Abstract
Hydrocarbon oil with low vapor pressure has been used as a lubricant in high-vacuum conditions. Decomposition of the oil under boundary lubrication conditions was studied by investigating desorption of hydrogen and hydrocarbons, generated by tribochemical reaction occurred on nascent surface of 52100 steel during the sliding process in a ball-on-disk type sliding tester. Gaseous products by tribochemical decomposition were monitored by a quadrupole mass spectrometer (Q-MS), which would reveal the decomposition mechanism of hydrocarbon oil. It is found that tribochemical reactions of hydrocarbon oil occurred on active sites on steel generated by sliding, the desorption amount of hydrogen and gaseous hydrocarbons increased linearly with sliding velocity, and parabolically with load. A critical load for the activation of decomposition of the hydrocarbon oil on bearing steel was found to be about 1.1 N. In this paper, the decomposition mechanism of hydrocarbon oil was also explored.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tribochemistry is generally defined as the chemical reactions that occur between the lubricant/environment and the material surfaces under boundary lubrication conditions. It is regarded that there are two main causes for tribochemistry: (1) thermal and pressure effects at mechanical contacts, i.e., high temperature and pressure at the contact area, (2) enhanced activity of contacting surfaces, for example, nascent surfaces and emissions [1–5]. Under boundary lubrication conditions, the surface layers on solids such as metal oxides and organic contaminants are removed by mechanical stimulation, resulting in the formation of nascent surfaces. There are many kinds of active sites on the nascent surfaces such as cation, anion, radical sites, and defects [6–10]. We have studied many chemical reactions on the nascent surface of metals such as steel, nickel, gold, and ceramics such as Si3N4, Al2O3. The nascent surface has high activity to catalyze and accelerate the decomposition of organic compounds. The adsorption activity is dependent on the fundamental electronic structure of both the metal and compound [3,9–11]. But the decomposition of actual lubricants on nascent surface was rarely confirmed.
As well known, atomic hydrogen produced by decomposition of lubricants interacts with steel to induce subcritical crack growth leading to ductility diminution and causes hydrogen embrittlement [12,13]. Hydrogen embrittlement of metals will cause trouble in the gas and oil industry where high concentrations of hydrogen are present. Especially in outer space, many moving mechanical assemblies (MMAs) for space applications rely on a small, initial charge of lubricant for the entire mission lifetime, often in excess of 5 years [14]. The lubricants used in this field are synthetic hydrocarbon oils with low vapor pressure [15–17]. Tribological failure of MMAs occurs when the lubricant degrades or evaporates and therefore loses its ability to lubricate tribological contacts, for example raceways contacting with bearing balls. Moreover, contamination by low molecular weight products generated from the lubricant degradation will be harmful to the vacuum systems.
In this work, decomposition of hydrocarbon oil on the nascent surface of bearing steel under boundary lubrication conditions in high-vacuum was studied, and the decomposition mechanism was discussed.
Experimental section
In this study, ball and disk specimens were made of bearing steel 52100. The diameter of the ball was 6.35 mm. The disk was 24 mm in diameter and 7 mm in thickness. The disk was polished and its surface roughness was controlled to Ra 0.02 μm. The specimens were cleaned for 10 min in an ultrasonic bath with petroleum ether and hexane respectively before sliding test.
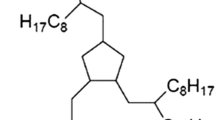
Synthetic hydrocarbon (1, 2, 4-Tris (2-Octyl-1-Dodecyl) Cyclopentane) oil intended for precision applications in aerospace or high-vacuum environments was selected as lubricant. It was marked as Pennzane® (Nye 2001A), and fully described in Ref. [18]. Main physical properties of the oil are shown in Table 1.
Schematic diagram of the test apparatus used in this study is illustrated in figure 1. A ball-on-disk type sliding tester was installed in a high-vacuum chamber with a base pressure of <2 × 10−5 Pa. The pressure was achieved by a turbo molecular pump. The lubricant oil was coated on the disk with the average thickness of 10 μm. During the rubbing test, the pressure in vacuum chamber was measured by a Bayard-Alpert ion gauge, and intensities of the molecular and fragment ions coming from reaction products were monitored by a quadrupole mass spectrometer (Q-MS). All the tests were carried out on the same friction track by using the same ball at room temperature. To examine the effect of mechanical conditions on decomposition of hydrocarbon oil, experiments were performed at the sliding velocity of 0.02, 0.03, 0.04, 0.05, and 0.06 m/s, respectively. The load was changed by the order of 2, 4, 8, 12 and 16 N at each of the constant sliding velocity as mentioned above. Details of the experiments have been reported elsewhere [9–11].
Figure 2 shows a typical chart from a mass spectrometer. For the first 3 min, the sliding tester was left at rest and it was confirmed that the baselines of ion intensities were stable. For the next 6 min, the sliding test was carried out and the intensity changes were recorded. For the last 6 min, the sliding tester was left at rest and the stability of the baselines was confirmed again.
Results and discussion
Desorption of gases during sliding process
Figure 3 shows desorption of hydrogen during sliding experiment. Initially, no obvious gas desorption was observed except for hydrogen when the ball was sliding on the disk. Then hydrogen desorption decreased gradually. Subsequently it increased sharply and became stable after about 20 h of sliding. In addition to hydrogen, evolution of gaseous hydrocarbons, such fragment ions as CH +3 , C2H +3 , C2H +4 and C3H +7 , were detected after 20 h of sliding time repeated as shown in figure 2.
From figure 2, we can find that intensities of those ions increased immediately when sliding test was started and then became constant after a few seconds. Once the sliding process was terminated, it returned to the initial value within several seconds. In the case of hydrogen, the intensity increased and decreased gradually.
The above results indicate that chemical reactions were initiated at sliding contacts. The surface of the steel disk was covered with a layer composed of oxides, chemisorbed water, and organic contaminants, which were less active than nascent surface. Hydrogen evolution at the initial stage did not originate from a catalytic decomposition of lubricant, but from the thermal decomposition of surface hydroxyl groups and chemisorbed water. With removal of the contaminant, desorption of hydrogen decreased until about 7 h. While contaminant and oxide layer were removed and the nascent surface with active sites were generated by friction for 7 h, desorption of hydrogen increased remarkably and reached a constant value as shown in figure 3. Hydrogen and gaseous hydrocarbons desorbed as tribochemical reaction products simultaneously as shown in figure 2. Differences of intensity change of hydrogen and gaseous hydrocarbons possibly due to hydrogen penetration into steel [13].
Desorption amount of reaction products
From figure 2, we can find that all the molecular ion intensities of the reaction products increased exponentially due to desorption with sliding time, and then came to a steady state, at which the formation rate of nascent surface was balanced by the diminishing rate of active sites with adsorbed molecules. According to equation (1), desorption rate (R d, molecules/s) of reaction products by mechanical activation can be estimated from the pressure change (ΔP) due to desorption [9,10].
where, C is a conductance at the gas outlet of the vacuum chamber; ΔP is pressure change due to desorption at the steady state and is proportional to the intensity change of the molecular and fragment ions; k is Boltzmann’s constant; and T is absolute temperature.
Desorption amount (A d) was obtained from the hatched area shown in figure 4. It can be calculated by the following equation.
where, A p is the peak area of partial pressure change during sliding.
The dependence of desorption amount of hydrogen on mechanical conditions is shown in figure 5. It can be deduced that desorption amount of hydrogen by mechanical activation increased proportionally with sliding velocity at any load tested in this study. On the other hand, desorption amount increased in a parabolic relation with load.
Figure 6 shows the dependence of desorption amount of hydrogen on the cube root of load. It can be seen that all curves are linear, intersecting with the axis of the cube root of load at the same point of 1.1 N. Therefore, this point is named as a critical load for the activation. In other words, no hydrogen desorption would happen if load was below 1.1 N.
Figure 7 presents the dependence of the desorption amount of methane on mechanical conditions. It can be seen that desorption amount of methane on nascent surface of bearing steel increased proportionally with sliding velocity, and parabolically with load. Figure 8 shows the dependence of desorption amount of methane on the cube root of load. Critical load for the activation is also observed at load of 1.1 N. For other gaseous hydrocarbons, such as C2H +3 , C2H +4 , and C3H +7 , similar results were observed.
These phenomena can be explained as follows: When a ball slides on a disk, the relation between contact radius and load can be shown as following equation.
where, d is the contact diameter, W is the load, R is the ball radius, E′ is the modulus of elasticity.
If \( 2\left( {\frac{{3R}} {{2E'}}} \right)^{{\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 3}}\right.\kern-\nulldelimiterspace} \!\lower0.7ex\hbox{$3$}}} \) is defined as c, equation (3) will be described as:
During sliding process, total area (S) of nascent surface generated by friction can be calculated by the equation (5).
where, v is the sliding velocity, t is the sliding time.
From equations (4) and (5), total area of nascent surface can be calculated as follows,
The generation rate of active sites is proportional to the sliding velocity [9]. Desorption amount must be proportional to the total area of nascent surface correspondingly. This relation is shown as follows,
which means,
The relations of equation (8) are clearly shown in figures 5–8.
Decomposition mechanism of hydrocarbon oil on bearing steel surface
The temperature rise at sliding contact may be one of the important factors for the activation cause of the decomposition reaction. As shown in figure 2, the decomposition reaction continued for a few seconds after the sliding process was terminated. Since the temperature rise should cease after the termination of sliding, the main part of the decomposition could not be caused only by the temperature rise due to friction, but also should be attributed to the catalytic activity of the fresh metal surface [10]. The main part of hydrogen evolution was observed after friction tests were continued for more than 7 h as shown in figure 3. It may need 7 h to form enough active sites on nascent surface for the reaction. After the experiments, wear scar was observed and it was about 180 μm in width.
A possible process of tribochemical reaction and decomposition of hydrocarbon oil on the nascent surface of bearing steel is proposed as described in figure 9. First, active sites on the nascent surface are generated by rubbing. It is expected that defects are generated on nascent surfaces by mechanical contacts. It is well known that surface defects act as an active site for certain reactions [19]. We have reported that aromatic compounds and olefins decomposed on nascent gold surface even at room temperature [1]. Second, the adsorption of hydrocarbon oil followed by C–C bond rupture leads to unzipping of molecule chain in contact with the nascent surface and the molecular weight of the oil molecule is reduced [20]. As a result, hydrogen and gaseous hydrocarbons are formed. It is clear that molecules decompose by the catalysis of the active sites. Since saturated hydrocarbon molecules cannot chemisorb even on nascent steel surface at room temperature [3], the decomposition should be affected by heat generated at the contact. Organic deposits formed on nascent surface will be removed easily even at low friction force, and the corresponding load is the critical load for the activation which is mentioned above.
In a certain decomposition mechanism, distribution of products may be uniform. The ratio of desorption amount of different ions represents desorption mechanism. Figure 10 shows the dependence of ratio of desorption amount of methane to hydrogen on mechanical conditions. It is found that the ratio of desorption amount of methane to hydrogen was not limited by sliding velocity and load in this study. For other gaseous hydrocarbons, similar results were obtained. This means that desorption mechanism of products generated from decomposition of hydrocarbon oil at mechanical contact of steel was not affected by mechanical conditions in this study.
Conclusions
Tribochemical reactions of hydrocarbon oil on the nascent surface of bearing steel 52100 under boundary lubrication conditions were investigated. Active sites were generated on the nascent surface of bearing steel after the removal of surface oxide layer during the rubbing process. Tribochemical decomposition of hydrocarbon oil occurred on those active sites. Hydrogen and gaseous hydrocarbons desorbed as tribochemical reaction products. The desorption amount of hydrogen and gaseous hydrocarbons increased linearly with sliding velocity, and parabolically with load. A critical load for the activation of decomposition of hydrocarbon oil on bearing steel was about 1.1 N. The ratio of desorption amount of gaseous hydrocarbon to hydrogen was independent of sliding velocity and load, which substantiated desorption mechanism of products generated by decomposition was not affected by mechanical conditions in this study.
Reference
X. Wu, N. Kobayashi, H. Nanao, S. Mori, Tribol. Lett. 18 (2005) 239
B. Vick, M.J. Furey, Wear 254 (2003) 1155
S. Mori, Y. Imaizumi, STLE Trans. 31 (1988) 449
K. Nakayama, H. Hashimoto, Tribol. Int. 29 (1996) 385
S.M. Hsu, J. Zhang, Z. Yin, Tribol. Lett. 13 (2002) 131
T.H. Ballinger, Jr. J.T. Yates, Langmuir 7 (1991) 3041
X. Liu, R.E. Truitt, J. Am. Chem. Soc. 119 (1997) 9856
S.S. Deshmukh, V.I. Kovalchuk, V.Y. Borovkov, J.L. d’Itri, J. Phys. Chem. B 104 (2000) 1277
S. Mori, M. Suginoya, Y. Tamai, ASLE Trans. 25 (1982) 261
S. Mori, Appl. Surf. Sci. 27 (1987) 401
X. Wu, P. Cong, H. Nanao, K. Kobayashi, S. Mori, Langmuir 18 (2002) 10122
G. Atxaga, A. Pelayo, A.M. Irisarri, Eng. Fail. Anal. 13 (2006) 226
M. Kohara, T. Kawamura, M. Egami, Tribol. Trans. 49 (2006) 53
M.J. Jansen, W.R. Jones and S.V. Pepper, NASA Glenn Research Center Technical Reports, NASA/TM—2002-211683, 1
M. Marchetti, W.R. Jones, K.W. Street, D. Wheeler, D. Dixon, M.J. Jansen, H. Kimura, Tribol. Lett. 12 (2002) 209
S. W. Chun, F.E. Talke, H.J. Kang, W.K. Kim, Tribol. Trans. 46 (2003) 70
Y. Yoshii, H. Hattori, Tribol. Online, 1 (2006) 14
C.G. Venier, E.W. Casserly, Lubri. Engr. 47 (1991) 586
G.A. Somorjai 1994 Introduction to surface chemistry and catalysis. Wiley-Interscience, New York
P.J. John, J.N. Cutler, J.H. Sanders, Tribol. Lett. 9 (2000) 167
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, R., Minami, I., Nanao, H. et al. Investigation of decomposition of hydrocarbon oil on the nascent surface of steel. Tribol Lett 27, 25–30 (2007). https://doi.org/10.1007/s11249-007-9203-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11249-007-9203-3