Abstract
Dinitroanilines represent a class of compounds that are widely used in herbicide formulations as they depolymerise plant microtubles, causing chromosome doubling. The potential of microtubule depolymerising herbicides trifluralin, oryzalin, and amiprophosmethyl (APM) for in vitro chromosome doubling of Rosa was studied. Five concentrations (0, 3, 6, 12 and 24 μM) and three exposure periods (12, 24 and 48 h) for each of the compounds were compared. Oryzalin, trifluralin and APM were not significantly different in their ability to induce chromosome doubling of R. hybrida cv Iceberg. At concentration of 6 μM and exposure period of 24 h, chromosome doubling of R. hybrida cv Iceberg was not significantly different with each of the polyplodising agents. At higher concentration (24 μM) and longer exposure period (48 h), 66.7% and 62.5% chromosome doubling was achieved with APM and trifluralin, respectively. However, the application of 6 μM oryzalin to R. persica (2n = 2x), R. hybrida cv Iceberg (2n = 3x) and R. hybrida cv Akito (2n = 4x), resulted in 60.0%, 6.3% and 0% chromosome doubling, respectively, which suggest that chromosome doubling is genotype dependent and plants with lower ploidy level have a higher propensity for chromosome doubling. Flow cytometry results at 18 and 24 weeks after herbicide treatment, indicated that the best time to test the treated plants was after 24 weeks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Some of the tremendous success of polyploid plants, ranging from superior environmental adaptation to horticultural and agricultural performance are due to their unique genetic attributes. Soltis and Soltis (2000) explained that (i) polyploids, both as individuals and as populations, generally maintain higher levels of heterozygosity than do their diploid progenitors; (ii) polyploids exhibit less inbreeding depression than do their diploid parents and can therefore tolerate higher levels of selfing, although polyploid angiosperms do not differ in outcrossing rates from their diploid parents; (iii) most polyploid species are polyphyletic, having formed recurrently from genetically different diploid parents.
Roses are one of the most widely grown and valued of all ornamental crops. Most cultivated garden, cut flower and pot roses are tetraploid (2n = 4x = 28), while most species roses and some cultivars are diploid (2n = 2x = 14) (Cairns 2000; Krüssmann 1981). Tetraploid cultivars have been crossed with wild diploids, often with the objective of introgressing the fungal disease resistance of wild roses into cultivated varieties, but the resulting triploids have low fertility (Rowley 1960; Shahare and Shastry 1963). Chromosome doubling of the triploid roses might be expected to produce fertile hexaploids.
The use of colchicine on shoot meristems has been the primary method of somatic polyploidisation in roses (Basye 1990; Ma et al. 1997; Roberts et al. 1990; Semeniuk and Arisumi 1968). Because colchicine is a highly toxic carcinogen and is relatively expensive, anti-microtubule herbicides like amiprophosmethyl (APM), oryzalin, and trifluralin have been explored as alternatives for polyploidisation (Eeckaut et al. 2004; Ramulu et al. 1991; Wan et al. 1991). The dinitroaniline herbicides oryzalin and trifluralin inhibit the rapid phase of paclitaxel (Taxol)-induced polymerisation of rose tubulin at low micromolar concentrations (Morejohn et al. 1987). The phosphoric thioamide herbicide APM also inhibits the Taxol-induced assembly of rose tubulin in vitro at low micromolar concentrations (Morejohn and Fosket 1984). Compared to colchicine, the efficiency of such herbicides in polyploidisation of plants has been variable but promising (Zlesak et al. 2005). Stanys et al. (2005) found plants with changed chromosome numbers were obtained at 0.25–38 mM colchicine and 10–50 μM oryzalin concentrations. We recently reported the successful in vitro chromosome doubling of diploid and triploid roses, using oryzalin (Kermani et al. 2003).
Following mitotic inhibitor treatment, cells arrested at metaphase may recover and enter the next mitotic cycle with twice number of chromosomes. When chromosome doubling agents are topically applied, chimerism is common and isolating polyploid sectors and ploidy characterisation of the meristematic layers is necessary (Semeniuk and Arisumi 1968; Tilney-Bassett 1986).
Roberts and Allum (2005) reported that the accessibility of the shoot meristems, the concentration of the spindle inhibitor and the exposure time are factors that are critical to success. In the present investigation, the efficiency of three antimicrotuble inhibitors (oryzalin, trifluralin and APM), were compared at different concentrations and for different exposure periods, on chromosome doubling of 3 mm nodal segments of Rosa persica (2n = 2x = 14), R. hybrida cv Iceberg (2n = 3x = 21) and R. hybrida cv Akito (2n = 4x = 28).
Materials and methods
Plant material and general procedures
Explants of R. persica, R. hybrida cv Iceberg and R. hybrida cv Akito were supplied by the Rose Germplasm Collection at the Agricultural Biotechnology Research Institute of Iran (ABRII). The ploidy level of explants was determined by flow cyotmetry. Nodal segments (1–1.5 cm) were washed thoroughly under running tap water for half an hour and surface sterilised for 30 s in 70% ethanol, followed by a 15 min soak in 2.5% sodium hypochlorite solution with a few drops of Tween-20 as a wetting agent, and rinsed three times with sterile distilled water. MS (Murashige and Skoog 1962) basal medium +30 g/l sucrose was used for introduction of plantlets to in vitro culture. After establishment in vitro, the plantlets were maintained on shoot proliferation medium, which contained full strength VS (van der Salm et al. 1994) salts and vitamins with 4 μM 6-benzylaminopurine (BAP), 0.5 μM 1-naphthaleneacetic acid (NAA) and 30 g/l sucrose. The pH of all media was adjusted to 5.8 before adding 8 g/l plant agar (Duchefa, Netherlands), and autoclaved for 15 min at 121°C and 1.2 kPa. Cultures were placed under high pressure metal halide lamps (PPFD 60 μmol m−2 s−1 at the plant surface) on a 16/8 h light/dark cycle in a culture room maintained at 21 ± 1°C.
In vitro shoots were then cultured on solidified shoot elongation medium (growth regulator-free shoot proliferation medium) for 6 weeks prior to chromosome doubling treatment.
Oryzalin, trifluralin and APM treatment of nodal sections
Stock solutions (50 mM) of oryzalin (Sigma-Aldrich, USA), trifluralin (Duchefa, Netherlands) and APM (Duchefa, Netherlands) were dissolved in dimethyl sulfoxide and filter sterilized (0.2 μM pore size, Sartorius, Germany). Appropriate amounts were added to autoclaved media after they had been cooled to 60°C. Nodal sections (approx. 3 mm thick) from the third, fourth and fifth nodes below the apex were used for polyploidisation treatments. Leaves were removed and the nodal sections were treated with a spindle inhibitor on a two-phase (liquid and semi-solid) shoot proliferation medium in glass 90 mm Petri dishes. Liquid media were used to help raise the concentration of the spindle inhibitor within the plant tissues as rapidly as possible and semi-solid media with the same concentrations of spindle inhibitors were used to secure the plants in position. After the treatment, shoots were washed and transferred to fresh semi-solid shoot proliferation medium. Each treatment involved 5 repeats with 4 explants (total of 20 explants). Survival and chromosome doubling rates were recorded after 18 and 24 weeks.
In order to investigate role of genotype on chromosome doubling, in the first experiment, nodal sections of R. persica, R. hybrida cv Iceberg and R. hybrida cv Akito were treated with 6 μM oryzalin for 24 h. In the second experiment, effect of different spindle inhibitors on chromosome doubling was studied; nodal sections of R. hybrida cv Iceberg were treated with 6 μM of oryzalin, trifluralin and APM for 24 h. In the third experiment, nodal sections of R. hybrida cv Iceberg were exposed to 0, 3, 6, 12 and 24 μM trifluralin and APM for 12, 24 and 48 h and interactive effect of different concentrations of spindle inhibitors and exposure periods were compared.
Assessments of oryzalin, trifluralin and APM effects on ploidy
After spindle inhibitor treatment, all the resulting shoots arised from the meristems of axillary buds that had been exposed to spindle inhibitors were sub-cultured on shoot proliferation medium every 3 weeks. According to Kermani et al. (2003), it was established, by micro-dissection, that both in vitro shoot tips and axillary buds contained 5–6 leaf primordia. It was expected that ploidy changes in these preformed leaves would be independent of the axillary buds they subtended, whereas in subsequently initiated nodes, the ploidy of a leaf and its axillary bud would be the same because they arose, after treatment, from the same meristem initials. At week 18, survival rate for all of the treatments was recorded and three leaves from each of the proliferated shoots (typically 15–20 shoots had arisen from each survived explant) were tested by flow cytometry. Chimerism, either between or within the leaves was considered as mixoploidy. For calculations, all proliferated shoots descended from one survived explant were considered as one, and mixoploidy and chromosome doubling were calculated as percentages of the survivors. The shoots were sub-cultured every 3 weeks again and, at week 24, three leaves from each shoot were tested and percentages of mixoploids and polyploids were recorded.
Ploidy was assessed by flow cytometry (PAI, Partec, Germany) using the same procedures as Yokoya et al. (2000). Parsley, Petroselinum crispum cv Champion Moss Curled (2n = 2x = 22; 2C DNA amount = 4.46 pg), was used as an internal calibration standard because its DNA amount was similar to, but distinct from, those of the roses under investigation. 4′, 6-diamidino-2-phenylindole (DAPI) was used as the flurochrome. Nuclei of rose leaves are mostly at the G1 (pre-replicative) stage of cell division and few are at the G2 (post-replicative) stage, so mixoploids can be easily identified in flow cytometry histograms by the presence of two peaks. For chromosome counting, in vitro shoots (from triploid and hexaploid R. hybrida cv Icerberg) were placed in rooting medium (half-strength VS mineral salts and vitamins, 15 g/l sucrose, 0.5 μM NAA and 8 g/l plant agar) 24 weeks after the spindle inhibitor treatment. In vitro root tip meristems from plantlets were collected, pretreated with 2 mM 8-hydroxyquinoline at room temperature for 2 h and fixed at 4°C for 48 h in 1:1 chromic acid (1%) and formalin (10%). The root tips were subsequently stored at 4°C in 70% ethanol until the cytological analysis. For chromosome staining, roots were hydrolyzed in 1 NaOH at 60°C for 10 min and stained using the technique described by Agayev (1998), after which the root tip meristems were macerated in a drop of cytase, and then squashed on a slide. Observation of metaphase chromosomes were made to support the flow cytometry results.
Experimental design and statistical analysis
Experiments were analyzed in a factorial based on completely randomized design. Analysis of variance was performed and comparisons of means were conducted using least significant difference (LSD). All analyses were performed using SAS and MSTATC software. Differences were regarded as significant at P ≤ 0.05.
Results
Role of genotype on chromosome doubling
In order to study the role of genotype on chromosome doubling, three different roses; R. persica, R. hybrida cv Iceberg and R. hybrida cv Akito were treated with oryzalin. Results indicated that when 6 μM of oryzalin was used for 24 h, 60.0% chromosome doubling was achieved in R. persica, 6.3% in R. hybrida cv Iceberg and 0% in R. hybrida cv Akito (Table 1). The survival rates of treated plantlets were 100% in R. persica and 80% in the other two genotypes. Table 1 also shows that when the plantlets were tested 18 and 24 weeks, after treatment higher rates of chromosome doubling were observed with the later, suggesting that some of the mixoploids were converted to chromosome doubled plants.
Effect of oryzalin, trifluralin and APM on R. hybrida cv Iceberg
When R. hybrida cv Iceberg was treated with 6 μM of oryzalin, trifluralin and APM for 24 h, survival rates of treated shoots and percentage of resulting hexaploids were not significantly different (Table 2). The results also indicated that percentages of chromosome doubled plants recorded 18 weeks after treatments were 0%, but these increased to 7.1%, 6.7% and 6.3% for trifluralin, APM and oryzalin, respectively by week 24, which further support testing of the treated plantlets after 24 weeks.
Interactive effects of concentrations and exposure periods
R. hybrida cv Iceberg was treated with 0, 3, 6, 12 and 24 μM trifluralin and APM for 12, 24 and 48 h. Mean comparison test indicated that as the exposure period and concentration of polyploidizing agents were increased, higher rates of chromosome doubling were achieved. At a concentration of 24 μM for 48 h, 66.7% and 62.5% chromosome doubling was detected for APM and trifluralin, respectively (Table 3). Statistical analysis showed that, at the treatment parameters used, trifluralin and APM were not significantly different in their ability to induce chromosome doubling (Table 4). Figure 1 showed that, although high concentrations of both polyplodising agents increased the chromosome doubling rates, they had negative effects on the survival rates. The survival rates were reduced from 100% in the control to 40% and 30% in the plantlets treated with higher concentration (24 μM) and longer exposure period (48 h) for trifluralin and APM respectively.
Assessments of the effects of oryzalin, trifluralin and APM on ploidy
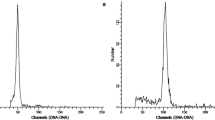
The 2C peaks of rose nuclei stained with DAPI had lower fluorescence intensities than those of parsley and could easily be distinguished, examples for each ploidy level is given in Fig. 2. The 4C (G2 stage of the cell cycle) peaks of rose and parsley were either small or undetectable. The ratio of the fluorescence intensities of the rose and parsley nuclei (P 1/P 2 values), were calculated for each histogram. Table 5 shows that the P1/P2 values for chromosome doubled plants was nearly twice compared to their progenitors.
Flow cytometry histograms showing (A) 2n = 2x = 14 in diploid R. persica (B) 2n = 4x = 28 in herbicide-induced tetraploid R. persica (C) 2n = 3x = 21 in triploid R. hybrida cv Iceberg (D) 2n = 6x = 42 in herbicide-induced hexaploid R. hybrida cv Iceberg. Each histogram shows the peak of the internal standard, parsley (Petroselinum crispum cv Champion Moss Curled), which always has a greater fluorescence intensity relative to the rose nuclei encountered in this study
The results obtained from root tip preparations confirmed the flow cytometry results. The chromosome number for triploid and hexaploid R. hybrida cv Iceberg were 21 and 42, respectively (Fig. 3).
Discussion
The highest rates of chromosome doubling, 62.5% and 66.7%, were achieved when the explants were treated with 24 μM of trifluralin and APM for 48 h respectively, which was associated with the lowest survival rates (40% and 30% for trifluralin and APM, respectively). This inverse relationship was expected and has been described by other researchers (Bartels and Hilton 1973; Hugdahl and Morejohn 1993; Kermani et al. 2003; Chen and Gao 2007). Lower survival rates may be due to a physiological disturbance caused by spindle inhibitors, resulting in a reduced rate of cell division (Swanson 1957). In addition, the nucleotypic theory of Bennett (1972) suggests that cell DNA content and developmental rates are inversely related. Bennett (1972) stated that DNA influences development in two ways, directly through its informational content, and indirectly by the physical-mechanical effects of its mass. The term ‘nucleotype’ was used to describe those conditions of the nucleus, which affect the phenotype independently of the informational content of the DNA. It was suggested that cell cycle time, meiotic duration, and minimum generation time were determined by the nucleotype. Our results showed that although oryzalin treatments of R. hybrida cv Akito, resulted in 80% survival rate, all of the survivors remained as tetraploids, which could be due to an inability of the mixoploids and octaploids to recover and resume growth after the polyploidisation events. Growth inhibition after colchicine treatment has already been shown by Yan (2001) who treated shoot tips of wax flower by immersion in 0.05% colchicine and found deformation of new growth. Stebbin (1984) also stated that the decrease rate of polyploid was caused by the reduced ratio of cell division.
Exposure of genotypes with different ploidy levels indicated that chromosome doubling is genotype dependent and chromosome doubled plants originated from progenitors with lower ploidy level have a greater ability to recover from treatments. Since Kermani et al. (2003) also obtained 66% tetraploids from nodal sections of diploid R. hybrida cv Thérèse Bugnet after exposure to 5 μM oryzalin for 24 h, 5–6 μM oryzalin for 24 h seems to be a successful treatment for chromosome doubling of diploid roses. The existence of genotype, environmental and genotype × environment interaction effects on chromosome doubling ability of sugarbeet (Beta vulgaris) haploids were investigated by Ragot and Steen (1992). Although they showed that there was no significant interaction between genotype and environment, both genetic and environmental factors were found to affect the chromosome doubling ability of sugarbeet haploids.
Treatments of R. hybrida cv Iceberg with different polyplodising agents showed that there were no significant differences in the capability of these agents in inducing chromosome doubling. This is in accordance with the findings described by Hansen and Andersen (1996), who studied colchicine, as well as trifluralin, oryzalin, and APM for their potential for in vitro chromosome doubling of Brassica napus microspore culture and reported that the polyploidising effect of mM concentrations of colchicine was comparable to μM concentrations of other polyploidising agents. They also showed that there was no significant difference in chromosome doubling efficiency of the three herbicides, although APM was less toxic than trifluralin and oryzalin.
In the present investigation flow cytometry greatly facilitated the screening for ploidy levels after treatment with spindle inhibitors, and enabled the effect of the treatments with trifluralin, APM and oryzalin to be rigorously evaluated. Also, using flow cytometry, plants could be screened for altered ploidy levels at the in vitro stage, when it would have been difficult, laborious and time consuming to obtain roots with a high mitotic index (Kermani 2001). Conversion of some of the mixoploids to the original and chromosome doubled plants indicated that testing the plantlets, 24 weeks after the treatment, allowed time for chimeras to segregate, and some of them were converted to chromosome doubled plants.
In conclusion, the present investigation resulted in new tetraploid and hexaploid plants from R. persica and R. hybrida cv Iceberg. R. persica is one of the wild native species of Iran, with a distinctive red eye, and R. hybrida cv Iceberg is a fragrant rose well adapted to the Iranian climates, growing most of the year with few disease problems, which makes them and their chromosome doubled plants to be good candidates to add to the breeding pool of roses in Iran.
References
Agayev YM (1998) Advanced squash methods for investigation of plant chromosomes. In: Fourth Iranian Congress of Crop Production and Breeding Sciences, Aug 25–27 1996, Esfehan University of Tech, Esfehan, 1–20
Bartels PG, Hilton JL (1973) Comparison of trifluralin, oryzalin, pronamide, propham and colchicine treatments on microtubules. Pest Biochem Physiol 3:462–472
Basye RE (1990) An amphidiploid of Rosa banksiae and Rosa laevigata induced by colchicines. Amer Rose Ann 75:83–87
Bennett MD (1972) Nuclear DNA content and minimum generation time in herbaceous plants. Proc R Soc London Ser B 181:109–135
Cairns T (ed) (2000) Modern roses XI. Academic Press, London
Chen LL, Gao SL (2007) In vitro tetraploid induction and generation of tetraploids from mixoploids in Astragalus membranaceus. Scientia Horticulturae 112:339–344
Eeckhaut TGR, Werbrouck SPO, Leus LWH, Van Bockstaele EJ, Deberg PC (2004) Chemically induced polyploidisation in Spathiphyllum wallisii Regel through somatic embryogenesis. Plant Cell Tiss Org Cult 78:241–246
Hansen NJP, Anderson SB (1996) In vitro chromosome doubling potential of colchicine, oryzalin, trifluralin, and APM in Brassica napus microspore culture. Euphytica 88:159–164
Hugdahl JD, Morejohn LC (1993) Rapid and reversible high-affinity binding of the dinitroaniline herbicide oryzalin to tubulin from Zea mays L. Plant Physiol 102:725–740
Kermani MJ, Sarasan V, Roberts AV, Yokoya A, Wentworth J, Sieber VK (2003) Oryzalin-induced chromosome doubling in Rosa and its effects on plant morphology and pollen viability. Theor Appl Genet 107:1195–1200
Kermani MJ (2001) Chromosome doubling and the breeding of disease resistant roses. PhD Thesis University of East London, London, UK
Krüssmann G (1981) The complete book of roses. Timber press, Oregon, USA
Ma Y, Byrne DH, Chen J (1997) Amphidiploid induction from diploid rose interspecific hybrids. Hort Sci 32:292–295
Morejohn L, Bureau T, Mole-Bajer J, Bajer A, Fosket D (1987) Oryzalin, a dinitroaniline herbicide, binds to plant tubulin and inhibits microtubule polymerization in vitro. Planta 172:252–264
Morejohn L, Fosket D (1984) Inhibition of plant microtubule polymerization in vitro by the phosphoric amide herbicide amiprophos-methyl. Science (Wash DC) 224:874–876
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Ragot M, Steen P (1992) Genetic and environmental effects on chromosome doubling of sugarbeet (Beta vulgaris L.) haploids. Euphytica 63:233–237
Ramulu KS, Verhoeven HA, Dijkhuis P (1991) Mitotic blocking, micronucleation, and chromosome doubling by oryzalin, amiprophos-methyl, and colchicine in potato. Protoplasma 160:65–71
Roberts AV, LIoyd D, Short KC (1990) In vitro procedures for the induction of tetraploidy in a diploid rose. Euphytica 49:33–38
Roberts AV, Allum JF (2005) Chromosome doubling in roses: how and why? In: Fourth international symposium on rose research and cultivat. California, USA
Rowley GD (1960) Triploid garden roses. Am Rose Ann 45:108–113
Shahare ML, Shastry SVS (1963) Meiosis in garden roses. Chromosoma 13:702–724
Soltis PS, Soltis DE (2000) The role of genetic and genomic attributes in the success of polyploids. PNAS 97:7051–7057
Semeniuk P, Arisumi T (1968) Colchicine-induced tetraploid and cytochimeral roses. Botan Gaz 129:190–193
Stanys V, Weckman A, Staniene G, Duchovskis P (2005) In vitro induction of polyploidy in japanese quince (Chaenomeles japonica). Plant Cell Tiss Org Cult 84:263–268
Stebbins GL (2001) Polyploidy and distributed of actic-alpine flora: new evidence and new approaches. Botanica Helvestica 94:1–13
Swanson CP (1957) Cytology and ctogenetics. Prentice Hall, New Jersey
Tilney-Bassett RAE (1986) Plant cimeras. Edward Arnold, London, UK
van der Salm TPM, van der Toorn CJG, Hanisch ten Cate CH, Dubois LAM, De Vries DP, Dons HJM (1994) Importance of the iron chelate formula for micropropagation of Rosa hybrida L dMoney wayT. Plant Cell Tissue Organ Cult 37:73–7
Wan Y, Duncan DR, Rayburn AL, Petolino JF, Widholm JM (1991) The use of antimicrotubule herbicides for the production of doubled haploid plants from anther-derived maize callus. Theor Appl Genet 81:205–211
Yan G (2001) Chromosome doubling of wax flower. Plant regenerated in vitro. Proc biol of wax flower:11–20
Yokoya K, Roberts AV, Mottley J, Lewis R, Brandham PE (2000) Nuclear DNA amounts in roses. Ann Bot 85:557–561
Zlesak DC, Thill CA, Anderson NO (2005) Trifluralin-mediated polyploidization of Rosa chinensis minima (Sims) Voss seedling. Euphytica 141:281–290
Acknowledgment
This work was funded by Agricultural Biotechnology Research Institute of Iran (ABRII) (Project number: 2-013-140000-01-0000-84004). The authors wish to thank professor Agayev for his assistance in cytogenetic studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khosravi, P., Kermani, M.J., Nematzadeh, G.A. et al. Role of mitotic inhibitors and genotype on chromosome doubling of Rosa . Euphytica 160, 267–275 (2008). https://doi.org/10.1007/s10681-007-9571-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-007-9571-7