces caused by friction was suppressed by boundary films comprising calcium phosphate, iron phosphate and calcium carbonate as well as metal oxides, thereby reducing tribochemical decomposition effected by nascent surfaces. Since boundary films formed as a result of using OBCS alone principally comprise calcium carbonate and are relatively soft, it is assumed that the films’ protection effects were low and decomposition suppression action was at a low level. Meanwhile, the combined use of OBCS and phosphate ester was highly effective in suppressing decomposition. It is surmised that boundary films of calcium phosphate formed on rubbed surfaces reduced the exposure of nascent surfaces and suppressed the decomposition.
4 Conclusion
A friction test was conducted under vacuum conditions by applying an MAC oil, a synthetic hydrocarbon oil, to bearing steel AISI52100. In the test, decomposition of the MAC oil and the formation of hydrogen gas and low molecular weight hydrocarbons such as methane and ethane were detected with a quadrupole mass spectrometer. Use of phosphate ester and OBCS as additives was effective in suppressing the decomposition reaction and the following findings were obtained.
-
1.
The rate of hydrogen gas formation resulting from decomposition gradually increased and exhibited an almost steady value beyond a certain rubbing distance.
-
2.
After reaching a steady state, the hydrogen gas formation rate increased linearly with the cube root of the load and the line representing the hydrogen gas formation rate intercepted the load axis. This revealed a certain critical load below which no decomposition was observed.
-
3.
The use of additives, namely phosphate ester, OBCS and a mixture of these, each reduced the hydrogen gas formation rate by an order of magnitude or more. Their effects ranked in the following order: OBCS < phosphate ester < mixture. The mixture exhibited a specifically high decomposition suppression effect under severe contact conditions.
-
4.
The results of analyzing rubbed surfaces using XPS and TOF–SIMS showed that in the case of using the mixture of additives, films of calcium phosphate were formed on the wear tracks. It was assumed that the films suppressed the exposure of nascent surfaces and as a result reduced the decomposition of the hydrocarbon oil.
References
Venier, C.G., Casserly, E.W.: Multiply alkylated cyclopentanes (MACs): a new class of synthesized hydrocarbon fluids. Lubr. Eng. 47, 586 (1991)
Chun, S.W., Talke, F.E., Kang, H.J., Kim, W.K.: Thermal characteristics of multiply alkylated cyclopentane and perfluoropolyether. Tribol. Trans. 46, 70 (2003)
Jansen, M. J., Jones, W. R., Pepper, S. V.: Evaluation of an in situ, liquid lubrication system for space mechanisms using a vacuum spiral orbit tribometer. NASA Glenn Research Center Technical Reports, NASA/TM-2002-211683.
Marchetti, M., Jones, W.R., Street, K.W., Wheeler, D., Dixon, D., Jansen, M.J., Kimura, H.: Tribological performance of some pennzane- based greases for vacuum applications. Tribol. Lett. 12, 209 (2002)
John, P.J., Cutler, J.N., Sanders, J.H.: Tribological behavior of a multialkylated cyclopentane oil under ultrahigh vacuum condition. Tribol. Lett. 9, 167 (2000)
Lu, R., Minami, I., Nanao, H., Mori, S.: Investivation of decomposition of hydrocarbon oil on the nascent surface of steel. Tribol. Lett. 27, 25 (2007)
Atxaga, G., Pelayo, A., Irisarri, A.M.: Failure analysis of a set of stainless steel disk springs. Eng. Fail. Anal. 13, 226 (2006)
Kohara, M., Kawamura, T., Egami, M.: Study on mechanism of hydrogen generation from lubricant. Tribol. Trans. 49, 53 (2006)
Mori, S., Imaizumi, Y.: Adsorption of model compounds of lubricant on nascent surfaces of mild and stainless steel under dynamic conditions. STLE Trans. 31, 449 (1988)
Mori, S., Nanao, H.: Relationship between chemical properties of nascent surfaces of non-ferrous metals and their boundary lubricating properties. J. Jpn. Soc. Tribol. 55, 841 (2010)
Mori, S.: Boundary lubrication from the viewpoint of surface chemistry—role of nascent surface on tribochemical reaction of lubricant additives. JTEKT Eng. J. 1008, 2 (2010)
Mori, S., Suginoya, M., Tamai, Y.: Chemisorption of organic compounds on a clean aluminum surface prepared by cutting under high vacuum. ASLE Trans. 25, 261 (1982)
Mori, S.: Adsorption of benzene on the fresh surface formed by cutting under high vacuum. Appl. Surf. Sci. 27, 401 (1987)
Wu, X., Cong, P., Nanao, H., Kobayashi, K., Mori, S.: Chemisorption and tribochemical reaction mechanism of HFC-134a on nascent ceramic surfaces. Langmuir 18, 10122 (2002)
Mori, S.: Tribochemical activity of nascent surfaces. In: Proceedings of international tribology conference, Yokohama, pp. 37–42 (1995)
Lu, R., Nanao, H., Kobayashi, K., Kubo, T., Mori, S.: Effect of lubricant additives on tribochemical decomposition of hydrocarbon oil on nascent steel surfaces. J. Jpn. Petrol. Inst. 53, 1 (2010)
Kubo, T., Nanao, H., Mori, S., Ichihashi, T.: TOF–SIMS analysis of boundary lubrication film derived from multi additives (part 2). J. Jpn. Soc. Tribol. 52, 12 (2007)
Goodman, D.W.: Chemical modification of chemisorptive and catalytic properties of nickel. Appl. Surf. Sci. 19, 1 (1984)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fukushima, Y., Tada, I., Nanao, H. et al. Combined Effect of Phosphate Ester and OBCS on Tribochemical Decomposition of Hydrocarbon Oil on Nascent Steel Surfaces. Tribol Lett 63, 3 (2016). https://doi.org/10.1007/s11249-016-0687-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11249-016-0687-6