Abstract
Phytophthora root and stem rot (PRR) caused by Phytophthora sojae is one of the most devastating diseases reducing soybean (Glycine max) production all over the world. Harpin proteins in many plant pathogenic bacteria were confirmed to enhance disease and insect resistance in crop plants. Here, a harpin protein-encoding gene hrpZpsta from the P. syringae pv. tabaci strain Psta218 was codon-optimized (renamed hrpZm) and introduced into soybean cultivars Williams 82 and Shennong 9 by Agrobacterium-mediated transformation. Three independent transgenic lines over-expressing hrpZm were obtained and exhibited stable and enhanced tolerance to P. sojae infection in T2–T4 generations compared to the non-transformed (NT) and empty vector (EV)-transformed plants. Quantitative real-time PCR (qRT-PCR) analysis revealed that the expression of salicylic acid-dependent genes PR1, PR12, and PAL, jasmonic acid-dependent gene PPO, and hypersensitive response (HR)-related genes GmNPR1 and RAR was significantly up-regulated after P. sojae inoculation. Moreover, the activities of defense-related enzymes such as phenylalanine ammonia lyase (PAL), polyphenoloxidase (PPO), peroxidase, and superoxide dismutase also increased significantly in the transgenic lines compared to the NT and EV-transformed plants after inoculation. Our results suggest that over-expression of the hrpZm gene significantly enhances PRR tolerance in soybean by eliciting resistance responses mediated by multiple defense signaling pathways, thus providing an alternative approach for development of soybean varieties with improved tolerance against the soil-borne pathogen PRR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soybean (Glycine max) is one of the most important sources of vegetable oil and protein for human and livestock consumption. Phytophthora root and stem rot (PRR) caused by the soil-borne hemibiotrophic oomycete pathogen P. sojae, is currently one of the most devastating diseases of soybean and results in approximately 1–2 billion dollars of losses every year (Tyler 2007; Lin et al. 2013). PRR can lower soybean yield by 10–50% (Shen and Su 1991; Zuo et al. 2002) and poses a major threat to soybean production in China (Zhao et al. 2014). P. sojae can infect soybean plants at various developmental stages throughout much of the growing season (Schmitthenner et al. 1994). Symptoms caused by the pathogen include damping off of seedlings that causes emerging seedlings to collapse, rotting of roots and stems in established plants. PRR is usually controlled in the field by improving drainage, crop rotation, and the use of fungicides. Although crop rotation and tillage may have some positive effects on PRR management, these methods are not always practical. Chemical fungicides are not environmentally friendly and tend to raise public health concerns and cause the development of fungicide resistance in pathogens. Soybean germplasm with partial resistance to PRR were also used to develop resistant soybean cultivars (Qutob et al. 2000; Sugimoto et al. 2012); however, because of the complexity of physiological races (to date, at least 55 races identified) in P. sojae, partial resistance is not always adequate in preventing significant crop loss caused by PRR (Burnham et al. 2003). Genetic engineering utilizing alien genes that confer disease resistance offers an alternative to conventional breeding methods for improving resistance against plant pathogens. To date, several attempts have been made to generate transgenic soybean with enhanced PRR resistance by over-expressing the pathogenesis-related class 10 protein, Gly m 4l (Fan et al. 2015), and ethylene response factor (ERF) (Dong et al. 2015).
Harpins are a group of glycine-rich and heat-stable proteins encoded by the hrp (hypersensitive response and pathogenicity) genes in Gram-negative plant pathogenic bacteria. The proteins are secreted through a Type-III protein secretion system and act as translocators or helper proteins for effector proteins during host–pathogen interactions (Tampakaki et al. 2010). When externally applied to plants or produced endogenously after stable or transient expression, harpins can induce systemically acquired resistance (SAR) in host plants by activating defense responses mediated by salicylic acid (SA), jasmonic acid (JA), and ethylene signaling pathways (Sohn et al. 2007; Shao et al. 2008; Pavli et al. 2011). Overexpression of the harpin protein-encoding genes can confer an enhanced resistance to diseases and insects in plants such as tobacco, rice, rape, and cotton (Li et al. 2002, 2004; Fu et al. 2014; Huo et al. 2010; Miao et al. 2010; Wei et al. 1992). Transgenic tobacco plants expressing the hrpN gene from Erwinia amylovora showed increased resistance to the necrotrophic fungal pathogen Botrytis cinerea (Jang et al. 2006). The exogenous expression of the Xanthomonas oryzae hpa1Xoo gene in tobacco improved plant resistance to both Alternaria alternata and tobacco mosaic virus (Peng et al. 2004). Moreover, the hpa1Xoo also enhanced resistance to all predominant pathotypes of Magnaporthe oryzae in transgenic rice grown in China (Shao et al. 2008).
The hrpZpsta gene was first isolated from the P. syringae pv. tabaci strain Psta218 (Jiang et al. 2009), which encodes a 14.7 kDa glycine-rich protein similar to the harpins from Xanthomonas, Erwinia and Ralstonia solanacearum species (Miao et al. 2010). In previous studies, the external application of the recombinant hrpZpsta protein was reported to trigger the hypersensitive response (HR) in common tobacco (Jiang et al. 2009). Here, the codon-optimized hrpZpsta (renamed hrpZm) was introduced into soybean by Agrobacterium-mediated transformation, and transgenic soybean lines over-expressing hrpZm were evaluated for their tolerance to PRR. Furthermore, the changes in resistance response genes and plant defense-related enzymes elicited by hrpZm expression were also investigated. Our results indicate that over-expression of hrpZm in soybean significantly enhanced PRR tolerance by eliciting resistance responses mediated by multiple signaling pathways.
Materials and methods
Codon optimization of hrpZpsta and vector construction
The nucleic sequence of hrpZpsta (GenBank ID: FJ605454.1) from P. syringae was optimized based on soybean codon usage with the OptimumGene™ algorithm (GenScript USA Inc., USA) and renamed as hrpZm. For construction of the expression vector, 1.42 kb of the soybean constitutive promoter Gmubi3 (Glyma20g27950.1) which was reported to give strong constitutive expression in soybean (Hernandez-Garcia et al. 2009), was excised from the pUC57-Gmubi3 plasmid with the restriction enzymes Pst I and Xba I and then cloned into the compatible pTF101 plasmid. The optimized version of hrpZm was amplified with specific primer pairs (forward primer, 5′-GCTCTAGAATGCAAAGCCTTAGTTTGAACT CTTC-3′; reverse primer, 5′-CAGAGCTCTCACCATTGAAACTGCTGTTGCTG-3′), and subcloned into the pTF101-Gmubi3 at the Xba I and Sac I sites. In the resulting construct pTF101-Gmubi3-hrpZm, the hrpZm is located between the Gmubi3 promoter and the nos terminator (Fig. 1a). The construct also contains a bar gene which confers resistance to the herbicide phosphinothricin (PPT). The construct was then introduced into the Agrobacterium tumefaciens strain EHA101 using the freeze–thaw method as described by Holsters (1978).
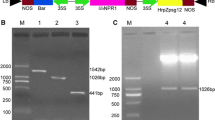
Generation and characterization of the transgenic plants over-expressing the hrpZm gene. a Schematic representation of the recombinant plasmid pTF101-Gmubi3-hrpZm. RB and LB indicate the right and left borders of the T-DNA. The hrpZm and bar genes were driven by soybean polyubiquitin promoter Gmubi3 and modified CaMV 35S promoters, respectively. b LibertyLink® strip detection of transgenic plants. W82 and SN9, non-transformed control Williams 82 and Shennong 9; 1–15, T0 transgenic plants. Arrows indicated control and test lines. c PCR analysis of the T1–T4 transgenic plants. M, DNA marker; Ctl+, positive control; Bk, blank control; NT, non-transformed plants; 1–20, transgenic plants. d Herbicide tolerance screening of the transgenic lines using 500 mg/L glufosinate spray. NT, non-transformed plants; HP49, HP116, and HP127, independent transgeniclines. e Southern blot analysis of the T2 transgenic plants. M, DNA marker; Ctl+, positive control; W82 and SN9, non-transformed control Williams 82 and Shennong 9; HP127, HP89, HP97, HP116, HP49, HP38, HP68, HP86 and HP173, T2 transgenic plants. f RT-PCR analysis of the transgenic plants. M, DNA marker; Ctl+, positive control; Bk, blank control; HP49, HP116, and HP127, T2 transgenic plants. g Western blot analysis of the transgenic plants. The polyclonal antibodies developed against the recombinant HRPZm and BAR proteins respectively were used to detect the expression of the foreign genes in the transgenic plants, with purified recombinant hrpZm and BAR protein as positive controls. M, protein ladder; Bk, blank control; Ctl+, recombinant hrpZm or BAR protein; NT, non-transformed plants; HP49, HP116, and HP127, T2 transgenic plants
Regeneration and screening of the transgenic plants
Two soybean cultivars, Williams 82(W82) and Shengnong 9(SN9), kindly provided by Prof. Fudi Xie from Shenyang Agricultural University in China, were used for Agrobacterium-mediated transformation as described by Zhang et al. (2014). As the negative control, the empty vector (EV) pTF101 without hrpZm was also transformed into the soybean. The regenerated PPT-tolerant plantlets were transplanted into a greenhouse, and transgenic seeds were produced by self-pollination. The T0 transgenic plants were screened by polymerase chain reaction (PCR) and LibertyLink® strip (EnviroLogix Inc., cat #AS 013 LS, Portland, ME, USA). Total genomic DNA was extracted from the young leaves of individual plants using a simple and quick DNA extracting method developed by Edwards et al. (1991). PCR was performed to verify the presence of hrpZ m using the primers HRP-F1/HRP-R1(5′-ATTACCCGTGTCATAGGCACCAAG-3′, 5′-CGCATTATCAGCAGACGCTCC-3′). PCR amplification was conducted with 2 × Taq PCR MasterMix (TansGen Biotech, Beijing, China) according to the manufacturer’s directions. The LibertyLink® strip detection was used to confirm expression of the bar gene product, phosphinothricin acetyltransferase, according to the manufacturer’s directions. From T1 to T4 generations, the leaf-spraying assay was used to screen for herbicide tolerance with 500 mg/L glufosinate when the first trifoliate leaves were fully expanded. Herbicide-tolerant plants were further subjected to PCR analysis using the aforementioned primers until homozygous transgenic plants were obtained.
Southern blot analysis
Southern blot analysis was performed with the DIG High Prime DNA Labeling and Detection Starter Kit I (Labeling and Detection Starter Kit I, 11745832910, Roche Applied Science, Indianapolis, IN, USA) according to the manufacturer’s instructions. The genomic DNA was prepared from T2 transgenic plants using a modified cetyl-trimethyl ammonium bromide method with high salt (Tel-Zur et al. 1999). Approximately 30 μg of the genomic DNA was digested completely with Xba I (New England Biolabs Inc., Beverly, Massachusetts) and separated on a 1.0% agarose gel. After denaturation with an alkaline solution, the digested DNA was transferred onto positively charged nylon membranes (GE Amersham, RPN303B, USA). The hybridization probe was amplified from the hrpZm fragment (423 bp) with the primers HRP-F2/HRP-R2(5′-ATGCAAAGCCTTAGTTTGAACTCTTC-3′, 5′-TCACCATTGAAACTGCTGTTG CTG-3′), and then labeled with digoxigenin using digoxigenin-(DIG)11-dUTP. Hybridization was carried out at 42 °C for 12–16 h. Chemical staining was carried out at room temperature with BCIP/NBT as substrate until signal detected clearly.
Real time-PCR (RT-PCR) and western blot analysis
RT-PCR was used to determine the expression of hrpZm in transgenic soybean plants. The young leaves of 2-week-old T2 transgenic plants were collected individually. Total RNA was prepared using the EasyPure PlantRNA Kit (TransGen Biotech, Beijing, China) and genomic DNA contaminations were eliminated with DNase I. The cDNA was then synthesized using the ThermoScript RT-PCR system (Invitrogen, USA) according to the manufacturer’s instructions. RT-PCR products were amplified with the primers HRP-F3/HRP-R3 (5′-AAACAACTGGTCCATCTACGAG-3′, 5′-AACTGCTGTTGCTGACGAG G-3′). The Gapdh gene of soybean (XM_003526927) was amplified with the primers (5′-CACCGGAGTTTTCACCGATA-3′,5′-AGGAATGATGTTAAATGAAGCAG-3′) as an internal reference for the standardization of gene expression. Three biological replicates were performed for each transgenic plant.
For western blot analysis, 100 mg of fresh leaves from T2 transgenic plants were frozen in liquid nitrogen and homogenized with a mortar and pestle in 1 mL of extraction buffer (100 mM NaCl, 10 mM EDTA, 200 mM Tris–HCl, 0.05% Tween-20, 0.1% SDS, 14 mM β-mercaptoethanol, 400 mM sucrose, and 2 mM phenylmethanesulfonyl fluoride). The homogenate was centrifuged at 10,000 rpm for 10 min and the total protein in the supernatant was quantified using the Bradford method (Bradford 1976). The protein samples were separated in a 12% SDS-PAGE gel and transferred electrophoretically onto a PVDF membrane (Thermo Fisher). After blocking with 3% dried skimmed milk diluted in PBST (1 × PBS, 0.1% Tween-20), the membrane was blotted with the rabbit polyclonal antibody against the recombinant HRPZm protein. Then, the membrane was incubated with horseradish peroxidase (HRP)-labeled goat-anti-rabbit IgG (1:5000 dilution, Abcam Trading Company Ltd., Shanghai, China) at room temperature and the color was developed using 3,3′-Diaminobenzidine (DAB). The expression of the bar was also analyzed with the mouse anti-BAR polyclonal antibody and goat anti-mouse IgG-HRP antibody as mentioned above. The polyclonal antibodies against HRPZm and BAR were developed and kindly provided by Dr. Yongzhi Wang from Jilin Academy of Agricultural Sciences.
Evaluation of tolerance to PRR under greenhouse conditions
The T2–T4 transgenic soybean lines and negative controls (NT and EV-transformed plants) were evaluated for PRR tolerance according to Schmitthenner et al. (1994). P. sojae race 1 was isolated from the infected soybean plants in the fields and cultured on the V8 agar medium (200 mL V8 juice, 3 g CaCO3, 1.5% agar per liter) at 25 °C (Akamatsu et al. 2010). Strain was allowed to grow on V8 agar medium until full growth was observed. The mycelia were prepared with a hole puncher with a diameter of 0.6 cm at the periphery of the petri dish to ensure the same amount of infectious materials used for inoculation. The hypocotyls of 15-day-old soybean seedlings grown in the greenhouse were inoculated. The inoculated plants were then kept in a humid environment for 15–24 h before being transferred to the greenhouse for symptom development at 25 °C in a photoperiod of 18 h light/6 h dark cycle. After 5–10 days of inoculation, the infected plants were numbered and the mortality rate of the plants was calculated (Zhu and Wang 1999). All of the experiments were performed three times with 20 plants inoculated for each replicate.
Quantitative RT-PCR analysis of the disease-responsive genes
The fully grown leaves from the T3 transgenic and negative control plants were harvested and frozen in liquid nitrogen at 0, 1, 2, 3, 4, 5 days after inoculation with P. sojae mycelia. Total RNA and cDNA was prepared as previously mentioned. qRT-PCR was performed to analyze relative expression levels of the defense-related genes including PR1 (AF136636), PR12 (BU964598), PAL (X52953), PPO (EF158428), GmNPR1 (FG418594), and GmRAR1 (FJ222386) with GmACT (U60500) as the internal control, using a SYBR Green-based One-Step qRT-PCR kit (TransGen Biotech, China). The amplification reactions were conducted in a final reaction volume of 20 µL containing 10 µL SYBR Green PCR Master Mix, 2 µL 80 ng cDNA, and 0.4 µL each of forward and reverse primers. The primers used in this study were provided in Supplementary Table S1. The reaction conditions were as follows: 50 °C for 2 min; 95 °C for 10 min; 45 cycles of 95 °C for 2 min, 62 °C for 30 s, and 72 °C for 30 s. The relative quantitative expression was determined using the 2−ΔΔCt method (Livak and Schmittgen 2001). All experiments were performed in three biological replicates and three technical replicates.
Activity analysis of defense-related enzymes PAL, PPO, POD, and SOD
Protein samples were extracted from the leaves of T3 transgenic and negative control plants mentioned above at 0, 1, 2, 3, 4, 5, 6 and 7 days after inoculation with P. sojae. The enzyme activity assays were performed as previously reported for PAL (Li et al. 2008), POD (Abeles and Biles 1991), PPO (Coseteng and Lee 1987), and SOD (Beauchamp and Fridovich 1971). All the samples were analyzed in three biological replicates.
Agronomic performance of the transgenic lines
T3 transgenic lines and their NT counterparts were grown at the experimental station in Jilin province, China. At maturity, twenty plants from each transgenic line were randomly sampled. Plant height, branch number, node number, pod number, seed number, seed weight, and 100-seed weights were measured and recorded.
Statistical analysis
For quantitative analysis, the data were analyzed with a t test and P = 0.05 or 0.01 using the Microsoft Analysis Tool. Differences in mortality rate and agronomic traits in each transgenic line were compared to those in negative controls (NT and EV-transformed plants).
Results and analysis
Generation of the transgenic soybean plants over-expressing the hrpZm gene
For optimal expression in soybean, the nucleic acid sequence of hrpZpsta from the P. syringae pv. tabaci strain Psta218 was optimized based on the soybean codon bias. The optimized version of hrpZm showed 73.52% identity with the original sequence and contained a GC content of 47.8%. A total of 173 PPT-tolerant plantlets were generated and screened by LibertyLink strip detection and PCR analysis. With the LibertyLink strip detection, the appearance of two red lines simultaneously in a sample indicated expression of the bar gene at the translational level (Fig. 1b). The presence of the expected amplicons (1.09 kb) was further confirmed in the LibertyLink® strip positive plants by PCR analysis (Fig. 1c). In this study, almost all the T0 transgenic plants flowered normally and produced fertile and viable seeds. From T1 to T4 generations, the transgenic lines were screened by glufosinate spraying and PCR analysis (Fig. 1c, d).
Southern blot analysis further confirmed the integration of the transgene in the transgenic soybean genomes with approximately 1–4 copies of T-DNA insertions in the selected independent transgenic plants (Fig. 1e). All the bands were greater than the expected fragment size of 2.57 kb fragment, which was located between the left border and the unique Xba I site near the right border. In contrast, no positive signal was detected in the NT plants. After preliminary PRR tolerance screening of T1 transgenic lines, the three independent transgenic lines HP49, HP116, and HP127 which exhibited higher tolerance to P. sojae infection were selected for further analysis.
The expression of hrpZm in transgenic plants was analyzed using RT-PCR and western blot analysis. A 332-bp RT-PCR fragment was detected in all three transgenic lines HP49, HP116, and HP127, but absent in the NT plants (Fig. 1f). Western blot analysis was further carried out to confirmed expression of the transgenes. As shown in Fig. 1g, a 14.76 kDa of hrpZm and 20 kDa of BAR were detected in the three transgenic plants with the corresponding polyclonal antibodies respectively, while no positive signal was detected in the NT plants (Fig. 1g). Taken together, all these results indicated that hrpZm was successfully transformed into soybean and expressed in the transgenic lines HP49, HP116, and HP127.
Three transgenic lines exhibited stable and enhanced tolerance to PRR
Tolerance of the three independent transgenic lines and their corresponding NT and EV- transformed plants to PRR was analyzed under greenhouse conditions. After 5–10 days of inoculation with P. sojae mycelia, the typical symptoms of PRR were observed in the NT and EV-transformed plants, including yellowing and wilting leaves, plant stunting and brown soft rot of the seedling stems. As the pathogen progressed, the wilted leaves bended towards the plant and succumbed to death (Fig. 2a, b). Comparatively, most of the transgenic plants showed slightly chlorotic leaves and developed normally (Fig. 2a, b). The mortality rates were further counted and calculated. As shown in Fig. 2c, three T2 transgenic lines showed a considerable decrease in mortality rates (ranging from 6.67 to 17.24%) compared to both the EV-transformed (36.26%) and NT plants (19.18% for Williams 82 and 33.33% for Shennong 9). Three T3 transgenic lines showed a considerable decrease in mortality rates (ranging from 15.38 to 40.00%) compared to both the EV-transformed (61.58%) and NT plants (43.33% for Williams 82 and 66.67% for Shennong 9). And T4 transgenic lines showed a considerable decrease in mortality rates (ranging from 14.28 to 35.71%) compared to both the EV-transformed (68.57%) and NT plants (33.33% for Williams 82 and 66.67% for Shennong 9).Throughout three generations (from T2 to T4), the transgenic lines exhibited stable and significantly enhanced tolerance to P. sojae compared to the NT and EV-transformed plants (Fig. 2c). However, when no P. sojae inoculation were involved, no agronomic traits or phenotypic changes such as leaf morphology, flower color, hilum color, plant height, podding height, node numbers, 100 seed weight, and maturity period were observed in the transgenic lines compared to the NT plants (Table 1). Taken together, our results showed that over-expression of the hrpZm confered stable and enhanced tolerance to PRR, and caused no unexpected visible changes in agronomic traits in transgenic soybean plants.
Tolerance evaluation of the T2–T4 transgenic lines to PRR under greenhouse conditions. a Tolerance responses of the transgenic lines at 10th day after inoculation with P. sojae race 1. W82 and SN9, non-transformed control Williams 82 and Shennong 9; EV, empty vector transformed plants; HP49, HP116, and HP127, T2 transgenic lines. b Reduction in mortality rate of three generations of transgenic lines inoculated with P. sojae. The hypocotyls of 15-day-old soybean seedlings were inoculated with macerated mycelia. All of the experiments were performed three times with 20 plants inoculated for in each replicate. c The average mortality rate for each transgenic line was calculated 7 days after inoculation with error bars indicating standard errors. Asterisks indicate significant differences between each transgenic line and the corresponding NT plants at the level of 0.01
Over-expression of hrpZm induced up-regulation of the disease-responsive genes in the transgenic soybean lines
Previous studies showed that harpin proteins could induce defense responses mediated by different signaling pathways (Sohn et al. 2007; Pavli et al. 2011). In this study, we investigated the expression levels of several disease-responsive genes in the transgenic plants after inoculation with P. sojae. As shown in Fig. 3, before inoculation, the basal expression levels of RAR, PR1 and PAL were higher in the transgenic plants compared to the NT and EV transformed plants; whereas GmNPR1, PPO and PR12 showed no dramatic changes in basal expression levels. These results suggested that the constitutive expression of hrpZm driven by the Gmubi3 promoter might up-regulate the HR-related and SA-dependent genes before inoculation. After inoculation with P. sojae, the expression levels of PR1, PR12, PAL which were thought to be mediated in SA signaling, and PPO which was involved in JA-dependent signaling, were all up-regulated in the transgenic plants relative to the NT and EV transformed plants. In addition, the expression of GmNPR1 and RAR, which were all marker genes for HR, were also significantly up-regulated in transgenic plants after P. sojae infection (Fig. 3a, f). The expression patterns of these defense-related genes in transgenic soybean plants differed from those in plants expressing the Erwinia pyrifoliae hrpN (EP) gene, in which the expression of NPR1 did not increase after inoculation with Botrytis cinerea (Sohn et al. 2007). Moreover, we found that the expression of GmNPR1, RAR, and PPO were up-regulated earlier than other SA-dependent disease-responsive genes such as PR12, PAL, and PR1, suggesting that the SA-mediated defense responses were induced later than those mediated by HR and JA after P. sojae inoculation. Our results suggested that the over-expression of hrpZm induced up-regulation of the disease-responsive genes in the transgenic soybean plants and different harpin proteins could elicit different defense signaling pathways during host–pathogen interactions.
The expression levels of the disease-responsive genes in transgenic soybean plants after P. sojae inoculation. Total RNA was extracted from the fully grown leaves of the T3 transgenic plants at 0, 1, 2, 3, 4, 5 days after inoculation with P. sojae mycelia. Quantification of each gene was calculated using the formula 2ΔΔCt for the expression levels relative to the internal control GmACT. The data represent means of three biological replicates, with error bars indicating standard errors. a–f indicated the expression levels of RAR, PR1, PPO, PAL, PR12 and GmNPR1, respectively. SN9, non-transformed control Shennong 9; EV, empty vector transformed plants; HP116, T3 transgenic plants
Over-expression of hrpZm increased the activities of defense-related enzymes
Plant defense-related enzymes play an important role in plant disease resistances (Wei and Beer 1993; He et al. 1993). In this study, four defense-related enzymes, PAL, POD, PPO and SOD, were analyzed in the transgenic line HP116 which contained one copy of T-DNA insertion. The results showed that the activities of the four defense-related enzymes significantly increased in the transgenic plants compared with NT and EV transformed plants after inoculation with P. sojae. PAL and POD showed similar activity patterns, indicated by the three peaks at 2, 4 and 7 dpi, respectively (Fig. 4a, b). In contrast, PPO activity had two peaks at 2 and 6 dpi (Fig. 4c). SOD activity reached a maximum level on the second day after inoculation but declined sharply on the fourth day, and then increased at 5 dpi (Fig. 4d). Despite the activity changes on different days after inoculation, all these four defense-related enzymes exhibited higher activities in the transgenic plants than in the negative controls including the NT and EV-transformed plants. Our results suggested that over-expression of hrpZm increased the activities of these defense-related enzymes, which were at least partly responsible for the enhanced PRR tolerance of the transgenic soybean lines.
Activity analysis of defense-related enzymes PAL, PPO, POD, and SOD. The enzymes were extracted from the leaves of T3 transgenic plants at 0, 1, 2, 3, 4, 5, 6 and 7 days after inoculation with P. sojae. The data represent means of three biological replicates and error bars indicate standard errors. a–d indicated the activities of PAL PPO, POD and SOD, respectively. SN9, non-transformed control Shennong 9; EV, empty vector transformed plants; HP116, T3 transgenic plants
Discussions
Harpin proteins have been reported to enhance resistance to diseases and insects in plants such as tobacco, rice, rape, and cotton (Li et al. 2002, 2004; Fu et al. 2014; Huo et al. 2010; Miao et al. 2010; Wei et al. 1992). In this study, we demonstrate that the transgenic soybean lines over-expression hrpZm from P. syringae enhanced the tolerance to the hemibiotrophic oomycete pathogen P. sojae, which causes serious yield losses in soybean crops all over the world every year. The enhanced tolerance to pathogen infection in the transgenic lines may result from the substantial increase in the expression of defense-related genes that are involved in multiple defense pathways. The basal expression levels of RAR, PR1, and PAL are higher in the un-inoculated transgenic plants compared to the NT and EV transformed plants. After inoculation with P. sojae, expression levels of the defense genes involved in HR and JA signaling were up-regulated earlier than those involved in SA signaling. Consistent with the expression of the defense genes, the activities of several defense-related enzymes, such as PAL, PPO, POD and SOD also increase significantly in transgenic lines compared to the negative control plants after P. sojae inoculation. These results indicate that hrpZm can induce a different set of defense-related genes and enzymes that enhanced tolerance to P. sojae in soybean.
The introduction of different harpin genes can affect the expression of various defense-related genes to facilitate resistance to plant pathogens (Sohn et al. 2007). In this study, the expression patterns of defense-related genes in hrpZm-expressing soybean lines are different from those observed in plants expressing the E. pyrifoliae hrpN (EP) gene. Specifically, the expression of NPR1 is significantly up-regulated in transgenic soybean after P. sojae inoculation; however, there are no expression changes of the NPR1 in hrpN-expressing plants after inoculation with B. cinerea (Sohn et al. 2007). The differences in the expression pattern of NPR1 in transgenic plants expressing different harpin genes could be due to variation in the receptors for the genes targeted by harpin proteins (Miao et al. 2010).
It should be noted that transgenic soybean lines expressing hrpZm are not entirely immune to P. sojae under our experimental conditions. Similarly, several other harpin-expressing transgenic plants only exhibit enhanced but not complete resistance to a wide range of pathogens (Peng et al. 2004; Shao et al. 2008; Sohn et al. 2007; Miao et al. 2010). The reason for this may be that PRR resistance assays are performed with the hypocotyl inoculation which uses a high inoculum dose of P. sojae. And it is possible that the incomplete pathogen resistance observed in hrpZm-expressing soybean plants is partially due to the dose of inoculum used in our laboratory conditions. PRR tolerance conferred by hrpZm needs to be further confirmed under field conditions.
Previous studies have shown that constitutive expression of some stress-response genes frequently results in growth retardation (Shen et al. 2003; Ito et al. 2006) or enhancement, as indicated by some harpins such as hrpN, which induces plant growth through the ethylene-mediated signaling pathway (Chuang et al. 2010; Dong et al. 2004; Oh et al. 2007). However, our results showed that hrpZm-expressing lines exhibited no obvious differences in the agricultural traits when compared to the NT plants. The results may be associated with the integration sites of the hrpZm in the soybean genome or differences in the signaling pathways and defense-related genes expression mediated by hrpZm compared to other harpin proteins.
References
Abeles FB, Biles CL (1991) Characterization of peroxidases in lignifying peach fruit endocarp. Plant Physiol 95:269–273
Akamatsu A, Chilvers M, Stewatr J, Peever T (2010) Identifacation and function of a polyketide synthase gene responsible for 1,8-dihydroxynaphthalene-melanin pigment biosynthesis in Ascochyta rabiei. Curr Genet 56:349–360
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and assay applicable to acrylamide gels. Anay Biochem 44:276–287
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Burnham KD (2003) Quantitative trait loci for partial resistance to Phytophthora sojae in soybean. Crop Sci 43:1610–1617
Chuang HW, Harnrak A, Chen YC, Hsu CM (2010) A harpin-induced ethylene-responsive factor regulates plant growth and responses to biotic and abiotic stresses. Biochem Bioph Res Commun 402:414–420
Coseteng MY, Lee CY (1987) Change in apple polyphenoloxidase and polyphenol concentrations in relation to degree of browning. J Food Sci 52:985–989
Dong H, Peng J, Bao Z, Meng X, Bonasera JM, Chen GY, Beer SV, Dong H (2004) Downstream divergence of the ethylene signaling pathway for harpin-stimulated arabidopsis growth and insect defense. Plant Physiol 136:3628–3638
Dong L, Cheng Y, Wu J, Cheng Q, Li W, Fan S, Jiang L, Xu Z, Kong F, Zhang D, Xu P, Zhang S (2015) Overexpression of GmERF5, a new member of the soybean ear motif-containing erf transcription factor, enhances resistance to Phytophthora sojae in soybean. J Exp Bot 66:2635–2647
Edwards K, Johnstone C, Thompson C (1991) A simple and rapid method for the preparation of plant genomic DNA for pcr analysis. Nucleic Acids Res 19:1349
Fan S, Jiang L, Wu J, Dong L, Cheng Q, Xu P, Zhang S (2015) A novel pathogenesis-related class 10 protein gly m 4 l, increases resistance upon Phytophthora sojae infection in soybean (Glycine max [L] merr.). PLoS ONE 10(10):e0140364. https://doi.org/10.1371/journal.pone.0140364
Fu M, Xu M, Zhou T, Wang D, Tian S, Han L, Dong H, Zhang C (2014) Transgenic expression of a functional fragment of harpin protein hpa1 in wheat induces the phloem-based defence against english grain aphid. J Exp Bot 65:1439–1453
He SY, Huang HC, Collmer A (1993) Pseudomonas syringae pv. Syringae harpinpss: a protein that is secreted via the hrp pathway and elicits the hypertensive response in plants. Cell 73:1255–1266
Hernandez-Garcia CM, Martinelli AP, Bouchard RA, Finer JJ (2009) A soybean (Glycine max) polyubiquitin promoter gives strong constitutive expression in transgenic soybean. Plant Cell Rep 28:837–849
Holsters M, Waele Dd, Depicker A, Messens E, Montagu MV, Schell J (1978) Transfection and transformation of agrobacterium tumefaciens. Mol Gen Genet 63:181–187
Huo R, Wang Y, Ma LL, Qiao JQ, Shao M, Gao XW (2010) Assessment of inheritance pattern and agronomic performance of transgenic rapeseed having harpin xooc-encoding hrf2 gene. Transgenic Res 19:841–847
Ito Y, Katsura K, Maruyama K, Taji T, Kobayashi M, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2006) Functional analysis of rice dreb1/cbf-type transcription factors involved in cold-responsive gene expression in transgenic rice. Plant Cell Physiol 47:141–153
Jang YS, Sohn SI, Wang MH (2006) The hrpN gene of Erwinia amylovora stimulates tobacco growth and enhances resistance to Botrytis cinerea. Planta 223:449–456
Jiang Z, Zou X, Gao J (2009) Cloning and expression of hrpZ gene from Pseudomonas syringae pv. Tabaci (in chinese). J Jilin Agric Univ 31:700–704
Li X, He Y, Chen S, Zhao Z, Li S, Li R (2002) Analysis of late blight resistance in transgenic potato expressing harpinea gene (in chinese). J Yunnan Agric Union 17:252–257
Li P, Lu X, Shao M, Long J, Wang J (2004) Genetic diversity of harpins from Xanthomonas oryzae and their activity to induce hypersensitive response and disease resistance in tobacco. Sci China Ser C 47:461–469
Li SM, Hua GG, Liu HX, Guo JH (2008) Analysis of defence enzymes induced by antagonistic bacterium Bacillus subtilis strain ar12 towards Ralstonia solanacearum in tomato. Ann Microbiol 58:573–578
Lin F, Zhao M, Ping J, Johnson A, Zhang B, Abney TS, Hughes TJ, Ma J (2013) Molecular mapping of two genes conferring resistance to Phytophthora sojae in a soybean landrace pi 567139b. Theor Appl Genet 126:2177–2185
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C(T)) Method. Methods 25:402–408
Miao W, Wang X, Li M, Song C, Wang Y, Hu D, Wang J (2010) Genetic transformation of cotton with a harpin-encoding gene hpaxoo confers an enhanced defense response against different pathogens through a priming mechanism. BMC Plant Biol 10:67
Oh CS, Beer SV (2007) AtHIPM, an ortholog of the apple hrpn interacting protein, is a negative regulator of plant growth and mediates the growth-enhancing effect of hrpN in Arabidopsis. Plant Physiol 145:426–436
Pavli OI, Kelaidi GI, Ampakaki AP, Skaracis GN (2011) The hrpZ gene of Pseudomonas syringae pv. Phaseolicola enhances resistance to rhizomania disease in transgenic Nicotiana benthamiana and sugar beet. PLoS ONE 3:e17306
Peng JL, Bao ZL, Ren HY, Wang JS, Dong HS (2004) Expression of harpinxoo in transgenic tobacco induces pathogen defense in the absence of hypersensitive cell death. Phytopathology 94:1048–1055
Qutob D, Hraber PT, Sobral BWS, Gijzen M (2000) Comparative analysis of expressed sequences in Phytophthora sojae. Plant Physiol 123:243–253
Schmitthenner AF, Hobe M, Bhat RG (1994) Phytophthora sojae races in ohio over 10-year interval. Plant Dis 78:269–276
Shao M, Wang J, Dean RA, Lin Y, Gao X, Hu S (2008) Expression of a harpin-encoding gene in rice confers durable nonspecific resistance to Magnaporthe grisea. Plant Biotechnol J 6:73–81
Shen CY, Su YC (1991) Discovery and preliminary studies of Phytophora megasperma on soybean in China (in chinese). Acta Phytopathol Sin 21:298
Shen YG, Zhan WK, He SJ, Zhang JS, Liu Q, Chen SY (2003) An EREBP/AP2-type protein in Triticum aestivum was a DRE-binding transcription factor induced by cold, dehydration and ABA stress. Theor Appl Genet 106:923–930
Sohn SI, Kim YH, Kim BR, Lee SY, Lim CK, Hur JH, Lee JY (2007) Transgenic tobacco expressing the hrpn ep gene from Erwinia pyrifoliae triggers defense responses against Botrytis cinerea. Mol Cells 24:232–239
Sugimoto K, Fujita S, Miyazawa T, Nishi H, Okada M, Takemura T (2012) Pediatric left renal vein entrapment syndrome diagnosed by 99mtc-albumin-conjugate scintigraphy. Nephron Clin Pract 122:122–126
Tampakaki AP, Skandalis N, Gazi AD, Bastaki MN, Sarris PF, Charova SN, Kokkinidis M, Panopoulos JN (2010) Playing the ‘harp’: evolution of our understanding of hrp/hrc genes. Annu Rev Phytopathol 48:347–370
Tel-Zur N, Abbo S, Myslabodski D, Mizrahi Y (1999) Modified ctab procedure for DNA isolation from epiphytic cacti of the general Hylocereus and Selenicereus (cactaceae). Plant Mol Biol Rep 17:249–254
Tyler MB (2007) Phytophthora sojae: root rot pathogen of soybean and model oomycete. Mol Plant Pathol 8:1–8
Wei ZM, Beer SV (1993) HrpI of Erwinia amylovora functions in secretion of harpin and is a member of a new protein family. J Bacteriol 175:7958–7967
Wei ZM, Laby JR, Zumoff CH, Bauer DW, He SY, Beer SV (1992) Harpin, elicitor of the hypersensitive response produced by the plant pathogen Envinia amylovora. Science 257:85–88
Zhang L, Yang XD, Zhang YY, Yang J, Qi GX, Guo DQ, Xing GJ, Yao Y, Xu WJ, Li HY, Li QY, Dong YS (2014) Changes in oleic acid content of transgenic soybeans by antisense rna mediated posttranscriptional gene silencing. Int J Genom 2014:921950
Zhao X, Sun M, Han Y, Li X, Zhang H, Teng W, Li W (2014) Identification of loci underlying tolerance to Phytophthora root rot in soybean germplasm (in chinese). Soybean Sci 33:488–491
Zhu Z, Wang X (1999) An identification method of soybean resistance to phytophthora root rot (in chinese). Chinese J oil crop sci 21:52–54
Zuo Y, Xue C, Han W, Liu T (2002) The infections characteristics of Phytophthora sojae to soybean seedlings (in chinese). Acta Phytophylacica Sin 12:377–378
Acknowledgements
This work was supported by grants from China National Novel Transgenic Organisms Breeding Project (2016ZX08004-004), Jilin Provincial Science and Technology Development Project (20150204011NY) and Jilin Provincial Agricultural Science and Technology Innovation Project (CXGC2017JQ013). We also thank Dr. Yongzhi Wang for providing the polyclonal antibodies and standard proteins.
Author information
Authors and Affiliations
Contributions
Hongyu Pan, Yingshan Dong and Qiyun Li designed the experiments. Qian Du, Xiangdong Yang, Xiaofang Zhong and Kyung Seok Kim performed the experiments, analyzed the data and drafted the manuscript. Jing Yang and Guojie Xing conducted the Agrobacterium-mediated transformation experiments. Jinhua Zhang, Xiaoyu Li and Zhaoyuan Jiang conducted the inoculation assay. All the authors participated in the revision of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors claim no conflict of interest in the publication of this paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Du, Q., Yang, X., Zhang, J. et al. Over-expression of the Pseudomonas syringae harpin-encoding gene hrpZm confers enhanced tolerance to Phytophthora root and stem rot in transgenic soybean. Transgenic Res 27, 277–288 (2018). https://doi.org/10.1007/s11248-018-0071-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-018-0071-4