Abstract
The reduction of tris(2,2-bipyridine)iron(III) by hydrazine in aqueous acidic medium exhibits first-order kinetics in both \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) and hydrazine. Saturation kinetics, consistent with reactant association prior to the redox step, has been observed. Because this reaction takes place while both the reactant, \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \), and the reduced product, \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \), decompose, a study of the irreversible decomposition of \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) and \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) has also been performed. The decomposition rate of the \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) and \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) species is, respectively, at least ca. 50- and ca. 40-fold slower than the N2H4-induced reduction of tris(2,2-bipyridine)iron(III) (k 2 = 2.1 M−1s−1 at [H+] = 0.0273 M, µ = 1.0 M and T = 25.0 ± 0.1 °C) under comparable conditions. An increase in [H+] of the reaction medium has a decreasing effect on the rate of the \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) reduction with hydrazine. A kinetically determined K a1 value for the dissociation of \( {\text{N}}_{2} {\text{H}}_{6}^{2 + } \) has been obtained as K a1 = 0.16 ± 0.01 M (pK a1 = 0.80) at µ = 1.0 M. \( {\text{N}}_{2} {\text{H}}_{5}^{ + } \) was found to be the more reactive hydrazine species in aqueous acidic medium. Activation parameters, applicable to the utilized experimental conditions for the hydrazine-induced reduction of \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) , have been determined as ΔH ‡ = 54 ± 2 kJ mol−1 and ΔS ‡ = −102 ± 6 J K−1 mol−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The benefits of substitution-inert one-electron transfer complexes, such as \( {\text{Mo}}\left( {\text{CN}} \right)_{8}^{n - } \), \( {\text{W}}\left( {\text{CN}} \right)_{8}^{n - } \) and \( {\text{Fe}}\left( {\text{CN}} \right)_{6}^{n - } \) (n = 3 or 4), in studying the kinetics of multi-electron transfer reactions are widely appreciated [1–3]. They react predominantly via an outer-sphere electron transfer mechanism.
The hydrazinium ion, \( {\text{N}}_{2} {\text{H}}_{5}^{ + } \), is known [4] to be a more powerful reducing agent than \( {\text{N}}_{2} {\text{H}}_{6}^{2 + } \) in relatively strong aqueous acidic medium, while in aqueous alkaline medium, neutral hydrazine, N2H4, is considered a stronger reducing agent than \( {\text{N}}_{2} {\text{H}}_{5}^{ + } \). It has been shown that the in the hydrazine-induced reduction of \( {\text{Fe}}\left( {\text{CN}} \right)_{6}^{3 - } \), \( {\text{W}}\left( {\text{CN}} \right)_{8}^{3 - } \), \( {\text{Mo}}\left( {\text{CN}} \right)_{8}^{3 - } \) [5, 6], \( {\text{Ir}}\left( {\text{Cl}} \right)_{6}^{2 - } \) [7] and the tyrosil radical of the R2 fragment of ribonucleotide reductase [8] in the pH range of 2 < pH < 10, N2H4 rather than \( {\text{N}}_{2} {\text{H}}_{5}^{ + } \) is the reactive hydrazine species. In contrast, in the relatively stronger acidity range 0.1 < [H+] < 0.3 mol dm−3, \( {\text{N}}_{2} {\text{H}}_{5}^{ + } \) was found to reduce \( {\text{Fe}}^{\text{III}} \left( {\text{phen}} \right)_{3}^{3 + } \) much faster than \( {\text{N}}_{2} {\text{H}}_{6}^{2 + } \) [9]. In all of these studies, the previously detected [10, 11] radical cation,  , is a reaction intermediate species. Nitrogen gas (N2) has been suggested as the nitrogen product in all the studies mentioned above. In fact, Stanbury [12] concluded that substitution-inert one-electron oxidants uniformly yield N2(g) as the sole nitrogen reaction product for reductions with hydrazine. In support of this, N2(g) was proved to be a reaction product by mass spectroscopy in the reduction of \( {\text{Fe}}^{\text{III}} \left( {\text{phen}} \right)_{3}^{3 + } \) by hydrazine [9].
, is a reaction intermediate species. Nitrogen gas (N2) has been suggested as the nitrogen product in all the studies mentioned above. In fact, Stanbury [12] concluded that substitution-inert one-electron oxidants uniformly yield N2(g) as the sole nitrogen reaction product for reductions with hydrazine. In support of this, N2(g) was proved to be a reaction product by mass spectroscopy in the reduction of \( {\text{Fe}}^{\text{III}} \left( {\text{phen}} \right)_{3}^{3 + } \) by hydrazine [9].
Although tris(2,2-bipyridine)iron(III) is a strong one-electron oxidant (E° = 1.06 V in 0.1 M acid solution [13]), the kinetics of \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) reduction by hydrazine has never been explored. Only kinetic studies of the reduction of \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) by hydroxide ions [14, 15], cobaloxime (Co(dmgBF2)2(H2O)2) [16] and nitropentacyanocobaltate(III) [17] have been reported. These reactions were found to be first order in both oxidizing and reducing agents. Irreversible decomposition of \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) [18, 19] and \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) could cause a major interference and could be of primary importance in systems involving \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) and \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) [14–17]. Basolo [18] qualitatively showed that at low [H2SO4], the decomposition rate of \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) increases with increasing [H+] and reaches a constant rate at [H+] > 1.0 M (H2SO4). It was also established that increasing the temperature of reaction mixtures increases the decomposition rate of \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \).
The \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) complex is frequently used for colorimetric detection of iron. However, the iron to be analyzed must be present as Fe2+ [20] before bipy will complex with it. This implies that before iron can be determined colorimetrically in any sample, a reduction of any Fe3+ present is necessary to allow formation of the \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) complex. After the formation of \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \), it must be reoxidized to \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) for the iron colorimetric analysis [20]. \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) is known to catalyze self-oscillation in polymer chains as a result of the Belousov–Zhabotinsky reaction [21]. These and other applications make knowledge about the stability and redox processes not only of \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) [18], but also of \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \), important.
In this study, we first describe the decomposition of the complex ions \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) and \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \). After the conditions were identified where these decompositions would least impact on the chemical reduction of \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) by a suitable reductant, we report on the kinetics and mechanism of the \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) reduction with hydrazine as a function of acid strength, ionic strength and temperature.
Experimental
Compounds
Tris(2,2-bipyridine)iron(III) perchlorate was synthesized from tris(2,2-bipyridine)iron(II) perchlorate by oxidation with lead(IV) oxide, adapting the described procedure for the synthesis of tris(1,10-phenantroline)iron(III) perchlorate [22]. The complex was used as a primary standard after recrystallization. Hydrazine solutions were prepared from hydrazine hydrate (Fluka Chemica) and were standardized by potassium iodate [23, 24]. A stock solution of sulfuric acid was prepared and standardized with borax using methyl red as indicator [24] to provide strong acidic conditions during synthesis [25] and kinetic studies. This allowed comparison of the obtained kinetic results of the hydrazine-induced reduction of \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) with those of the reduction of a similar complex, \( {\text{Fe}}^{\text{III}} \left( {\text{phen}} \right)_{3}^{3 + } \) [9] that was studied before. The free [H+] in reaction mixtures was calculated by assuming that H2SO4 quantitatively dissociates in the first dissociation step to H+ and \( {\text{HSO}}_{4}^{ - } \) in aqueous solution. For the dissociation of \( {\text{HSO}}_{4}^{ - } \) to H+ and \( {\text{SO}}_{4}^{2 - } \), the dissociation constant of 1.2 × 10−2 M [24] was utilized. This means that a 0.1 mol dm−3 H2SO4 solution does not have free [H+] of 0.2 mol dm−3 but rather [H+] = 0.1384 mol dm−3.
Kinetic measurements
Absorbance measurements to obtain extinction coefficients and kinetic data were performed on a Shimadzu 1650 UV–Vis spectrophotometer. Rate data for the decomposition of \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) have been obtained at 522 nm [ε522{\( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \)} = 8301(57) M−1 cm−1] and that of \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) at 610 nm [ε610{\( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \)} = 203(5) M−1 cm−1]. Kinetic data for the reduction of \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) by hydrazine under pseudo-first-order conditions at constant ionic strength have been obtained by monitoring the absorbance increase in the product, \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \), at 522 nm. The low extinction coefficient of the \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) complex at 522 nm [ε522{\( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) = 100(3) M−1 cm−1] implies that no significant \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) interference (less than 2 %) was observed while monitoring the formation of \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) at 522 nm. The purity of the \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) complex was assessed by comparing our measured extinction coefficient with that reported in the literature [ε610{\( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \)} = 220 M−1 cm−1] [16, 17]. The temperature was controlled to within 0.1 °C by a Shimadzu Peltier system temperature regulator.
Pseudo-first-order reaction rate constants have been obtained by a nonlinear least squares fit of absorbance data to the equation
The value of k obsd was also confirmed with ln-time plots.
Reaction runs have been performed at various temperatures between 20 °C and 35 °C to obtain activation parameters for the reactions. The activation parameters ΔH ≠ and ΔS ≠ have been obtained from the linearized Eyring equation (Eq. 2) [26], where k B and h are the Boltzman and Planck constants, respectively. T and R are the Kelvin temperature and gas constant (8.314 J K−1 mol−1), respectively, while k 2 is the second-order rate constant of the electron transfer reaction.
A linear plot of ln (k 2/T) versus 1/T yielded ΔH ≠ from the slope, −ΔH #/R, while ΔS ≠ was obtained from the intercept, −ΔS #/R + ln (k B/h).
Scientist 3.0, a general least square fitting program by Micromath [27] was used for fitting of kinetic data and calculation of parameters.
Results and discussion
Decomposition of \( {\text{Fe}}^{n} \left( {\text{bipy}} \right)_{3}^{n + } \) (n = 2, 3)
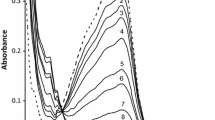
The reaction profile for the reaction between \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) and hydrazine shown in Fig. 1 does not resemble a typical pseudo-first-order reaction trace. At the end of the reaction, decomposition of the reduced product, \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \), is responsible for the observed absorbance decrease. Decomposition of the oxidant, \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \), may also contribute to the observed reaction profile in the initial stages of the reaction. These decomposition processes have a direct effect on the determination of the rate of reduction of \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) by hydrazine.
Reaction profile of the reduction of \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) by hydrazine at λ = 522 nm. The decrease in absorbance at the end of the reaction profile is associated with the decomposition of the reaction product, [\( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \)] Inset A ln-time plot from data of approximately the first half-life of the reaction. [\( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \)] = 0.0003 M; [N2H4] = 0.005 M; [H+] = 0.0692 M; µ(NaCl) = 1.0 M; T = 25.0 ± 0.1 °C. For the calculation of free H+ content, see “Experimental” section
To determine convenient experimental conditions for the reduction of tris(2,2-bipyridine)iron(III) with hydrazine that would at the same time limit interferences by the decomposition of \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) and \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) to a minimum, a study of the kinetics of the decomposition of the complex ions \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) and \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) was performed first. The decomposition kinetic data of both these complex ions in solution (Table 1; Fig. 2) indicate first-order kinetics. It was found that an increase in the ionic strength of the complex solution for both the complexes \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) and \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) has no effect on the decomposition rate (Table 1, entries 11–15). A decrease in the [H+] of solutions by decreasing the concentration of sulfuric acid (Table 1, entries 1–10), causes a decrease in the rate of decomposition for both the complexes.
Reaction trace of the decomposition at 30.0 ± 0.1 °C of \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) ([\( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \)] = 0.003 M; [H+] = 0.572 M; µ(NaCl) = 2.0 M; λ = 610 nm) and \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) ([\( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \)] = 0.0001 M; µ(NaCl) = 1.0 M; [H+] = 0.0692 M; λ = 522 nm). Insets A ln-time plot of the data. [Note that the concentration of \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) is more than ten times higher than that of \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \)] to observe the data on the same timescale
A mechanism for the decomposition of \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) has been suggested by Basolo and co-workers [18], and their mechanism is mutually consistent with the results of Tachiyashiki and Yamatera [19]. Our present results for the \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) decomposition are in agreement with the results of these two research groups, although an additional equilibrium involving protonation of an intermediate species has been identified and quantified in the proposed decomposition mechanism (Scheme 1). New in this scheme is the acid equilibrium involving the \( k_{3}^{{\prime }} \) and \( k_{ - 3}^{{\prime }} \) steps. The quantification of the acid dependence of the decomposition enabled us to solve all the other rate constants, \( k_{1}^{{\prime }} \), \( k_{ - 1}^{{\prime }} \), \( k_{2}^{{\prime }} \), \( k_{3}^{{\prime }} \), \( k_{ - 3}^{{\prime }} \) and \( k_{4}^{{\prime }} \) as well as the equilibrium constant \( K_{\text{a}}^{{\prime }} \) in the decomposition mechanism of \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) (values are shown in Scheme 1). This was not possible before. Previously, except for \( k_{1}^{{\prime }} \), only the ratios \( k_{2}^{{\prime }} /k_{ - 1}^{{\prime }} \), \( k_{3}^{{\prime }} /k_{ - 1}^{{\prime }} \) and \( - \left( {\frac{{k'_{ - 1} + k'_{2} }}{{k'_{3} }}} \right) \) could be obtained (Table 2) [19]. No similar data were available before for the decomposition of \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \), which were also obtained in this study.
Reaction mechanism for the decomposition of tris(2,2-bipyridine)iron(II) and iron(III), adapted from Ref. [18]. For \( k_{3}^{{\prime }} \) values, the first value was obtained utilizing Eq. (4) in Table 2 (\( k_{3}^{{\prime }} /k_{ - 1}^{{\prime }} \) = 1.79, with \( k_{ - 1}^{{\prime }} = k_{2}^{{\prime }} /0.133 \), the 3rd eq. in Table 2; and \( k_{2}^{{\prime }} \) obtained from the [H+] dependence of the reaction), while the second value was obtained from the gradient of the graphs shown in Fig. 4 [Eq. (1) in Table 2]. The obtained values are mutually consistent, lending credibility to the data treatment method
According to the originally proposed decomposition mechanism for \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) (i.e., by ignoring the \( k_{ - 3}^{{\prime }} \) step in the mechanism), with application of the steady-state approximation, a rate law (Eq. 3) for the observed decomposition was derived [18, 19] as
A quantitative fit of the kinetic data of Table 1 to Eq. (4) to resolve values for \( k_{ - 1}^{{\prime }} \), \( k_{2}^{{\prime }} \) and \( k_{3}^{{\prime }} \) is not possible. Failure to do this is probably due to activity effects of nonideal solutions which cannot be taken into account [19]. However, when [H+] is sufficiently low, the term \( k_{3}^{{\prime }} \)[H+] in Eq. (4) becomes negligibly small and the value of \( k_{{{\text{dec}},{\text{obsd}}}}^{\prime } \) will be equal to the limiting value described by Eq. (5).
The expression for \( k_{{{\text{dec}},{\text{obsd}}}}^{{\prime }} \) (Eq. 4) can be linearized (Eq. 6):
A plot of \( k_{{{\text{dec}},{\text{obsd}}}}^{\prime } \) versus \( \frac{{k_{{{\text{dec}},{\text{obsd}}}}^{{\prime }} - k_{{{\text{dec}},{ \lim }}}^{{\prime }} }}{{\left[ {{\text{H}}^{ + } } \right]}} \) for the variation of [H+] should be a straight line with gradient, m = \( - \left( {\frac{{k_{ - 1}^{\prime } + k_{2}^{\prime } }}{{k_{3}^{\prime } }}} \right) \), and the intercept yields a value for \( k_{1}^{{\prime }} \) (Fig. 3).
Relationship between k dec,obsd and \( \frac{{k_{\text{dec,obsd}} - k_{\text{dec,lim}} }}{{\left[ {{\text{H}}^{ + } } \right]}} \) at 20.0 ± 0.1 °C for the decomposition of \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{3 + } \) (triangular points: [\( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \)] = 0.0001 M; µ(NaCl) = 1.0 M; λ = 522 nm) and \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) (circular points: [\( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \)] = 0.003 M; µ(NaCl) = 2.0 M; λ = 610 nm)
Values for \( - \left( {\frac{{k_{ - 1}^{{\prime }} + k_{2}^{{\prime }} }}{{k_{3}^{{\prime }} }}} \right) \) and \( k_{1}^{{\prime }} \) as well as the calculated values for the ratios \( k_{2}^{{\prime }} /k_{ - 1}^{{\prime }} \) and \( k_{3}^{{\prime }} /k_{ - 1}^{{\prime }} \) for the decomposition of both \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) and \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) in aqueous solution are given in Table 2. The values obtained for the \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) decomposition are in agreement with values calculated by Tachiyashiki and Yamatera [19]. Similar data for the decomposition of \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) are also reported in Table 2 for the first time. To separate \( k_{ - 1}^{{\prime }} \), \( k_{2}^{{\prime }} \) and \( k_{3}^{{\prime }} \), an evaluation of the [H+] dependency of the decompositions was necessary.
Variation of [H+] during the decomposition of both \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) and \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) (Table 1, entries 1–10) clearly indicates a protonation equilibrium. The amended mechanism we propose, Scheme 1, makes provision for this previously unaccounted protonation equilibrium in the \( k_{3}^{{\prime }} \) and \( k_{ - 3}^{{\prime }} \) steps.
A nonlinear least squares fit of the [H+] variation data in Table 1 and Fig. 4 to Eq. (7) [28]
applied to the \( k_{2}^{{\prime }} \) and \( k_{4}^{{\prime }} \) reaction steps and the equilibrium step involving \( K_{\text{a}}^{{\prime }} \) and \( k_{3}^{{\prime }} /k_{ - 3}^{{\prime }} \) of Scheme 1 (normally one would use the notations k A, k HA and K a [28] for these steps and equilibrium, but we chose to use the notations of Scheme 1 so as not to lead to confusion with our data obtained later for the reduction of \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) with hydrazine), yields \( k_{4}^{{\prime }} = k_{\text{HA}} = \, \left( {5.7 \, \pm \, 0.2} \right) \times 10^{ - 4} \;{\text{s}}^{ - 1} \), \( k_{2}^{{\prime }} = k_{\text{A}} = \, \left( {5.3 \, \pm \, 0.3} \right) \times 10^{ - 5} \;{\text{s}}^{ - 1} \) and \( k_{3}^{{\prime }} /k_{ - 3}^{{\prime }} = K_{\text{a}}^{{\prime }} = \, 0.43 \, \pm \, 0.03\;{\text{M }}\left( {pK_{\text{a}} = \, 0.37} \right) \) for the \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) complex. For the \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) complex, values of \( k_{4}^{{\prime }} = k_{\text{HA}} = \, \left( {4.4 \, \pm \, 0.2} \right) \times 10^{ - 4} \;{\text{s}}^{ - 1} \), \( k_{2}^{{\prime }} = k_{\text{A}} = \, \left( {7.0 \, \pm \, 0.3} \right) \times 10^{ - 5} \;{\text{s}}^{ - 1} \) and \( k_{3}^{{\prime }} /k_{ - 3}^{{\prime }} = K_{\text{a}}^{{\prime }} = 0.38 \, \pm \, 0.04\;{\text{M}} \) (pK a = 0.42) were obtained.
Relationship between \( k_{\text{dec,obsd}} \) and [H+] at 20.0 ± 0.1 °C for the decomposition of \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) (triangular points; λ = 522 nm; [\( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \)] = 1.0 × 10−4 M; µ(NaCl) = 1.0 M) and \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) (circular points; λ = 610 nm; [\( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \)] = 3 × 10−3 M; µ(NaCl) = 2.0 M). For the calculation of free H+ content, see “Experimental” section
By utilizing \( k_{2}^{{\prime }} \) obtained from the acid dependence of \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) and \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) and substituting this in the equations yielding the values for \( k_{2}^{{\prime }} /k_{ - 1}^{{\prime }} \) and \( k_{3}^{{\prime }} /k_{ - 1}^{{\prime }} \) from Table 2, the rate constants \( k_{ - 1}^{{\prime }} \) and \( k_{3}^{{\prime }} \) could also be calculated, see Scheme 1 for the values. The proposed decomposition mechanism that is shown in Scheme 1 also contains all determinable rate constants. To independently verify these rate constants and those from the [H+] study, the obtained rate constants were substituted into the expression \( - \left( {\frac{{k_{ - 1}^{{\prime }} + k_{2}^{{\prime }} }}{{k_{3}^{{\prime }} }}} \right) \), the gradient in Fig. 3, of Table 2. The values obtained this way (−0.64 for \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) and −0.54 for \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \)) were mutually consistent with the experimental values reported in Table 2 (−0.57 and −0.46, respectively).
The decomposition of both the complexes, \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) and \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \), in aqueous solution is enhanced by an increase in the temperature of the complex ion solution (Table 1, entries 16–23). From the linearized Eyring equation (Eq. 2; Fig. 5), the activation parameters for \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \), under the experimental conditions in Table 1, have been determined as ΔH ≠ = 102 ± 2 kJ mol−1 and ΔS ≠ = 30 ± 7 J K−1 mol−1 at µ = 1.0 M (NaCl). The activation parameters for \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) have been determined as ΔH ≠ = 77 ± 2 kJ mol−1 and ΔS ≠ = 58 ± 5 J K−1 mol−1 at µ = 2.0 M (NaCl). The small size of these ΔH ≠ values (ΔH ≠ ≤ 102 kJ mol−1 for both complexes) are consistent with only one rate-determining decomposition step, shown in Scheme 1 as \( k_{2}^{{\prime }} \). All other components of the decomposition are at least one order of magnitude faster than \( k_{2}^{{\prime }} \), as in the case, for example, with \( k_{4}^{{\prime }} \). Consistent with this conclusion are the positive ΔS ≠ values of both complexes. Any positive ΔS ≠ value is consistent with bond breaking. The rate-determining step in Scheme 1, \( k_{2}^{{\prime }} \), consists of bond breaking between Fen+ and a “half-bonded” bipy ligand.
Relationship between ln(k obsd/T) and 1/T from the linearized Eyring equation for the decomposition of \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) (circular points) and \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) (triangular points). ([\( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \)] = 0.0001 M; free [H+] = 0.0692 M; µ(NaCl) = 1.0 M), and [\( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \)] = 0.003 M; free [H+] = 0.572 M; µ(NaCl) = 2.0 M)
The decomposition results of \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) and \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) indicated that convenient experimental conditions for the reduction of tris(2,2-bipyridine)iron(III) with hydrazine that would minimize the influence of decomposition of \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) and \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) on this redox process, are low [H+] and relatively low reaction temperatures. We therefore chose the general experimental conditions for the redox reaction between \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) and hydrazine as [H+] = 0.0237 M and a temperature of 25.0 ± 0.1 °C.
Reduction of \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \)
The reduction of \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) with hydrazine has been followed by monitoring the formation of \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) at 522 nm [ε522{\( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \)} = 8301(57) M−1 cm−1] under pseudo-first-order conditions, with [N2H4] at least a factor of 10 larger than [\( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \)]. Results are summarized in Table 3. Due to the decomposition of the complexes \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) and \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) in solution, albeit at least 40 times slower than the rate of electron transfer, only rate data processed up to the first half-life of the reaction were utilized. The decomposition of \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) and \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) may be considered as insignificant up to this point. To explain this, from the decomposition results of \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) and \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) (Table 1), at the chosen reaction conditions of [H] = 0.0273 M and 25.0 ± 0.1 °C, half-lives for the decompositions were calculated as 8.5 × 103 s and 6.7 × 103 s, respectively. Since the slowest pseudo-first-order hydrazine-induced reduction of \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) resulted in k = 0.44 × 10−2 s−1 (Table 3, entry 1) with a half-life of 157 s, it is reasonable to assume that during the first half-life of the redox reaction, the decomposition of \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) and \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) can be neglected.
Linear ln(A ∞ − A t) versus time plots indicate that the reaction is first order in the complex ion \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \). The ionic strength of the reaction medium does not have an effect on the oxidation reaction rate (Table 3, entries 16–20). Pseudo-first-order reaction rate constants, k obsd, for the variation of [N2H4] in the reaction mixture give a nonlinear dependence on [N2H4] (Table 3, entries 1–7; Fig. 6). This is consistent with a reaction mechanism involving saturation kinetics [29], Eqs. (8) and (9).
Dependence of k obsd on [N2H4]. A linear reciprocal plot (inset), consistent with saturation kinetic behavior from Eq. (11), is obtained. [\( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \)] = 0.0003 M; µ(NaCl) = 1.0 M; free [H+] = 0.0692 M; T = 25.0 ± 0.1 °C

The radical cation intermediate,  , has previously been proposed as an intermediate in the oxidation of hydrazine in several studies, and its detection has been described [7, 10, 11].
, has previously been proposed as an intermediate in the oxidation of hydrazine in several studies, and its detection has been described [7, 10, 11].
The saturation rate dependence at constant [H+] from Eqs. (8) and (9) can be derived as [29]:
The reciprocal equation,
implies that a plot of (1/k obsd vs. 1/[N2H4]) should be linear with slope = 1/k 2 K ass and y-intercept = 1/k 2. This dependence between k −1obsd and [N2H4]−1 was observed (inset Fig. 6), and a nonlinear least squares fit of the variation data of [N2H4] (Table 3) to Eq. (10) yielded K ass = 38 ± 4 M−1 and the redox step rate constant, k 2 = 0.046 ± 0.004 s−1. The initial slope in the curve of Fig. 6 is k 2 K ass = 1.8 ± 0.2 M−1s−1. In terms of N2H4 oxidation with \( {\text{Fe}}^{\text{III}} \left( {\text{phen}} \right)_{3}^{3 + } \), the redox rate constant was found to be k 2 = 0.041 ± 0.001 s−1, and the value of K ass was 55 ± 6 M−1s−1 [9].
Results in Table 3 (entries 8–15) indicate a decrease in reaction rate with an increase in hydrogen ion concentration. This indicates that a protonated hydrazine species should be involved in the reaction mechanism. Two protonated hydrazine species are known (Eqs. 12, 13).
Hydrazine is a weak base, and \( {\text{N}}_{2} {\text{H}}_{5}^{ + } \) has a pK a2 of 7.89 [12, 30–32]. The lowest experimental [H+] used, 0.0088 M (Table 3, entry 8), is high enough that N2H4 as reactive species is not possible under the conditions used, as 99.9 % of the added hydrazine in the reaction mixture will be present as the hydrazinium ion, \( {\text{N}}_{2} {\text{H}}_{5}^{ + } \). This means that Eq. (13) will not have a noticeable effect on the redox reaction rate under the experimental conditions reported in Table 3. With respect to Eq. (12), several K a1 values for \( {\text{N}}_{2} {\text{H}}_{6}^{2 + } \) have been reported [12, 30], but the value of K a1 = 0.57 M reported by Zhongjiang and Margerum [30] stands out as the most reliable. By applying K a1 = 0.57 M to Eq. (12), it can be calculated that ± 8 % of the added hydrazine will be present as \( {\text{N}}_{2} {\text{H}}_{6}^{2 + } \) and ± 92 % will be \( {\text{N}}_{2} {\text{H}}_{5}^{ + } \) at [H+] = 0.0088, the lowest [H+] used. The decrease in the reaction rate with increase in [H+] suggests that \( {\text{N}}_{2} {\text{H}}_{6}^{2 + } \) is less reactive in the reduction of \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) than the \( {\text{N}}_{2} {\text{H}}_{5}^{ + } \) species.
From Eq (12), two hydrazine species can therefore be involved in the reduction of \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) as per Eqs. (14) and (15).

A nonlinear least squares fit of the kinetic data for the variation of [H+] in the reaction mixture (Table 3; Fig. 7) to Eq. (7) [28] yielded k 1 = (8 ± 2) × 10−4 s−1, k 2 = (1.17 ± 0.01) × 10−2 s−1 and a kinetically determined equilibrium constant, K a1 = 0.16 ± 0.01 M−1 (pK a1 = 0.80) at µ = 1.0 M (NaCl) for equilibrium 12. This acid dissociation constant is in the same order of magnitude as the literature value previously reported by Margerum and co-workers (K a1 = 0.57 ± 0.05 M, pK a = 0.24 [30]) and is also mutually consistent with the value obtained during the oxidation of hydrazine by \( {\text{Fe}}^{\text{III}} \left( {\text{phen}} \right)_{3}^{3 + } \) (K a1 = 0.42 ± 0.05 M, pK a = 0.38 [9]). The value obtained for k HA = (8 ± 2) × 10−4 s−1 could be considered as zero within an error of 4σ(I).
Relationship between k obsd and pH and in the inset, [H+], for the reduction of \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) by N2H4. [\( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \)] = 0.0003 M; [N2H4] = 0.005 M; µ(NaCl) = 1.0 M; T = 25.0 ± 0.1 °C. For the calculation of free [H+], see “Experimental” section
Due to the decomposition of \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) and \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) in solution, the stoichiometry of the hydrazine-induced reduction of \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) could not be determined volumetrically. Stanbury [12], however, reported that N2(g) is the sole nitrogen product of the oxidation of hydrazine by an inert one-electron oxidant. This was confirmed in the kinetic study of the oxidation of hydrazine by \( {\text{Fe}}^{\text{III}} \left( {\text{phen}} \right)_{3}^{3 + } \) [9], where the formation of N2(g) was confirmed by mass spectrometry.
In this study, utilizing reagent concentrations substantially larger than that for the kinetic experiments, gas bubbles have also been observed forming in the reaction mixtures for the reduction of \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) with hydrazine. Since \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \), like \( {\text{Fe}}^{\text{III}} \left( {\text{phen}} \right)_{3}^{3 + } \), is also a one-electron oxidant and together with Stanbury’s observation [12], it is reasonable to assume that the observed gas bubbles are N2 gas. N2(g) as the sole nitrogen reaction product necessitates the following hydrazine redox half-reaction:
This indicates a consumption rate of 4 mol \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) for 1 mol N2H4. The overall reaction to satisfy such a stoichiometry is
This stoichiometry implies Eq. (18), following the rate-determining step Eq. (15) (which is composed of Eqs. 8, 9), represents three consecutive one-electron transfer steps k 3, k 4 and k 5, each faster than the rate-determining step (k 2), Eq. (15), to form the product N2(g) as explained by Stanbury [7].

Activation parameters for the electron transfer reaction between \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) and hydrazine applicable to the conditions employed in this study (Table 3) have been obtained by variation of the reaction temperature between 20 °C and 35 °C (Table 3, entries 20–27). Higher temperatures were not possible due to interference of the decomposition of \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) and \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) in solution, as it becomes too fast to ignore. The activation parameters ΔH ≠ and ΔS ≠ for the specific reaction conditions (Table 3) have been obtained from a nonlinear least squares fit to the linearized Eyring equation (Eq. 2; Fig. 8). This yielded an activation enthalpy, ΔH ≠ = 54 ± 2 kJ mol−1, and an activation entropy, ΔS ≠ = −102 ± 6 J K−1 mol−1. These thermodynamic parameters are associated with the fast equilibrium (Eq. 8) and the ensuing rate-determining electron transfer step (Eq. 9), in the overall reduction mechanism of the \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \). The smallness of ΔH ≠ is consistent with only one elementary rate-determining step. The negative value of ΔS ≠ is consistent with this reaction step involving bond formation. This is depicted in Eq. (8), which highlights the fast association between hydrazine and \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) to form the intermediate transition state, [FeIII(bipy)3·N2H4]3+, prior to the slow electron transfer step (Eq. 9).
Linear relationship between ln (k obsd/T) and 1/T from the linearized Eyring equation (the inset is a direct fit to the Eyring equation) for the electron transfer reaction of \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) and N2H4. Specific reaction conditions are [\( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \)] = 0.0001 M; [N2H4] = 0.005 M; free [H+] = 0.0692 M; µ(NaCl) = 1.0 M
The obtained thermodynamic values have been validated by the method of Lente et al. [26] utilizing Eq. (19).
where σΔS ≠ and σΔH ≠ are the standard errors of the activation parameters, and T av is the average temperature (in Kelvin) at which the experiments have been performed.
Conclusion
Under appropriate reaction conditions (relatively low reaction temperatures and high [H+]), the reduction of \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) with N2H4 proceeds fast enough to be independent of any influences of the decomposition of both reactant \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) and product \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \). The kinetically determined pK a of \( {\text{N}}_{2} {\text{H}}_{5}^{ + } \) from the reduction of \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) with N2H4 were mutually consistent with published values. The hitherto unreported quantification of the [H+] dependence of the decomposition of \( {\text{Fe}}^{\text{II}} \left( {\text{bipy}} \right)_{3}^{2 + } \) and \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) allowed for the unique determination of all rate constants in the decomposition mechanism. The huge change in the N-binding sphere from \( {\text{N}}_{2} {\text{H}}_{5}^{ + } \) to N2(g) in the chemical reduction of \( {\text{Fe}}^{\text{III}} \left( {\text{bipy}} \right)_{3}^{3 + } \) with hydrazine, the slowness of the electron transfer rate (\( t_{1/2} = \, 157\;{\text{s}} \) if free [H+] = 0.0273 M and [N2H4] = 0.002 M, entry 1 Table 3) and the formation of a bridged intermediate, [FeIII(bipy)3·N2H5]4+, are consistent with an inner-sphere electron transfer reaction mechanism [33]. In support of this, the negative \( \Delta S^{ \ne } \) (−102 ± 6 J K−1 mol−1) is consistent with the electron transfer process favouring bond formation (association of reactants) and is thus also indicative of an inner-sphere redox process [34].
References
Barchardt D, Pool K, Wherland S (1982) Inorg Chem 21:93
Nielsen R, Wherland S (1984) Inorg Chem 23:1338
Pelizzetti E, Pramauro E, Blandamer MJ, Burgess J, Gosal N (1985) Inorg Chim Acta 102:163
Cotton FA, Wilkinson G (1967) Advanced inorganic chemistry. Wiley-Interscience, New York, p 208
Leipoldt JG, Bok LDC, Van Wyk AJ, Dennis CR (1977) Reac Kinet Catal Lett 6:467
Dennis CR, Van Wyk AJ, Basson SS, Leipoldt JG (1987) Inorg Chem 26:270
Stanbury DM (1984) Inorg Chem 23:2879
Han J-Y, Swarts JC, Sykes AG (1996) Inorg Chem 35:4629
Al Mahdi AAGA, Hussein MA, Joubert CC, Swarts JC, Dennis CR (2014) Polyhedron 81:409
Adams JQ, Thomas JR (1963) J Chem Phys 39:1904
Falle RH (1968) Can J Chem 46:1703
Stanbury DM (1998) In: Karlin KD (ed) Progress in Inorg Chem, vol 47. Wiley, New York, pp 551–561
Schilt AA (1963) Anal Chem 35:1599
Nord G, Wernberg O (1972) J Chem Soc Dalton 866
Nord G, Wernberg O (1975) J Chem Soc Dalton 845
Wangila GW, Jordan RB (2005) Inorg Chim Acta 358:2804
Ogunlusi GO, Ige J, Oyetunji OA (2013) Trans Met Chem 38:45
Basolo F, Hayes JC, Neuman HM (1954) J Am Chem Soc 76:3807
Tachiyashiki S, Yamatera H (1984) Bull Chem Soc Jpn 57:1070
Fries J, Getro H (1977) Organic reagents for trace analysis. E Merck, Darmstad
Hara Y, Fujimoto K, Mayama H (2014) J Phys Chem B 118:608
Balakumar P, Balakumar S, Subramaniam P (2012) Reac Kinet Mech Cat 107:257
Kolthoff JM, Belcher R (1987) Volumetric analysis, vol 3. Interscience Inc, New York
Vogel AI (1961) Quantitative inorganic analysis, 3rd edn. Longman, London
Beller G, Lente G, Fabian I (2010) Inorg Chem 49:3698
Lente G, Fabian I, Poe AJ (2005) N J Chem 29:759
Scientist 3.0 software, Micromath (2012) Saint Louis, Missouri, USA
Wilkens RG (1991) The study of kinetics and mechanism of reactions of transition metal complexes, 2nd edn. Allyn and Bacon, Boston
Swarts JC, Aquino MAS, Han J-Y, Lam KY, Sykes AG (1995) Biochim et Biophys Acta 1247:215
Zhongjiang J, Margerum DW (1999) Inorg Chem 38:5374
Smith RM, Martell E (1976) Critical stability constants. Plenum, New York
Mezek SP, Tateishi M, MacFarlane R, Bartels DM (1996) J Chem Soc, Faraday Trans 92:2451
Back RA (1984) Rev Chem Intermed 5:293
Goldstein S, Meyerstein D, Van Eldik R, Czapski G (1997) J Phys Chem A 101:7114
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al Mahdi, A.A.G.A., Hussein, M.A., Fourie, E. et al. Influence of the decomposition of Tris(2,2-bipyridine)iron(II) and (III) on the reduction of Tris(2,2-bipyridine)iron(III) by hydrazine in aqueous acidic medium: a kinetic study. Transition Met Chem 41, 25–34 (2016). https://doi.org/10.1007/s11243-015-9993-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-015-9993-3