Abstract
The kinetics and mechanism of substitution of some iron(II)–triazine complexes, namely \( {\text{Fe}}({\text{PDT}})_{3}^{2 + } \), \( {\text{Fe}}({\text{PDTS}})_{3}^{4 - } \), \( {\text{Fe}}({\text{PPDT}})_{3}^{2 + } \) and \( {\text{Fe}}({\text{PPDTS}})_{3}^{7 - } \) (PDT = 3-((2-phenyl)-5,6-diphenyl))-1,2,4-triazine, PDTS = 3-(2-pyridyl)-5,6-bis(4-phenylsulfonicacid)-1,2,4-triazine disodium salt, PPDT = 3-((4-phenyl-2-pyridyl)-5,6-diphenyl)-1,2,4-triazine and PPDTS = 3-(4-(4-phenylsulfonicacid)-2-pyridyl)-5,6-bis(4-phenylsulfonicacid)-1,2,4-triazine trisodium salt) by 1,10-phenanthroline (phen) have been studied by conventional spectrophotometry in acetate buffers over the pH range 3.6–5.6, by following the absorbance of the triazine (tz) complexes at their respective absorption maxima. The reactions were carried out under pseudo-first-order conditions, that is, [phen] ≫ [Fe(II)–triazine]. The reactions were found to be first order in both Fe(II)triazine complex and phen. The rates increase with increasing pH. Plots of k obs versus [phen] are linear with positive intercepts, and also plots of k obs against 1/[H+] are linear with positive intercepts on the rate axes, indicating that the reactions proceed by both phen– and hydrogen ion–dependent and independent paths. From the kinetic data obtained at 25–55 °C, the specific rate constants and thermodynamic parameters have been computed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The most widely used type of colorimetric reagent for the determination of iron is the ferroin chromogen. Several compounds based on ferroin have been prepared and characterized after the two earliest compounds of this class, 2,2′-bipyridine (bpy) and 1,10-phenanthroline (phen), were synthesized by Blau in the late nineteenth century. The related tridentate ligands 2,2′,6′,2″-terpyridine (terpy) and 2,4,6-tripyridyl 1,3,5-triazine (TPTZ) form 1:2 complexes with iron(II). Subsequently, Smith and Case [1–3] made a number of innovations in this group of compounds by introducing various substituents. The synthesis of 3-((2-pyridyl)-5,6-diphenyl)-1,2,4-triazine (PDT), 3-(4-(4-phenyl-2-pyridyl)-5,6-di-phenyl-1,2,4-triazine (PPDT) and 2,4-bis(5,6-bis(4-phenyl)-1,2,4-triazin-3-yl)pyridine (BDTP) resulted from such studies. The solubility of these compounds in water is low, and hence, their sulfonated derivatives, PDTS (3-(2-pyridyl)-5,6-bis(4-phenylsulfonicacid)-1,2,4-triazine disodium salt), PPDTS 3-(4-(4-phenylsulfonic-acid)-2-pyridyl)-5,6-bis(4-phenylsulfonicacid)-1,2,4-triazine and BDTPS (2,4-bis(5,6-bis(4-phenylsulfonic-acid)-1,2,4-triazin-3-yl)pyridine tetra sodium salt), have been prepared. These triazines form 1:3 complexes with iron(II) and are structurally similar to \( {\text{Fe}}({\text{bpy}})_{3}^{2 + } \) with three planar ligands coordinated to an octahedral iron(II) center. The intense absorptivities of triazine complexes indicate pronounced electron delocalization in these complexes, suggestive of a planar configuration for each ligand. The sulfonated triazines are extensively used in the determination iron(II) at trace concentrations. The spectral characteristics and stability constants of iron(II) complexes of bpy, phen, terpy, TPTZ, PDT, PDTS, PPDT and PPDTS are presented in Table 1.
It can be seen from Table 1 that the log β value of \( {\text{Fe}}({\text{TPTZ}})_{2}^{2 + } \) is the lowest. Hence, the substitution of TPTZ by bpy, phen, terpy, PDT, PDTS, PPDT or PPDTS is possible. Kinetic and mechanistic studies of substitution of \( {\text{Fe}}({\text{TPTZ}})_{2}^{2 + } \) by bpy and phen [10], terpy [11] and PDTS and PPDTS [12] have been reported. Phen reacts with these complexes in acetate buffers in the pH range 3.6–5.6, forming ferroin as the final product. Detailed kinetic studies of these reactions have been carried out in acetate buffers in the pH range 3.6–5.6, and the results are presented in this paper.
Experimental
Reagents and equipment
PDT, PDTS, PPDT, PPDTS and phen were obtained from GFS Chemicals Inc., USA and used without recrystallization. A standard phen monohydrate solution of 1.0 × 10−2 mol dm−3 was prepared by dissolving the requisite quantity of phen in 1.0 × 10−2 mol dm−3 HNO3. 1.0 × 10−2 mol dm−3 solutions of PDTS, PPDTS and phen were prepared by dissolving requisite quantities in water and stored in amber-colored bottles. Since PDT and PPDT are insoluble in water, 1.0 × 10−2 mol dm−3 solution of these reagents was prepared by dissolving the requisite quantities in methanol (Merck, GR). A 1.0 × 10−2 mol dm−3 solution of iron(II) was prepared by dissolving the requisite quantity of [Fe(NH4)2](SO4)2·6 H2O (BDH, AnalaR) in 1.0 × 10−2 mol dm−3 H2SO4.
A 2.0 mol dm−3 solution of sodium acetate was prepared by dissolving the required quantity of sodium acetate (BDH AnalaR) in distilled water. The strength of this solution was checked by passing 5.0-ml aliquots through a Dowex-50W-X8 cation-exchange resin column (H+ form) and estimating the resultant H+ ions after thoroughly washing the resin bed with distilled water. Glacial acetic acid was purified by repeated partial freezing and crystallization, then diluted with water and standardized with NaOH using methyl orange as indicator. A 59Fe solution (as ferric chloride in HCl, t ½ = 45 days with emission of two γ-rays of 1.10 and 1.29 MeV) obtained from Bhabha Atomic Research Centre, Mumbai, was reduced to iron(II) with NH2OH and used to label iron(II) solutions, \( {\text{Fe}}({\text{PDT}})_{3}^{2 + } \), \( {\text{Fe}}({\text{PDTS}})_{3}^{4 - } \), \( {\text{Fe}}({\text{PPDT}})_{3}^{2 + } \)and \( {\text{Fe}}({\text{PPDTS}})_{3}^{7 - } \).
Stock solutions of 2.0 × 10−4 mol dm−3 of \( {\text{Fe}}({\text{PDT}})_{3}^{2 + } \), \( {\text{Fe}}({\text{PDTS}})_{3}^{4 - } \), \( {\text{Fe}}({\text{PPDT}})_{3}^{2 + } \) and \( {\text{Fe}}({\text{PPDTS}})_{3}^{7 - } \) (hereafter generally referred to as \( {\text{Fe}}({\text{tz}})_{3} \) for convenience) were prepared by mixing iron(II) and triazines in 1:3 ratio. The formation of \( {\text{Fe}}({\text{PDT}})_{3}^{2 + } \) and \( {\text{Fe}}({\text{PPDT}})_{3}^{2 + } \) is rapid, while the formation of their sulfonated analogues \( {\text{Fe}}({\text{PDTS}})_{3}^{4 - } \) and \( {\text{Fe}}({\text{PPDTS}})_{3}^{7 - } \) is slightly slower, taking about 5 min for quantitative completion.
A Shimadzu UV–2450 UV–visible recording spectrophotometer with TCC 240-A thermostated Cell Controller in which the temperature was controlled to ±0.1 °C by Peltier effect was used to record the absorption spectra and to perform kinetic runs. An Orion Ionalyser/901 was used for pH measurements. Gamma activity measurements were made with a single-channel analyser SC 800 (ECIL) in conjunction with a 3″ well type NaI (Tl) crystal (Nuclear Chicago).
Product analysis and kinetic procedure
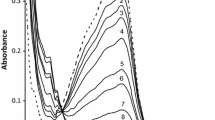
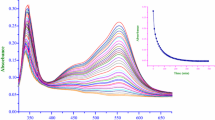
The visible absorption spectra of the reaction products show absorption maxima at 510 nm. The molar absorptivity for each of these products at 510 nm calculated on the basis of iron(II) content of the substrates corresponds to that of \( {\text{Fe(phen)}}_{ 3}^{{ 2 { + }}} \). The visible absorption spectra of all reactants and the final product(s) are shown in the Supplementary data.
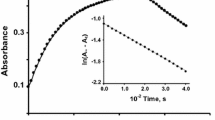
Requisite quantities of phen and acetic acid–sodium acetate buffer were taken in an amber-colored 25-ml volumetric flask and diluted with distilled water such that the total volume after the addition of iron(II)–triazine complex solution was 25 ml. The volumetric flask was suspended in a bath kept at the required temperature for a sufficient time to allow the reaction mixture to attain thermal equilibrium. Then, a known volume of iron(II)–triazine/sulfonated triazine solution, which was also equilibrated at the required temperature, was added, the contents were thoroughly mixed, and a portion of this solution was transferred quickly to the cell compartment of the spectrophotometer. The absorbance values at different time intervals were recorded against a water blank at 555, 562, 564 and 563 nm for \( {\text{Fe(PDT)}}_{ 3}^{{ 2 { + }}} \), \( {\text{Fe(PDTS)}}_{ 3}^{4 - } \), \( {\text{Fe(PPDT)}}_{ 3}^{{ 2 { + }}} \) and \( {\text{Fe(PPDTS)}}_{ 3}^{7 - } \) systems, respectively. The final product of the reaction, namely \( {\text{Fe(phen)}}_{ 3}^{{ 2 { + }}} \), has negligible absorbance at these wavelengths. The absorbance values at infinite time, A∞ (at 555, 562, 564 and 563 for the PDT, PDTS, PPDT and PPDTS systems, respectively), were obtained by allowing the reaction mixture to stand for sufficient time for the reactions to complete. The pseudo-first-order rate constants were evaluated from the slopes of plots of log (A t − A ∞) versus time, where A t is the absorbance value at time t and A ∞ is the absorbance at infinite time, that is, after the completion of reaction. About 60 % of the kinetic runs were performed in duplicate. The rate constants were reproducible to within ±8 %.
Results and discussion
The reactions were carried under pseudo-first-order conditions with [phen] ≫ [\( {\text{Fe}}({\text{tz}})_{3} \)] in acetate buffers in the pH range 3.6-5.6. Kinetic runs were performed at different initial concentrations of \( {\text{Fe}}({\text{tz}})_{3} \), keeping the concentrations of phen, pH and ionic strength constant. The k obs values were found to be independent of a fourfold increase in the initial concentration of \( {\text{Fe}}({\text{tz}})_{3} \), indicating that the reactions are first order in [Fe(tz)3]. The reactions were also carried out at different [phen], which revealed that an increase in [phen] increases the rate constant. These observations suggest that the reactions occur via two paths, namely phen dependent (associative) and phen independent (dissociative). Plots of k obs versus [phen] for the \( {\text{Fe}}({\text{PDT}})_{3}^{2 + } \)/\( {\text{Fe}}({\text{PDTS}})_{3}^{4 - } \)–phen reactions are presented in Figs. 1. Plots pertaining to the \( {\text{Fe}}({\text{PPDT}})_{3}^{2 + } \) and \( {\text{Fe}}({\text{PPDTS}})_{3}^{7 - } \) systems are given in the Supplementary data.
The rate increases with increase in pH. The plots of kobs versus 1/[H+] are linear with positive slopes on the ordinate, suggesting that these reactions also occur by pH-dependent and pH-independent paths. \( {\text{Fe}}({\text{PDT}})_{3}^{2 + } \)/\( {\text{Fe(PDTS)}}_{ 3}^{{ 4 { - }}} \)–phen reaction plots are shown in Fig. 2a and b, and those of other systems are given in the Supplementary data. The kinetic data for all four systems at 35 °C are presented in Table 2. The detailed data at all four temperatures of study namely 25, 35, 45 and 55 °C and the plots for \( {\text{Fe(PPDT)}}_{ 3}^{{ 2 { + }}} \)/\( {\text{Fe(PPDTS)}}_{ 3}^{{ 7 { - }}} \)–phen reactions are given in the Supplementary data.
Proposed mechanism for Fe(tz)3–phen
The pKa of phen is 5.02 at 25 °C [13]. Using this value, the concentrations of protonated and unprotonated forms of phen at different pH values in the range 3.6–5.6 have been computed using a simulation program. The data suggest that in this pH range, phen exists mostly in its protonated form. However, the unprotonated phen is considered to be the reactive species. We first consider the phen-independent pathway. It is well known that octahedral complexes containing bidentate ligands undergo dissociation with the formation of five-coordinated intermediates. It is considered that the iron(II)–triazine complexes also undergo such reversible dissociation in the rate-determining step with rate constant k 1 to give five-coordinated Fe(tz)2(tz-η1);
The phen molecules then react with this five-coordinated intermediate in a series of fast steps to give the final product, namely ferroin:
The substitution of Fe(tz)2(phen-η1) by water to give aqua species, which in turn reacts with phen to form \( {\text{Fe}}({\text{phen}})_{3}^{2 + } \), is also likely.
In the phen-dependent pathway, a phen molecule binds to the iron(II) center in Fe(tz)3 in the rate-determining step to give a seven-coordinate intermediate, Fe(tz)3(phen-η1);
Subsequently, Fe(tz)3(phen-η1) rearranges with the monodentate phen chelating at the iron(II) center after which the monodentate triazine dissociates;
The replacement of the other two triazine ligands by two more phen molecules, in view of large excess of [phen], occurs in a series of fast steps to give \( {\text{Fe}}({\text{phen}})_{3}^{2 + } \) as the final product.
This mechanism leads to the rate law:
Now
Hence
Therefore
Substituting the value of [phen]e in Eq. (7), we get
The terms [phen]t and [H+] e can be replaced by the initial concentrations of phen and hydrogen ion, respectively, written as [phen] and[H+]. Hence,
Since [H+] ≫ K a, the denominator in the above equation may be reduced to [H+]. Hence, Eq. (13) can be written as:
According to Eq. (14), plots of k obs versus [phen] and k obs versus 1/[H+] should be linear with positive slopes and intercepts, which is found to be the case. From these plots, the specific rate constants k 1 and k 2 have been evaluated using the pK a value of phen (K a values at 35, 45, 55 °C have been evaluated using the literature [13], pK a value of 5.02 at 25 °C and the E a and ΔS # values). The E a and ΔS # values for all the Fe(tz)3–phen reactions were evaluated and are presented in Table 3.
A comparison of specific rate constants for dissociative (k 1) and associative (k 2) paths indicates that the former is about 8–10 times faster than the latter. The data further show that in these sulfonated triazines, the k obs values and specific rate constants are about 10 times those of the corresponding unsulfonated analogues. This is due to the weakening of the iron(II)–nitrogen bonds as a result of the electron-withdrawing effects of the sulfonate groups. It is interesting to note that the specific rate constants for the unsulfonated and sulfonated triazines are almost equal for the associative path. This is because the rate-determining step involves addition of the incoming phen to the iron(II) center, forming a seven-coordinate intermediate, and does not involve breakage of an iron–nitrogen bond. The low E a values for the sulfonated triazines are also in consonance with this argument. The entropies of activation for the reaction of phen with iron(II)-sulfonated triazines are higher than those of their unsulfonated iron(II)–triazine complexes, indicating more ordered transition states in the case of the latter systems. The negative values of entropies for the phen-dependent path also support the associative pathway.
Table 3 also shows that the k 1 values are of the same order of magnitude as that for the dissociation of Fe(ferrozine)3. The dissociation of Fe(tz)3 [14] is very slow (e.g., the dissociation rate coefficient (k -1) for the reaction is 4.25 × 10−5 s−1). This is consistent with the mechanism suggested.
Ion-exchange studies
The proposed mechanism includes the formation of intermediates, Fe(tz)2(phen) and Fe(tz)(phen)2. Such complexes are reasonably stable and should be isolable. Fe(tz)3, the postulated intermediates, and the final product of all these reactions namely \( {\text{Fe}}({\text{phen}})_{3}^{2 + } \) all have different charges, which might be separated by ion-exchange chromatography. Hence, the reaction mixtures were subjected to ion-exchange separation to characterize the intermediate species. Since the intermediates separated with cation- or anion-exchange resins are very dilute and it will not be possible to confirm the presence of iron(II) species, 59Fe-labeled substrates were prepared for the ion-exchange studies. Stock solutions of 2.0 × 10−4 mol dm−3 concentration of Fe(tz)3 complexes were prepared by mixing 59Fe-labeled iron(II) and triazine solutions in the ratio of 1:3. Reaction mixtures that were 2.0 × 10−5 mol dm−3 in Fe(tz)3 and 1.0 × 10−3 mol dm−3 in phen were prepared and the pH of the solutions adjusted to ~4.0. These solutions were allowed to stand for about an hour and then cooled by adding ice. The solutions were successively passed through Dowex 50 W-X8 cation-exchange (H+ form) and Dowex 1X8 anion-exchange ([NO3]−form) resin columns (30 cm height and 1 cm dia).
For the \( {\text{Fe}}({\text{PDT}})_{3}^{2 + } \)/\( {\text{Fe}}({\text{PPDT}})_{3}^{2 + } \)–phen systems, a violet band was observed at the top of the cation-exchange column. The solutions that passed through both the cation and anion-exchange columns did not show any γ-activity, indicating the absence of neutral iron(II) species. The cation-exchange resin column was then washed with 1.0, 1.25, 1.50, 1.75 and 2.0 mol dm−3 HNO3 (cooled to 15 °C). The violet band moved slowly down the column on elution with 2.0 mol dm−3 HNO3 and was completely separated from the column with about 500 ml of 2.0 mol dm−3 HNO3. This eluate showed γ-activity. The desorbed solutions ahead of the violet band also showed γ-activity, probably due to \( {\text{Fe}}({\text{phen}})_{3}^{2 + } \). The total γ-activity activity of these two species corresponded to the γ-activity of the \( {\text{Fe}}({\text{PDT}})_{3}^{2 + } \)/\( {\text{Fe}}({\text{PPDT}})_{3}^{2 + } \) taken initially.
For the \( {\text{Fe}}({\text{PDTS}})_{3}^{4 - } \)/\( {\text{Fe}}({\text{PPDTS}})_{3}^{7 - } \)–phen systems, the solutions obtained after passing through the cation and anion-exchange columns showed γ-activity, indicating the presence of neutral iron(II) species. This neutral species on standing for a few hours after addition of phen turned orange red, and its visible absorption spectrum with λmax at 511 nm corresponded to that of ferroin. Hence, the neutral species separated may be Fe(PDTS)(phen)2. The cation-exchange resin column was washed with NaNO3 solutions of different concentrations. Gamma activity was noticed in eluents obtained on elution with 2.1 mol dm−3 NaNO3. The visible absorption spectrum of this solution recorded using a 10-cm quartz cell showed an absorption maximum at 510 nm. These ion-exchange results suggest that this species is dipositive, while the spectral characteristics reveal that it is \( {\text{Fe}}({\text{phen}})_{3}^{2 + } \). The anion-exchange resin bed was washed with solutions of different concentrations of NaNO3. The eluents obtained with 2.25 and 4.5 mol dm−3 NaNO3 showed γ-activity. From these results, it can be concluded that the species eluted with 2.1 mol dm−3 NaNO3 is dinegative, probably of the form Fe(PDTS)2(phen)2−, while the one separated with 4.5 mol dm−3 NaNO3 is the unreacted substrate, \( {\text{Fe}}({\text{PDTS}})_{3}^{4 - } \). The visible absorption spectrum of the species eluted with 4.5 mol dm−3 NaNO3 was recorded with a 10-cm quartz cell. This solution exhibited λmax at 562 nm, confirming that it is unreacted \( {\text{Fe}}({\text{PDTS}})_{3}^{4 - } \). To this solution, phen was added and allowed to stand for few hours, whereupon the solution turned orange red and its visible absorption spectrum corresponded to that of ferroin.
Similar ion-exchange studies were also carried out with Fe(PPDTS)3–phen reaction mixtures. The results suggest that only a single dipositive species with an absorption maximum at 510 nm could be desorbed from the cation-exchange column on elution with 2.25 mol dm−3 NaNO3. This corresponds to \( {\text{Fe}}({\text{phen}})_{3}^{2 + } \), the final product of these reactions. The results indicate the presence of mono (desorbed on elution with 1.0 mol dm−3 NaNO3)- and tetra-negative species (eluted with 4.5 mol dm−3 NaNO3) that can be attributed to \( {\text{Fe}}({\text{PPDTS}})({\text{phen}})_{2}^{ - } \) and Fe(PPDTS) (phen)4− intermediates. The anion-exchange resin column also showed a magenta species that could not be separated with either NaNO3 or Ca(NO3)2 of any concentration. On addition of phen to the putative mono-negative and tetra-negative species solutions and standing for a few hours, both solutions turned orange red and their visible spectra showed λmax at 510 nm, indicating the formation of ferroin. The solutions obtained after passing through both cation and anion-exchange resins did not show any γ-activity, indicating the absence of neutral reaction intermediates. All these results suggest that this substitution process occurs in successive steps. This is contrary to the mechanism envisioned by Burgess for the substitution of Fe(bpy)2(CN)2 by phen, which involved the formation of a high-spin aqua intermediate Fe(bpy)(CN)2(H2O)2 that gave aqua Fe2+, which in turn reacts with phen to form ferroin [15]. Burgess did not isolate any of the postulated intermediates.
Conclusion
In general, the substitution reactions at iron(II) centers are considered to occur by dissociative mechanisms. The present results are in accordance with this conclusion as the specific rate constants for the dissociative path (k 1) are much greater than the specific rate constants for the associative step (k 2). In the concentration range of phen used in these studies, the dissociative path is predominant. However, the kinetic data suggest clearly that at much higher concentrations of phen, the associative pathway would dominate.
References
Case FH (1960) A review of synthesis of organic compounds containing the ferroin group. GF Smith Chemical Company, Columbus
Diehl H, Smith GF, Mc Bride L, Cryberg R (1965) The iron reagents: bathophenanthroline, bathophenanthroline disulphonic acid, 2,4,6-tripyridyl-1-,3,5-triazine and phenyl-2-phenyl ketoxime, 2nd edn. GF Smith Chemical Company, Columbus
Schilt AA (1969) Analytical applications of 1,10-phenanthroline and related compounds. International series of monographs in analytical chemistry, vol 32. Pergman Press, New York
Schilt AA, Wong S-W (1984) J Coord Chem 13:331
Collins PF, Diehl H, Smith GF (1959) Anal Chem 31:1862
Ratnam S (1992) Kinetics and mechanism of some oxidation reactions of iron(III) in the presence of PDTS, PPDTS and BDTPS. PhD Thesis, Andhra University, Visakhapatnam, India
Stookey LL (1970) Anal Chem 42:779
Gibbs CR (1979) Anal Chem 48:1197
Traister GL, Schilt AA (1976) Anal Chem 48:1216
Rao GV, Sridhar Y, Hela PG, Padhi T, Anipindi NR (1999) Trans Met Chem 24:566
Rao GV, Bellam R, Anipindi NR (2012) Trans Met Chem 37:189
Anipindi NR (2012) Trans Met Chem 37:315
Danile PG, Ringano C, Sammartano S (1985) Talanta 32:78
Thompsen JC, Mottola HA (1984) Anal Chem 56:755
Burgess J (1969) Chem Commum 1422
Acknowledgments
Prof. T. Ramana, Coordinator, Advanced Analytical Laboratory, DST-Purse Programme, Andhra University, is thanked for making available the UV–visible spectrophotometer for carrying out this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bellam, R., Anipindi, N.R. Kinetics and mechanism of substitution of iron(II)–triazine complexes by 1,10-phenanthroline. Transition Met Chem 37, 489–495 (2012). https://doi.org/10.1007/s11243-012-9614-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-012-9614-3