Abstract
Phytophthora root and stem rot (PRR) caused by an oomycete pathogen Phytophthora sojae is one of the most devastating and widespread diseases throughout soybean-producing regions worldwide. The diversity and variability of P. sojae races make effective control of the pathogen challenging. Here, we introduced an elicitor of plant defense response, the harpinXooc-encoding hrf2 gene from the rice bacterial pathogen Xanthomonas oryzae pv. oryzicola into soybean and evaluated resistance to P. sojae infection. Molecular analysis confirmed the integration and expression of hrf2 in the transgenic soybean. After inoculation with P. sojae, non-transformed control (NC) plants exhibited typical PRR symptoms, including necrotic and wilting leaves, and plant death, whereas most of the transgenic plants showed slightly chlorotic leaves and developed normally. Through T3 to T5 generations, the transgenic events displayed milder disease symptoms and had higher survival rates compared to NC plants, indicating enhanced and stable resistance to P. sojae infection, whereas without P. sojae inoculation, no significant differences in agronomic traits were observed between the transgenic and non-transformed plants. Moreover, after inoculation with P. sojae, significant upregulation of a set of plant defense-related genes, including salicylic acid- and jasmonic acid-dependent and hypersensitive response-related genes was observed in the transgenic plants. Our results indicate that hrf2 expression in transgenic soybean significantly enhanced resistance to P. sojae by eliciting multiple defense responses mediated by different signaling pathways. The potential functional role of the hrf2 gene in plant defense against P. sojae and other pathogens makes it a promising tool for broadening disease resistance in soybean.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Phytophthora comprises over 100 species of parasitic oomycetes, the vast majority of which can cause severe diseases of various agriculturally important plants such as potato and soybean. Among them, Phytophthora sojae has a narrow host range and is primarily restricted to soybean, although some plant species such as lupin are also reported to be susceptible to the pathogen (Tyler 2007; Xiong et al. 2014). Phytophthora root and stem rot (PRR) caused by P. sojae is one of the most devastating and widespread diseases throughout many soybean-growing regions and is responsible for annual economic loss of 1–2 billion dollars (Sheng et al. 2015; Tyler 2007; Xiong et al. 2014). In recent years, PRR incidence has been increasing in several soybean-producing areas around the world, when soil conditions are suitable for pathogen development (Xiong et al. 2014). P. sojae can infect seeds, seedlings, and plants in all growth stages, causing seed decay, collapse of emerging seedlings, and root and stem rot in established plants. Presently, PRR is primarily managed based on host-mediated resistance provided by resistance (R) genes. In the P. sojae-soybean pathosystem, several types of resistance have been described, including race-specific resistance conferred by single dominant Rps (resistance to P. soja) genes and partial resistance provided by multiple genes (Qutob et al. 2000; Sugimoto et al. 2012). The resistance mediated by Rps genes encoding nucleotide-binding leucine-rich repeat (NB-LRR)-type proteins is race-specific and follows the gene-for-gene model (Bhattacharyya et al. 2005; Lin et al. 2014; Yin et al. 2013). To date, 18 Rps genes/alleles from soybean genetic sources have been identified and most of them have been mapped on the soybean genome (Lin et al. 2014; Sugimoto et al. 2012; Tyler 2007). Among them, seven Rps genes including Rps1a, Rps1b, Rps1c, Rps1 k, Rps3a, Rps6, and Rps8 are employed in commercial soybean production. However, PRR virulence pathotypes are very complex, with at least 55 P. sojae physiological races identified, and many virulence combinations occur because of mutations and sexual outcrossing (Abney et al. 2007; Burnham et al. 2003; Ryley et al. 1998; Tyler 2007). The diversity and variability of P. sojae races both within and between fields make it challenging to effectively control the pathogen based only on the Rsp gene-mediated resistance. Moreover, the use of resistant soybean cultivars with single Rsp genes can also promote the accumulation of new virulent pathogen strains, which is particularly evident in fields under continuous cultivation (Wang et al. 2006). Furthermore, although partial resistance which is inherited as quantitative trait loci (Wang et al. 2012), can provide more durable protection against P. sojae, it does not always prevent significant crop loss caused by the pathogen (Burnham et al. 2003). Therefore, new strategies for enhancing PRR resistance in soybean are still greatly needed.
To date, several attempts have been made to increase resistance of soybean to P. sojae infection by expressing foreign genes which confer resistance to fungal pathogens (Dong et al. 2015; Du et al. 2018; Fan et al. 2015). Overexpression of Gly m 4 l, a pathogenesis-related class 10 protein which plays an important role in the soybean defense system against P. sojae, was shown to enhance resistance to PRR (Fan et al. 2015). Expression of a novel ethylene response factor, GmERF5, in soybean significantly enhanced resistance to P. sojae and upregulated the expression of pathogenesis-related PR10, PR1-1, and PR10-1 genes involved in systemic acquired resistance (SAR) in plants (Dong et al. 2015). Recently, Du et al. (2018) showed that expression of the harpin-encoding gene hrpZm from Pseudomonas syringae enhanced resistance to P. sojae infections in transgenic soybean.
Harpin proteins encoded by the hrp (hypersensitive response and pathogenicity) genes are produced by several gram-negative plant pathogenic bacteria (Tampakaki et al. 2010). Harpins are secreted through type III secretion system and are mostly localized to the extracellular space in plant tissues, unlike bacterial effector proteins that act inside plant cells (Choi et al. 2013). Some harpins function as a part of translocator complexes involved in the translocation of effectors into plant cells (Choi et al. 2013). When harpins are directly applied to plants or expressed intracellularly, they trigger diverse beneficial processes such as induction of defense responses and enhancement of plant growth (Choi et al. 2013; Pavli et al. 2011; Peng et al. 2003; Shao et al. 2008; Sohn et al. 2007). Overexpression of harpin-encoding genes can enhance resistance to diseases and insects in plants such as tobacco, rice, rape, and cotton (Fu et al. 2014; Li et al. 2004; Miao et al. 2010; Rong et al. 2010; Wei et al. 1992). Recently, it has been reported that harpin overexpression in rice can even improve plant tolerance to abiotic stresses such as drought (Zhang et al. 2011). Multiple functions of harpin-encoding genes in plant development and defense against biotic and abiotic stresses make them a promising tool for enhancing plant disease resistance and improve crop yield through genetic engineering.
The harpinXooc-encoding hrf2 gene first isolated from the rice pathogenic bacterium Xanthomonas oryzae pv. oryzicola (Rong et al. 2010) has been shown to elicit hypersensitive response (HR)-related programmed cell death (PCD) in non-host plants and significantly enhance resistance to the fungal pathogen Sclerotinia sclerotiorum (Rong et al. 2010). In the present study, we generated transgenic soybean plants expressing hrf2 and assessed their resistance to the oomycete pathogen P. sojae.
Materials and methods
Agrobacterium tumefaciens-mediated transformation and generation of transgenic plants
A 414-bp fragment of the coding hrf2 region (as shown in Fig. S1) from X. oryzae pv. oryzicola strain RS105 (kindly provided by Prof. Xuewen Gao, Nanjing Agricultural University, China) was cloned into the SacI/XbaI sites of the compatible pCAMBIA3300 plasmid to generate the recombinant binary vector pCAMBIA3300-hrf2 (Fig. 1a). In the resulting construct, the total length of the T-DNA is 3.7 kb which covers the hrf2 coding region between the cauliflower mosaic virus (CaMV) 35S promoter and a polyA site, and the bar gene expression cassette which confers resistance to the herbicide glufosinate. The construct was then mobilized into A. tumefaciens strain EHA105. Soybean cultivar Shennong 9 which is susceptible to P. sojae, was used to generate transgenic soybean plants using the Agrobacterium-mediated transformation as described previously (Yang et al. 2017). The generated transgenic plants tolerant to glyfosinate (5 mg L−1) were transplanted into a greenhouse and analyzed by molecular screening.
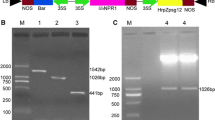
Generation and molecular analysis of transgenic soybean plants. a Schematic diagram of the pCAMBIA3300-hrf2 vector for Agrobacterium- mediated genetic transformation. The coding region of hrf2 was inserted between the constitutive promoter CaMV 35S and a polyA site. The construct also contained a selectable marker bar for selection of glufosinate-resistant transgenic plants. Solid bars indicate probes (bar and hrf2) used for Southern blotting. Small arrows indicate primers used for PCR screening. b, c Screening of transgenic plants using LibertyLink strip (b) and PCR detection (c). M, DNA marker (2 K); Ct + , positive control; NC, non-transformed plants; Bk, ddH2O; 1–12, T0 transgenic plants. d, e Southern blotting analysis of transgenic plants. Genomic DNA was digested with SacI or HindIII, and hybridized with the corresponding DIG-labeled hrf2 (d) and bar (e) probes, respectively. M, DNA marker (15 K); Ct + , positive control; NC, non-transformed plants; 1–4, T2 transgenic events L13, L44, L16, and L32. f Western blotting analysis of transgenic plants using a polyclonal antibody raised against the recombinant HRF2 protein; M, protein molecular weight markers; Ct + , recombinant HRF2 protein expressed in E. coli. NC, non-transformed plants; 1–4, transgenic events L13, L44, L16 and L32
Molecular analysis of transgenic soybean plants
Transgenic plants were first screened using LibertyLink® strips (EnviroLogix Inc., USA) and polymerase chain reaction (PCR) as described previously (Yang et al. 2018). Primers HRF2-F1 (5′-GTGGATTGATGTGATATCTCCACTG-3′) and HRF2-R1 (5′-CAAAGTCGCCGCCGCTGCTG-3′) specific for the CaMV 35S promoter and hrf2, respectively, were used to amplify a 492-bp fragment to confirm transgene presence. Glufosinate spray (1500 mg L−1) and PCR analysis were used to select positive progenies (generations T1 to T5). For Southern blotting analysis, total DNA was extracted from T2 transgenic and non-transformed control (NC) plants using a modified cetyltrimethylammonium bromide method (Telzur et al. 1999). Thirty micrograms of genomic DNA were digested with SacI or HindIII, subjected to electrophoresis in 0.8% agarose gels and subsequently transferred to positively charged nylon membranes (GE Amersham, USA). The transformation vector and non-transformed plants were used as positive and negative controls, respectively. Hybridization probes were prepared by amplifying the 414-bp hrf2 and 441-bp bar coding regions using primers HRF2-F2/HRF2-R2 (5′-ATGAACTCTTTGAACACAC. AATTC-3′/5′-TTACTGCATTGATGCGCTGTCGTTC-3′) and BAR-F1/BAR-R1 (5′-GCACCATCGTCAACCACTACATCGAG-3′/5′-TGAAGTCCAGCTGCCAGAAACCCAC-3′), respectively, and labeling them with digoxigenin (DIG)-High Prime (Roche, Germany). Hybridization was carried out at 42 °C for 12–16 h and staining was performed at room temperature with BCIP/NBT as substrate.
For western blotting analysis, leaf samples (0.1 g) were collected from T3 transgenic and NC plants and total proteins were extracted with buffer containing 100 mM NaCl, 10 mM EDTA, 200 mM Tris–HCl, 0.05% Tween-20, 0.1% SDS, 14 mM β-mercaptoethanol, 400 mM sucrose, and 2 mM phenylmethanesulfonyl fluoride. After sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in 12% gels, proteins were transferred onto PVDF membranes (GE Healthcare, USA) and probed with a rabbit polyclonal antibody against the recombinant HRF2 protein (with molecular weight of 13.63 kDa) expressed in E. coli (1:500 dilution, GenScript Co., Ltd., Nanjing, China), and then with horseradish peroxidase (HRP)-labeled goat-anti-rabbit IgG (1:5000 dilution; Abcam, UK) at room temperature for 4 h. After extensive washing, protein bands were visualized using BiodlightTM Western Chemiluminescent HRP substrate (Bioworld Technology, Inc. USA).
Evaluation of transgenic soybean plant resistance to P. sojae
P. sojae race 1, a prevalent strain in China, was cultured in 10% V8 media at 25 °C in the dark as described previously (Erwin and Ribeiro 1996), and used to infect unifoliate soybean leaves fully expanded in the greenhouse. Twenty hypocotyls of 15-day-old soybean seedlings of each transgenic and NC plants were inoculated with macerated mycelia (inoculum) of P. sojae using a standard hypocotyl inoculation method (Abney et al. 2007). The inoculated plants were maintained for 18–24 h in the dark, and then switched to the 18-h light/6-h dark regime at 25 °C for symptom development. PRR symptoms were evaluated approximately 7–10 days after inoculation and the percentage of surviving seedlings was calculated. The same number of non-transformed plants were mock-inoculated (wounded) without the pathogen and used as controls. All experiments were performed independently three times with 20 plants per replicate.
Expression analysis of defense-related genes
Quantitative real time (qRT) PCR was performed to assess transcription levels of defense-related SA-dependent genes such as GmPR1a (AF136636), GmPR2 (β-1, 3-glucanase, M37753), GmPR3 (chitinase class I, AF202731), GmPR5 (pathogenesis-related group 5 protein, BU765509), GmPR12 (defensin, M37753), and GmPAL (phenylalanine ammonia lyase, X52953), JA-dependent genes such as GmAOS (allene oxide synthase, DQ288260) and GmPPO (polyphenol oxidase, EF158428), and HR-related genes such as GmNPR1-1 (FJ418594), GmNPR1-2 (FJ418596), GmSGT1 (NM_001249656), and GmRAR1 (FJ222386). Three fresh leaves from randomly selected T3 transgenic and NC plants were harvested and frozen in liquid nitrogen at 0, 4, 8, and 12 h after inoculation with P. sojae. Total RNA was isolated using the EasyPure PlantRNA Kit (TransGen Biotech, China) and treated with DNase I to remove genomic DNA according to the manufacturer’s protocol. RNA integrity was confirmed by agarose gel electrophoresis and its concentration quantified by spectrophotometry (Nanodrop ND-1000, USA). First-strand cDNA was synthesized with the ThermoScript RT-PCR system (Invitrogen, USA) and used as a template for qRT-PCR performed at the following conditions: 50 °C for 2 min, 95 °C for 10 min, and 45 cycles of 95 °C for 2 min, 62 °C for 30 s, and 72 °C for 30 s. Primers used in this study are listed in Supplementary Table S1. Relative transcription levels of the target genes were calculated relative to that of the constitutively expressed native soybean gene GmACT11 (GenBank No. BW652479) using the 2ΔΔCt method as previously described (Yang et al. 2018). The results were statistically analyzed based on at least three independent biological replications.
Agronomic traits of transgenic plants in field conditions
For evaluation of the agronomic performance, the transgenic plants were planted in the field station in Jilin province, China. Three replicates of triple-row plots per transgenic event were grown using a randomized block design, with each row consisting of twenty-thirty plants. At maturity, ten plants from each transgenic event and NC control (without P. sojae inoculation) were randomly sampled and evaluated for agronomic traits such as plant height, branch, node, pod, and seed numbers, and 100-seed weights.
Statistical analysis
Least significant differences (LSD) between mean values were analyzed by the t test at P = 0.05 or 0.01 using SPSS software (v. 17.0) and each transgenic event was compared with the corresponding NC plants.
Results
Generation of transgenic soybean plants and molecular screening
Sixty-four plants of soybean genotype Shennong 9 were produced from glufosinate-tolerant green shoots following the Agrobacterium-mediated transformation. Among these, thirty-seven plants were confirmed positive by LibertyLink® strip detection and PCR analysis. LibertyLink strip detection showed expression of the bar gene with two red lines appearing simultaneously for transgenic plants (Fig. 1b). PCR analysis of LibertyLink-positive plants showed amplification of the expected 492-bp fragment corresponding to the CaMV 35S-hrf2 coding sequence (Fig. 1c). After preliminary PRR resistance screening, four independent transgenic events L13, L16, L32 and L44 which exhibited higher resistance, were selected for further molecular analysis and resistance evaluation. Integration of the foreign gene was confirmed by Southern blotting with both DIG-labeled hrf2 and bar probes. The results showed that hybridization signals appeared in the selected transgenic plants and all the bands were greater than the expected 1.56-kb (SacI-hrf2-RB, Fig. 1d) and 3.44-kb (LB-bar-hrf2-HindIII, Fig. 1e) fragments, respectively. In contrast, no hybridization signals were observed in NC plants. Moreover, low copy numbers of T-DNA insertions (single copies for L13 and L32, two copies for L16, three copies for L44) were detected in the four independent transformation events. Western blotting analysis revealed the presence of a 13.63-kDa polypeptide corresponding to the HRF2 monomer in transgenic plants, whereas no signals were observed in NC plants (Fig. 1f), confirming expression of the HRF2 protein in transgenic events.
Transgenic plants exhibited stably enhanced resistance to PRR under greenhouse conditions
Phenotypic reactions of the transgenic events and their corresponding NC plants to P. sojae were evaluated in the greenhouse. At 7 days after inoculation with P. sojae, typical PRR symptoms, including necrotic and wilting leaves and plant death, were observed in most NC plants of each experimental replicate. In contrast, most of transgenic plants showed no visible symptoms or only slightly chlorotic leaves and developed normally, and mock-inoculated transgenic seedlings were asymptomatic (Fig. 2a). The percentage of surviving seedlings calculated to assess disease severity caused by P. sojae inoculation was higher in transgenic events (73.35–92.86%) compared to NC plants (38.50–40.37%) (Fig. 2b), although slight variations in the proportion of surviving seedlings for each transgenic event across the three replicates were observed, which might be attributed to minor differences in environmental conditions. Furthermore, transgenic events consistently exhibited enhanced resistance to P. sojae compared to NC over three generations (from T3 to T5) (Fig. 2b). To analyze the influence of hrf2 expression on agronomic characteristics of transgenic plants, we evaluated several traits such as leaf morphology, flower and hilum color, plant and podding height, node numbers, weight of 100 seeds, and maturity period under field conditions without P. sojae inoculation. The data showed that there were no significant differences between transgenic and NC plants, indicating no visible influence on agronomic performance caused by hrf2 expression (Table 1).
PRR resistance evaluation of transgenic soybean plants expressing hrf2.a Resistance response of T3 transgenic plants to P. sojae infection. b Increased survival rate of three consecutive generations (T3–T5) of transgenic plants inoculated with P. sojae. NC, non-transformed plants; mock, non-transformed plant wounded without P. sojae inoculation. L13, L44, L16, and L32, transgenic events. The data are presented as the mean ± SE of three independent experiments (20 plants per each replicate); **P < 0.01 compared to corresponding NC plants
hrf2-expression upregulated multiple defense-related genes in transgenic plants
To investigate the molecular mechanisms underlying enhanced resistance to P. sojae in hrf2-expressing plants, we analyzed the transcription of 12 defense-related genes after P. sojae infection by qRT-PCR. First, we evaluated the time course of hrf2 expression in transgenic plants after inoculation with P. sojae. The results showed that hrf2 mRNA levels peaked at 4 h and remained high at 8 and 12 h post inoculation in all three examined transgenic soybean plants (Fig. 3a), although the abundance of the hrf2 transcript was slightly different among different transgenic events. Then, we compared mRNA levels of six SA-dependent genes (GmPR1, GmPR2, GmPR3, GmPR5, GmPR12 and GmPAL), two JA-dependent genes (GmAOS and GmPPO), and four HR-related genes (GmNPR1-1, GmNPR1-2, GmSGT1, and GmRAR) in transgenic and NC plants 4 h after P. sojae challenge. The results showed that the expression of all the 12 defense genes was significantly upregulated in response to P. sojae infection in transgenic plants but remained unchanged or increased to a lesser extent in NC plants (Fig. 3b–d). Especially high expression levels of the 12 defense-related genes were observed in transgenic event L13, which was consistent with the results of PRR resistance evaluation. Our data suggest that the expression of the hrf2 gene in soybean could elicit multiple resistance responses mediated by different signaling pathways, thus enhancing plant resistance to P. sojae infection and protecting soybean against PRR.
Relative expression of defense-related genes in transgenic soybean plants after P. sojae inoculation. a Expression changes of hrf2 mRNA in transgenic plants. Total RNA was extracted from fully grown leaves of T3 transgenic plants at 0, 4, 8, and 12 h after inoculation with P. sojae and mRNA levels of hrf2 were calculated relative to that of the GmACT11 gene. b–d Relative expression of six salicylic acid-dependent genes (b), two jasmonic acid-dependent genes (c), and four hypersensitive response-related genes (d) at 4 h after P. sojae inoculation. NC, non-transformed plants; L13, L16, and L32, transgenic events. The data are presented as the mean ± SE of three biological replicates; **P < 0.01 compared to corresponding NC plants
Discussion
Harpin proteins are known to increase resistance of plants to fungi and insects, improve their tolerance to abiotic stresses, and promote growth (Fu et al. 2014; Li et al. 2004; Miao et al. 2010; Rong et al. 2010; Wei et al. 1992; Zhang et al. 2011). It has been reported that the introduction of the harpinXooc-encoding hrf2 gene in rapeseed could effectively enhance its resistance to S. sclerotiorum, an oxalate-secreting necrotrophic fungal pathogen (Rong et al. 2010). In the present study, we showed that the transgenic plants constitutively expressing the hrf2 gene also exhibited significantly enhanced resistance to the oomycete pathogen P. sojae, as demonstrated by the hypocotyl inoculation assay. Compared with non-transformed plants, the transgenic events showed milder PRR symptoms and had higher survival rates. These results are consistent with the multiple functional activity of harpin proteins which were reported to induce plant resistance against diverse plant pathogens including fungi, bacteria, and viruses either after external application or stable expression (Choi et al. 2013; Pavli et al. 2011; Peng et al. 2003; Shao et al. 2008; Sohn et al. 2007). The potential function of hrf2 in plant defense against P. soja and other pathogens such as S. sclerotiorum makes this gene a promising candidate for engineering disease resistance in soybean breeding.
Mechanisms underlying increased resistance of harpin-expressing plants to various infections have to be characterized for future combination with other defense and resistance components. Phytohormones SA and JA are known to play crucial roles in regulating defense mechanisms in plants (Pieterse et al. 2009; 2012; Robert-Seilaniantz et al. 2011; Sugano et al. 2014). It was reported that an extensive crosstalk existing between phytohormone-controlled signaling pathways contributes to the induction of strong defense responses in plants (Koornneef and Pieterse 2008). SA plays a crucial role in the activation of defense mechanisms in response to biotrophic and hemibiotrophic pathogens, whereas JA is typically associated with defense against necrotrophic pathogens, and the respective pathways are often mutually antagonistic. However, synergistic interactions have also been reported in some pathosystems. In this study, we observed that six SA-dependent genes (GmPR1, GmPR2, GmPR3, GmPR, GmPR12, and GmPAL) and two JA-dependent genes (GmAOS and GmPPO) were simultaneously upregulated in hrf2-expressing transgenic plants compared with NC plants which showed no or little transcriptional changes in response to P. sojae infection. Moreover, transgenic plants also exhibited significantly increased expression of genes involved in HR signaling. These findings suggest that the enhanced resistance to P. sojae infection in transgenic soybean constitutively expressing hrf2 may be attributed to significant transcriptional upregulation of a panel of genes involved in different plant defense-related signaling pathways. In addition, it was also reported that external application of harpins such as HrpNEa to plants or constitutive expression of harpin-encoding genes promote plant growth through the ethylene-mediated signaling pathway (Chuang et al. 2010; Dong et al. 2004; Oh and Beer 2007). However, in this study, we did not observe significant differences in agronomic traits such as plant height in transgenic soybean events compared to NC plants in our preliminary field experiment. This discrepancy may be attributed to distinct signaling pathways mediated by HRF2 and other harpin proteins. The influence of expression of hrf2 on the agronomic performance of transgenic soybean needs to be further conducted larger field experiments.
Compared to exogenous application of harpins which can independently activate HR-related PCD and the SA-mediated SAR pathway in plants (Choi et al. 2013; Peng et al. 2003), endogenous expression of harpins may elicit more complicated responses to pathogens. Thus, transgenic plants expressing hrp genes show stronger reactivity to pathogens manifested by substantial increase in the expression of defense-related genes mediated by multiple signaling pathways (Pavli et al. 2011; Peng et al. 2004; Shao et al. 2008; Sohn et al. 2007), however, it should be noted that gene expression patterns might be distinct for different harpin proteins (Du et al. 2018). For example, although NPR1 is thought to be a key transcriptional regulator of multiple signaling pathways involved in plant defense (Peng et al. 2004), no changes in npr1 gene expression were detected in hrpN-transgenic plants inoculated with B. cinerea (Sohn et al. 2007). In contrast, in the present study, we observed a significant increase of npr1 expression in transgenic soybean after P. sojae infection. Similarly, transcriptional upregulation of the npr1 gene in response to pathogen infection was reported in transgenic tobacco plants expressing hpa1Xoo but not in those expressing hpaGEP (Peng et al. 2004; Sohn et al. 2007). This difference in npr1 regulation among harpin-expressing plants may be due to distinct receptors targeted by harpins of various origins (Miao et al. 2010).
In conclusion, our study suggests that the expression of the hrf2 gene encoding harpinXooc in soybean enhances plant resistance to P. sojae infection and protects against PRR through transcriptional upregulation of multiple defense-related genes. However, it should also be noted that transgenic soybean event expressing hrf2 are not entirely immune to P. sojae under our experimental conditions. For sustainability of PRR resistance, it is important to rely on multiple components of defense by pyramiding complementary resistance genes thorough breeding or genetic transformation.
References
Abney TS, Melgar JC, Richards TL, Scott DH, Grogan J, Young J (2007) New races of Phytophthora sojae with Rps1-d virulence. Plant Dis 81:653–655
Bhattacharyya MK, Narayanan NN, Gao H, Santra DK, Salimath SS, Kasuga T, Liu Y, Espinosa B, Ellison L, Marek L (2005) Identification of a large cluster of coiled coil-nucleotide binding site—leucine rich repeat-type genes from the Rps1 region containing Phytophthora resistance genes in soybean. Theor Appl Genet 111:75–86
Burnham KD, Dorrance AE, Vantoai TT, Martin S (2003) Quantitative trait loci for partial resistance to Phytophthora sojae in soybean. Crop Sci 43:1609–1617
Choi MS, Kim W, Lee C, Oh CS (2013) Harpins, multifunctional proteins secreted by gram-negative plant-pathogenic bacteria. Mol Plant Microbe Interact 26:1115–1122
Chuang H, Harnrak A, Chen YC, Hsu CM (2010) A harpin induced ethylene-responsive factor regulates plant growth and responses to biotic and abiotic stresses. Biochem Biophys Res Commun 402:414–420
Dong HP, Peng J, Bao Z, Meng X, Bonasera JM, Chen G, Beer SV, Dong H (2004) Downstream divergence of the ethylene signaling pathway for harpin-stimulated Arabidopsis growth and insect defense. Plant Physiol 136:3628–3638
Dong L, Cheng Y, Wu J, Cheng Q, Li W, Fan S, Jiang L, Xu Z, Kong F, Zhang D (2015) Overexpression of GmERF5, a new member of the soybean EAR motif-containing ERF transcription factor, enhances resistance to Phytophthora sojae in soybean. J Exp Bot 66:2635–2647
Du Q, Yang X, Zhang J, Zhong X, Kim KS, Yang J, Xing G, Li X, Jiang Z, Li Q, Dong Y, Pan H (2018) Over-expression of the Pseudomonas syringae harpin-encoding gene hrpZm confers enhanced tolerance to Phytophthora root and stem rot in transgenic soybean. Transgenic Res 27:277–288
Erwin DC, Ribeiro OK (1996) Phytophthora disease worldwide. American Phytopathological Society (APS Press), St. Paul
Fan S, Jiang L, Wu J, Dong L, Cheng Q, Xu P, Zhang S (2015) A novel pathogenesis-related class 10 protein Gly m 4 l, increases resistance upon Phytophthora sojae infection in soybean (Glycine max [L.] Merr.). Plos One 10:e0140364
Fu M, Xu M, Zhou T, Wang D, Shan T, Han L, Dong H, Zhang C (2014) Transgenic expression of a functional fragment of harpin protein Hpa1 in wheat induces the phloem-based defence against English grain aphid. J Exp Bot 65:1439–1453
Koornneef A, Pieterse CM (2008) Cross talk in defense signaling. Plant Physiol 146:839–844
Li P, Lu X, Shao M, Long J, Wang J (2004) Genetic diversity of harpins from Xanthomonas oryzae and their activity to induce hypersensitive response and disease resistance in tobacco. Sci China Ser C Life Sci 47:461–469
Lin F, Zhao M, Baumann DD, Ping J, Sun L, Liu Y, Zhang B, Tang Z, Hughes E, Doerge RW (2014) Molecular response to the pathogen Phytophthora sojae among ten soybean near isogenic lines revealed by comparative transcriptomics. BMC Genom 15:18. https://doi.org/10.1186/1471-2164-15-18
Miao WG, Wang XB, Li M, Congfeng S, Wang Y, Hu DW, Wang JS (2010) Genetic transformation of cotton with a harpin-encoding gene hpa Xoo confers an enhanced defense response against different pathogens through a priming mechanism. BMC Plant Biol 10:67. https://doi.org/10.1186/1471-2229-10-67
Oh CS, Beer SV (2007) AtHIPM, an ortholog of the apple HrpN-interacting protein, is a negative regulator of plant growth and mediates the growth-enhancing effect of HrpN in Arabidopsis. Plant Physiol 145:426–436
Pavli OI, Kelaidi GI, Tampakaki AP, Skaracis GN (2011) The hrpZ gene of Pseudomonas syringae pv. phaseolicola enhances resistance to rhizomania disease in transgenic Nicotiana benthamiana and sugar beet. Plos One 6:e17306
Peng JL, Dong HS, Dong HP, Delaney TP, Bonasera JM, Beer SV (2003) Harpin-elicited hypersensitive cell death and pathogen resistance require the NDR1 and EDS1 genes. Physiol Mol Plant Pathol 62:317–326
Peng JL, Bao ZL, Ren HY, Wang JS, Dong HS (2004) Expression of harpin(Xoo) in transgenic tobacco induces pathogen defense in the absence of hypersensitive cell death. Phytopathology 94:1048–1055
Pieterse CM, Leon-Reyes A, Van der ES, Van Wees SC (2009) Networking by small-molecule hormones in plant immunity. Nat Chem Biol 5:308–316
Pieterse CM, Van der DD, Zamioudis C, Leonreyes A, Van Wees SC (2012) Hormonal modulation of plant immunity. Annu Rev Cell Dev Biol 28:489–521
Qutob D, Hraber PT, Sobral BW, Gijzen M (2000) Comparative analysis of expressed sequences in Phytophthora sojae. Plant Physiol 123:243–254
Robert-Seilaniantz A, Grant M, Jones JD (2011) Hormone crosstalk in plant disease and defense: more than just jasmonate-salicylate antagonism. Annu Rev Phytopathol 49:317–343
Rong H, Yu W, Ma LL, Qiao JQ, Min S, Gao XW (2010) Assessment of inheritance pattern and agronomic performance of transgenic rapeseed having harpinXooc- encoding hrf2 gene. Transgenic Res 19:841–847
Ryley MJ, Obst NR, Irwin JAG, Drenth A (1998) Changes in the racial composition of Phytophthora sojae in Australia between 1979 and 1996. Plant Dis 82:1048–1054
Shao M, Wang J, Dean RA, Lin Y, Gao X, Hu S (2008) Expression of a harpin-encoding gene in rice confers durable nonspecific resistance to Magnaporthe grisea. Plant Biotechnol J 6:73–81
Sheng Y, Wang Y, Meijer HJG, Yang X, Hua C, Ye W, Tao K, Liu X, Govers F, Wang Y (2015) The heat shock transcription factor PsHSF1 of Phytophthora sojae is required for oxidative stress tolerance and detoxifying the plant oxidative burst. Environ Microbiol 17:1351–1364
Sohn SI, Kim YH, Kim BR, Lee SY, Lim CK, Hur JH, Lee JY (2007) Transgenic tobacco expressing the hrpN(EP) gene from Erwinia pyrifoliae triggers defense responses against Botrytis cinerea. Mol Cells 24:232–239
Sugano S, Sugimoto T, Takatsuji H, Jiang CJ (2014) Induction of resistance to Phytophthora sojae in soyabean (Glycine max) by salicylic acid and ethylene. Plant Pathol 62:1048–1056
Sugimoto T, Kato M, Yoshida S, Matsumoto I, Kobayashi T, Kaga A, Hajika M, Yamamoto R, Watanabe K, Aino M (2012) Pathogenic diversity of Phytophthora sojae and breeding strategies to develop Phytophthora-resistant soybeans. Breed Sci 61:511–522
Tampakaki AP, Skandalis N, Gazi AD, Bastaki MN, Sarris PF, Charova SN, Kokkinidis M, Panopoulos NJ (2010) Playing the “harp”: evolution of our understanding of hrp/hrc genes. Annu Rev Phytopathol 48:347–370
Telzur N, Abbo S, Myslabodski D, Mizrahi Y (1999) Modified CTAB procedure for DNA isolation from Epiphytic Cacti of the Genera Hylocereus and Selenicereus (Cactaceae). Plant Mol Biol Rep 17:249–254
Tyler BM (2007) Phytophthora sojae: root rot pathogen of soybean and model oomycete. Mol Plant Pathol 8:1–8
Wang Z, Wang Y, Chen X, Shen G, Zhang Z, Zheng X (2006) Differential screening reveals genes differentially expressed in low- and high-virulence near-isogenic Phytophthora sojae lines. Fungal Genet Biol 43:826–839
Wang H, Wijeratne A, Wijeratne S, Lee S, Dorrance AE (2012) Dissection of two soybean QTL conferring partial resistance to Phytophthora sojae through sequence and gene expression analysis. BMC Genom 13(1):428. https://doi.org/10.1186/1471-2164-13-428
Wei ZM, Laby RJ, Zumoff CH, Bauer DW, He SY, Collmer A, Beer SV (1992) Harpin, elicitor of the hypersensitive response produced by the plant pathogen Erwinia amylovora. Science 257:85–88
Xiong Q, Ye W, Choi D, Wong J, Qiao Y, Tao K, Wang Y, Ma W (2014) Phytophthora suppressor of RNA silencing 2 is a conserved RxLR effector that promotes infection in soybean and Arabidopsis thaliana. Mol Plant Microbe Interact 27:1379–1389
Yang X, Niu L, Zhang W, He H, Yang J, Xing G, Guo D, Du Q, Qian X, Yao Y, Li Q, Ying S (2017) Robust RNAi-mediated resistance to infection of seven potyvirids in soybean expressing an intron hairpin NIb RNA. Transgenic Res 26:665–676
Yang X, Niu L, Zhang W, Yang J, Xing G, He H, Guo D, Du Q, Qian X, Yao Y, Li Q, Ying S (2018) RNAi-mediated SMV P3 cistron silencing confers significantly enhanced resistance to multiple Potyvirus strains and isolates in transgenic soybean. Plant Cell Rep 37:103–114
Yin W, Dong S, Zhai L, Lin Y, Zheng X, Wang Y (2013) The Phytophthora sojae Avr1d gene encodes an RxLR-dEER effector with presence and absence polymorphisms among pathogen strains. Mol Plant Microbe Interact 26:958–968
Zhang L, Xiao S, Li W, Feng W, Li J, Wu Z, Gao X, Liu F, Shao M (2011) Overexpression of a Harpin-encoding gene hrf1 in rice enhances drought tolerance. J Exp Bot 62:4229–4238
Acknowledgements
This work was supported by Grants from Jilin Provincial Agricultural Science & Technology Innovation Project (c8223001010, c7208000307) and National Natural Science Foundation of China (31701448). We thank Prof. Xuewen Gao (Nanjing Agricultural University, China) for providing the pM18-hrf2 plasmid. We would also like to thank Editage (www.editage.cn) for English language editing.
Author information
Authors and Affiliations
Contributions
XY designed the experiments. LN conducted the experiments and wrote the manuscript. GX, DG, LS, and QZ performed Agrobacterium-mediated transformation experiments. JZ conducted the hypocotyl inoculation assay. HH, JY, and XZ participated in molecular screening and qRT-PCR analyses. All authors have read the manuscript and contributed to its revision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Niu, L., Yang, J., Zhang, J. et al. Introduction of the harpinXooc-encoding gene hrf2 in soybean enhances resistance against the oomycete pathogen Phytophthora sojae. Transgenic Res 28, 257–266 (2019). https://doi.org/10.1007/s11248-019-00119-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-019-00119-4