Abstract
An efficient in vitro plant regeneration system was established through somatic embryogenesis for Anoectochilus elatus Lindley, an endangered jewel orchid. Direct somatic embryogenesis was achieved from nodal explants (17.4 embryos per explant with 63.4% response) on Mitra medium supplemented with Morel vitamins, thidiazuron (4.54 µM) and ∞-naphthaleneacetic acid (2.69 µM). Simultaneously, a protocol was developed for indirect somatic embryogenesis from internodal explant, produced embryogenic calli and embryos (31.3 embryos with 76.4% response) on same medium amended with 50 mg/L peptone and 5% coconut water. Both types of embryogenic pathways, produced morphologically similar globular embryos in the form of protocorm like bodies and successfully germinated on hormone free Mitra medium supplemented with Morel vitamins. Morpho-histological investigation of the embryo revealed the initiation and developmental features of somatic embryos. In vitro regenerated plantlets were successfully established from heterotrophic to a photoautotrophic stage by reducing the nutrient content in culture media, adjusting temperature and humidity through three step method. During the process, no morphological and physiological abnormalities were observed. Hardened plantlets were successfully acclimatized at poly tunnel chamber with 95% of survival rate. Further, inter simple sequence repeats (ISSRs) molecular markers were used to analyse the genetic homogeneity of regenerated plants. Analysis with this method showed that the homogeneity is comparatively higher in direct somatic embryo regenerated plants (94.22%) as compared to plants elevated from an indirect somatic embryo (93.05%). The present study provides morpho-histological and genetically stable plants for germplasm conservation and further utility of this endangered jewel orchid.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Orchidaceae is highly evolved and world’s richest family considering diversity, belonging to the group of monocotyledons consisting of > 28,000 species in 736 genera (Christenhusz and Byng 2016). Orchids are cosmopolitan in distribution and show diverse habit, commercially valuable long shelf-life colourful flowers and are used in traditional system of medicine (Hossain et al. 2013).
Orchids include both terrestrial and epiphytes, grown for their attractive flowers, although some small group of terrestrial genera contains beautifully veined leaves often velvety with different colorization is grown for their foliage, such orchids are called ‘Jewel Orchids’ (Bhattacharjee and Chowdhery 2012). The genus Anoectochilus belongs to the group of ‘Jewel Orchids’ because of their dazzling foliage ornamentation. It has 40–45 species distributed particularly in Asiatic countries (Ket et al. 2004) and in India, seven species are available throughout Himalaya, Eastern and Western Ghats (Misra 2007). Anoectochilus elatus is an endangered terrestrial orchid commonly known as ‘South Indian Jewel Orchid’ with vernacular names of ‘Mayilraegai & Kairaegai Saedi’ in Tamil and Nagathali in Malayalam. It exhibits monopodial growth pattern with rhizomatous stem grown on humus rich soil slopes, narrowly distributed in the Eastern and Southern Western Ghats of Tamil Nadu, India at an elevation of 1300–2100 m (Sherif et al. 2016). This orchid has great aesthetic value due to its dark velvety foliage with silver and gold colour venation. Leaf of this plant contains rich flavonoidal compound (Maridass et al. 2008). In tribal medicine, it is used to treat snake bites, chest and abdominal pains (Sarkar 2012). The natural regeneration process is hampered in this species due to low fruit set, seed germination, slow vegetative growth and lack of suitable symbionts to sustain itself in adverse condition. Further, habitat destruction via rapid deforestation, landslides, and encroachment of forest land for anthropogenic activity, reduction of humidity and pollinator decrease makes decline in the number of locations and subpopulation of this species in nature. Due to these reasons wild population of this species is inaccessible and categorized as endangered orchid species in India and SriLanka (Jalal and Jayanthi 2012; Sherif et al. 2012, 2016).
Globally, in vitro techniques are efficiently used to protect horticultural and medicinally important rare and endangered orchid species. Direct and indirect somatic embryogenesis protocol offers several advantages in plant biotechnology to produce genetically uniform phenotypes (Bhattacharyya et al. 2014); to provide virus free tissue for genome repair (Pikulthong et al. 2016); to regenerate complete plants from single cells called plasmogamy and to develop synthetic seeds for long-term storage of germplasm (Baskaran et al. 2015). The plantlets derived from in vitro are unable to withstand ex vitro conditions, due to the effect of abiotic and biotic interferences. Besides, morphological abnormalities and physiological failures raised in in vitro regenerated plantlets cause serious losses in eco-restoration and commercial oriented applications (Kaur 2015). Hence, the study of morpho-histological features of in vitro raised plants, during hardening and acclimatization process ensure their ‘true-to-type’ of survival and success.
During the in vitro development, plant tissue undergoes several morpho-physiological changes, genetic alterations with chromosome abnormality, doubling of genetic elements like aneuploidy and polyploidy which is caused by exogenous plant growth regulators (PGRs) and other intrinsic factors originated gene mutation (Takagi et al. 2011). Genetic instability or somaclonal variation of a genotype affects long-term preservation of germplasm in vitro. Molecular markers such as random amplified polymorphic DNA (RAPD), amplified fragment length polymorphism (AFLP), inter-simple sequence repeat (ISSRs) and start codon targeted polymorphism (SCoT) were extensively used to evaluate genetic homogeneity of in vitro besides ex vivo plants (Milella et al. 2011; Bhattacharyya and Kumaria 2014; Khilwani et al. 2016; Seth et al. 2016). Among these molecular markers, ISSRs marker is frequently used to analyse the genetic homogeneity or variation studies in plant biology, because of its high reproducibility and low-cost efficiency (Dangi et al. 2014). Genetically stable plants are important for plant conservation studies, secondary metabolite production at in vitro and also for the preservation of genetic resources of rare and endangered plants. Thus, the analysis of genetic characters or stability of in vitro derived plants through molecular markers are invited.
Concerned to answer the difficulties met in propagating of A. elatus, and for further applications, the present study was focused on effective protocol for somatic embryogenesis via two different systems; to increase the survival rate of in vitro derived plantlets and to analyse the genetic homogeneity among the regenerated plants using ISSRs marker.
Materials and methods
Source of explants
The aerial parts of juvenile stem containing node and internodes (1.0 cm) were excised from 6-month-old in vitro raised plants of A. elatus, used as explant source (Sherif et al. 2012).
Culture medium and condition
For direct (node) and indirect (internode) somatic embryogenesis technique, respective explants were cultured on Mitra medium (Mitra et al. 1976) supplemented with 2% sucrose and different types of vitamin sources like MS vitamins (Murashige and Skoog 1962), Ma vitamins (Ma 1991) and Morel vitamins (Morel and Wetmore 1951). Various types of PGRs like N6-benzyleadenine (BA; 2.22–13.32 µM), thidiazuron (TDZ; 2.25–13.62 µM), N6-(2-isopentenyl) adenine (2iP; 2.46–14.76 µM), ∞-naphthaleneacetic acid (NAA; 2.69–16.11 µM), 2,4-diclorophenoxyacetic acid (2,4-D; 2.26–13.56 µM) were added to Mitra medium to induce direct embryos. As well organic elicitors such as peptone (PEP; 50–150 mg/L) and coconut water (CW; 5–20%) alone and combinations used for embryogenic callus induction, proliferation and embryo enhancement. The Mitra medium without vitamins, PGRs and organic elicitors served as control. All the tissue culture grade chemicals were purchased from Hi-media® Pvt. Ltd. hosted at Mumbai, India.
The pH of the medium was set as 5.7 ± 0.2 with the help of NaOH or HCl (0.1 N) before adding 0.7% tissue culture grade agar–agar to the medium. The medium was dispensed into culture tubes (1 × 6 inch), 250 ml flat bottom conical flask and Petri dishes (100 mm) (Borosil®, Chennai, India) after autoclaving at 121 °C for 15 min. All the cultures were established in laboratory condition providing 16-h photoperiod illuminated with cool white fluorescent tube lamps (Philips TL-D Super 80, Gurgaon, India; 40 µmol m−2 s−1). Culture room was maintained at a constant temperature (23 ± 2 °C) and humidity (80%).
Germination, hardening, and acclimatization
Mitra medium supplemented with Morel vitamins used for embryo germination and plantlet conversion. After 4 weeks of culture, the regenerated plantlets were removed from culture vessels and thoroughly washed in distilled water to eliminate surface residues. For preliminary hardening, the plantlets were established in paper cups containing sterile potting mixtures as previously described by Sherif et al. (2016) for 6 weeks. Further, the plants were established in a plant growth chamber (Sanyo, Japan) for secondary hardening. The plant growth chamber manually provided with 65–70% humidity, 26 ± 2 °C temperature and 16-h photoperiod illuminated with cool-white fluorescent light (Sanyo, 80 m−2 s−1) for 6 weeks. For the period of primary & secondary hardening experiment, plantlets were sprinkled with sterile distilled water once a week. Then the plants were shifted and acclimatized at poly tunnel chamber with 60% humidity and 28 ± 2 °C relative temperature (Saveer Biotech, New Delhi) for 3 months.
Morphology and histological analysis
Direct embryos and embryonic callus with various stages were selected for histology and microscopy analysis. The samples were fixed in FAA solution (Formalin: acetic acid: 70% ethanol at 1:1:18 v/v) for 24-h. Then the samples were hand sectioned using commercial blades (Wilkinson sword™, Gillette Company, USA) and stained with 1% safranin, 0.025% toluidine blue O (TBO), 0.5 µg/mL 4′,6-Diamidino-2-phenylindole dihydrochloride (DAPI) and mounted on a clean glass slide. The mounted specimens were examined and meanwhile the fresh embryonic callus having globular embryos with shoot meristem appearance, leaves of in vitro and acclimatized plants histological features were observed using light microscopy (Olympus® BX 41, Tokyo, Japan) at ×20 and ×40 magnifications. For scanning electron micrograph, fresh samples were frozen in liquid nitrogen and scanned in a low vacuum liquid scanning electron microscope (SEM; Hitachi® S3400, Tokyo, Japan) with the chamber pressure of 30 Pa and an accelerated voltage of 15 kV.
Determination of genetic homogeneity using ISSRs primer
Total genomic DNA was isolated from fresh leaves (100 mg) obtained from in vitro regenerated well acclimatized plants (each seven plants from DSE & IDSE) and parental plant material was used as control. Cetyltrimethylammonium bromide method used for DNA extraction (CTAB: Doyle and Doyle 1990). The isolated DNA samples were purified using plant DNA purification kit (Medox®, India) and stored in the refrigerator for further analysis. Fifteen ISSRs primers (Xcelris Genomics & Labs Ltd., Ahmedabad, India) were purchased and used for genetic homogeneity assessment of in vitro regenerated plants. The PCR reaction mixture contains 50 ng of genomic DNA, Taq 2x master mix comprises Tris HCl pH 8.5, (NH4)2SO4, 4 mM MgCl2, 0.2% Tween® 20, 0.4 mM of each dNTP, 0.2 units/µL of Taq DNA polymerase with inert red dye (Ampliqon, Denmark) and 5 pmol of specific primers. PCR amplification was carried out in 96 well thermal cycler (Eppendorf Pros, Hamburg, Germany) programmed with early denaturation at 96 °C (2 min), 40 cycles of denaturation at 94 °C (0.5 min), annealing at 52 °C (0.5 min), and then elongation terminated at 72 °C (7 min). Finally, PCR products were subjected to electrophoresis (Medox-Bio™, Mx-1251-0, India) contained 1.5% agarose gel prepared by using 1× TAE buffer with 10 mg/mL ethidium bromide (fluorescent dye). Accordingly, voltage (70 V) is set and the gel is allowed to run for 2 h. Standard 100 bp DNA ladder (Takara, USA) was used to determine the nucleotide fragment size of the amplicons. Further, the agarose gel fingerprinting results are documented in the computer using the gel documentation system (Alpha Imager® EP Gel Documentation System).
Data analysis
The experiment design is given as schematic representation in Fig. 1. The entire experiment was conducted as randomized design, pertaining control and treatment included with 20 replicates. Data regarding direct embryo and embryonic callus induction percentage, nature of embryonic callus, number of embryos per culture and plants recovered from each embryo was scored after treatment initiation and was continued at an interval of 10 days once until the completion of experiment. The obtained data were tabularized and analyzed by using analysis of variance (One-Way ANOVA). The significant differences between treatments were determined based on Duncan’s multiple range test at p < 0.05 using SPSS-ASW program ver. 18.0.0 (SPSS®, Chicago, IL, USA).
Based on the agarose gel documentation images, data regarding nucleotide fragment size (bp), the total number of bands amplified, average number of bands per individual, monomorphic and polymorphic bands, the percentage of monomorphism and polymorphism of individuals were manually calculated from each ISSRs primers separately and tabulated for genetic homogeneity analysis.
Results
Direct somatic embryogenesis (DSE)
Induction of somatic embryos and its enhancement
Direct somatic embryo (DSE) was achieved from nodal explants on Mitra medium supplemented with different types of vitamins and PGRs. After first subculture, direct embryo initiation starts from near the cut surface of the nodal explant. Mitra medium fortified with Morel vitamins induced 2.8 embryos/explant as light greenish yellow globular embryo like structure with 18.5% somatic embryo induction after 3 weeks of culture. Subsequently, the individual cytokinin and auxin tested, 4.54 µM TDZ produced 4.2 embryos/explant with 56.4% somatic embryo induction followed by 8.87 µM BA (2.7 embryos with 29.5%) and 2.69 µM NAA (3.0 embryos with 36.5%). Morphologically, the growth of globular somatic embryo was healthier on TDZ and NAA. Other PGRs yielded less number of somatic embryos with a low frequency of embryo induction (Table 1; Fig. 2a, b).
Morphological stages of direct somatic embryogenesis of A. elatus. a, b Direct globular embryo formation from nodal explant (Mitra medium + Morel vitamins + 4.54 µM TDZ), c, d enhanced formation of globular embryos (Mitra medium + Morel vitamins + 4.54 µM TDZ + 2.69 µM NAA), e, f germination of embryo (Mitra medium + Morel vitamins), g T.S. of node with embryo observed in light microscopy, SEM image reveals; h globular embryo initiation, i enhanced number of globular embryo, j globular embryo with shoot (SP) and root pole (RP). [All scale bars 1 mm; T.S transverse section]
Preliminary scrutiny of individual concentration of PGRs exhibited a very limited number of embryos. Hence, the DSE induction was further enhanced by transferring nodal explants on positively resulted cytokinin and auxin. The direct embryo multiplication starts after third subculture on Mitra medium containing Morel vitamins supplemented with TDZ, NAA and BA combination. After 6 weeks of culture, 17.4 embryos per explant recovered from 4.54 µM TDZ + 2.69 µM NAA with 63.4% response followed by 13.4 embryos with 38.5% on 4.54 µM TDZ + 8.87 µM BA and 7.8 embryos with 45.3% on 2.69 µM NAA + 8.87 µM BA (Table 1; Fig. 2c, d). During each sub-culture, the hyperplastic tissue was excised from the explants for effective absorption of nutrients. Increased level of PGRs and prolong culture maintenance interrupted direct embryo formation and multiplication.
Histology and SEM analysis
Histology and SEM analysis proved various developmental stages of DSE from nodal explant. The transverse section showed single globular cell initiation from the surface region (Fig. 2g) and SEM analysis revealed direct globular somatic embryo formation from epidermis of the nodal explant (Fig. 2h). The globular embryo with bipolar structure developed without any intermediate callus development. During bipolar development shoot pole produced shoot tip; however, root pole remained embedded without any root formation on protuberances (Fig. 2i, j).
Indirect somatic embryogenesis (IDSE)
Induction and proliferation of embryogenic callus
The internodal explants were cultured on Mitra medium containing Morel vitamins fortified with different concentrations of TDZ and NAA alone and combination for embryogenic callus induction and proliferation. From the cut regions of internodes callus initiation was developed with few embryos after 2 subcultures. Maximum 14.3% embryogenic callus induction (8.43 globular embryos) was observed on 4.54 µM TDZ followed by 12.67% embryogenic callus induction on 2.69 µM NAA (6.33 globular embryos). The embryogenic calli was proliferated on the combination of 4.54 µM TDZ and 2.69 µM NAA. The synergistic combinations produced a maximum of 32.3% embryogenic callus induction with 14.3 globular embryos (Table 2). The embryogenic callus derived from internodal explants showed remarkable differentiation in morphological features like green to yellowish semi-friable calli depends on the hormone type and combinations (Table 2; Fig. 3a, b). Decreased level of PGRs and short time maintenance of cultures in initiation and proliferation medium affected embryogenic callus.
Morphological and histological stages of indirect somatic embryogenesis of A. elatus. a Embryogenic callus induction from internodal explant (Mitra medium + Morel vitamins + 4.54 TDZ), b globular somatic embryo (Mitra medium + Morel vitamins + 4.54 µM TDZ + 2.69 µM NAA + 50 mg PEP + 5% CW), c embryo germination (Mitra medium + Morel Vitamins), d globular stage, e, f incipient shoot meristem, g shoots developed from globular embryos, light microscopy reveals; h globular embryo formation from embryogenic callus (GE), i globular embryo, j micro shoot primordium development (MSP), k T.S. of typical bipolar structure, l T.S. of callus showing anticlinal (AC) and periclinal (PC) cell divisions, m T.S. of embryogenic callus cells showing prominent nucleus, n T.S. of embryogenic callus cells showing nutritive storage materials. [All scale bars 1 mm; T.S transverse section]
Enhancement of somatic embryo induction
In the present study, during the induction and proliferation of embryogenic calli, very limited number of globular embryos obtained. In order to enhance somatic embryos, embryogenic callus was cultured on Mitra medium contains Morel vitamins fortified with cytokinins (BA & 2iP) and additives (PEP & CW) at different concentrations individually or in combination with 4.54 µM TDZ and 2.69 µM NAA. The embryo induction was occurred after 3 subcultures on BA (2.22 µM), 2iP (4.92 µM), PEP (50 mg/L) and CW (5%) produced minimum 1.3–3.2 embryos per culture (Table 3). When they are combined with TDZ and NAA, significantly increased embryos at high-frequency rate. After 8 weeks culture, maximum number of somatic embryos (31.3 with 76.4% somatic embryo induction) was recorded on 4.54 µM TDZ, 2.69 µM NAA, 50 mg/L PEP, and 5% CW combination of culture (Table 4).
Light microscopy and histological analysis
Light microscopy and histological studies of embryogenic calli showed different morpho-histological features. Direct observation of embryogenic calli in light microscope exhibited green protuberances originating from the sub-epidermal regions. Later it was formed as closely packed cluster of cells like a globular embryo and developed into complete plantlets (Fig. 2h–j). The histological observation of embryos showed typical bipolar structure (Fig. 3k). The embryogenic cells were compactly arranged with anticlinal and periclinal cells covering large vacuolated cells with the prominent nucleus and scattered nutritive storage components (Fig. 3l–n).
Germination, hardening, and acclimatization
Globular embryos derived from DSE and IDSE were successfully germinated and converted into plantlets on hormone free Mitra medium contains Morel vitamins (Figs. 2e, f, 3c–f, 4a). After 4 weeks of culture, an average of 15.8 plants/treatment was recovered in DSE and 23 plantlets/treatment in IDSE (Tables 1, 4). The plantlets were pre-hardened in paper cups covered with transparent polybags and maintained in culture room condition at 23 ± 2 °C and 80–85% humidity for 6 weeks (Fig. 4b). After initial maintenance, the polythene bags were removed and plantlets were maintained in plant growth chamber at 26 ± 2 °C with 65–70% relative humidity for 6 weeks to tolerate high temperature and low humidity (Fig. 4c). Histological investigation of in vitro raised plants leaf showed immature waxy cuticle, dysfunctional stomata (i.e. either fully opened or closed stomata) and less photo synthetic pigments (Fig. 4d–f). Finally, well-hardened plants were successfully established and acclimatized at poly tunnel chamber with 95% survival rate (Fig. 4g). Well acclimatized plants exhibit structurally organized leaf with well-developed waxy cuticle, fully functional stomata, and more photosynthetic pigments as compared to in vitro plants (Fig. 4h, i). The anatomical character of root did not show any abnormal differentiation between hardening and acclimatized plants (Fig. 4j).
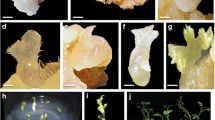
Hardening, acclimatization and histological analysis. a Well developed plantlets, b primary hardening of plantlets covered with transparent polyethylene bags, c secondary hardening of plantlets maintained in controlled plant growth chamber, d T.S. of epidermal region in in vitro grown plant showing pubescent epidermis and palisade parenchyma, e, f peel of in vitro leaf lower epidermis showing closed and open stomata, g acclimatized plants at poly tunnel chamber, h T.S. of epidermal region in acclimatized plant showing well developed and cuticlised pubescent epidermis and palisade parenchyma, i peel of well acclimatized plant leaf lower epidermis showing closed and open stomata, j T.S. of acclimatized plant root with abundant root hairs [Scale bars 50 µm; T.S transverse section]
Determination of genetic homogeneity using ISSRs
In the present protocol, 3 months old well acclimatized plant genotypes were compared with parental genotype for genetic homogeneity assessment by using 15 ISSRs primers. All the 15 genotypes DNA was successfully amplified with primers and produced distinct band patterns. The size of the amplified products ranged from 150 to 1500 bp. A total of 533 bands were amplified in DSE and 557 in IDSE raised plants, off which 503 (DSE) & 520 (IDSE) were monomorphic and 30 (DSE) & 37 (IDSE) were polymorphic. The highest number of bands obtained from UBC 842 (58 in DSE; 64 in IDSE) primer. An average number of bands per individual is 5.76 in DSE and 6.18 in IDSE derived plants. The number of polymorphic bands varied from 1 to 4. The percentage of monomorphism is 94.22 (DSE) & 93.05% (IDSE) and polymorphism is 5.77 (DSE) & 6.90% (IDSE) (Table 5; Fig. 5).
Discussion
In vitro somatic embryogenesis gives an opportunity to study the developmental phases of an embryo of a specific plant species. During the formation of somatic embryos, developmental phases differ in dicot and monocot taxa (George 1996; Ji et al. 2011). Orchids being monocotyledons are characterized by the absence of root meristem in the embryo (Misra 2007). Zygotic or somatic embryos of this family produce globular to bipolar structure called protocorm like bodies (PLBs) with an absence of embryonic tubercules (Mukerji et al. 2000). PLBs are important characteristic feature in orchid propagation and their morphogenesis resembles like somatic embryos of other taxa (Lee et al. 2013). Orchids are highly recalcitrant species and only a few genera were successfully respond for direct and indirect somatic embryogenesis system using different explants (Chen and Chang 2001; Feng and Chen 2014).
Direct and indirect somatic embryogenesis
Somatic cells of explant known to be deficient to vitamin synthesis and requires exogenous supply of specific vitamins to fulfil their metabolic needs (George 1996; Abrahamian and Kantharajah 2011). Hence, standardization of vitamin requirement is important for orchid PLBs formation and development (Parthibhan et al. 2015). In the present study, A. elatus node and internodal explants were cultured on Mitra medium supplemented with the different type of vitamins for DSE and IDSE. Morel vitamins containing ingredients differ from other media vitamin compositions unlike other vitamin source lack the potency for embryo formation. Morel vitamins have been successfully used in the production of somatic embryos of monocotyledons in vitro (Strosse et al. 2006). Vitamins like biotin and calcium pantothenate in diluted form gives an advantage to Morel vitamins to produce somatic embryos. Since, biotin and calcium pantothenate are essential cofactor vitamins used as the precursor in the biosynthesis of coenzymes and protein synthesis (George 1996). In Ricciocarpus species biotin with calcium pantothenate significantly enhancing the mass weight of shoots and biotin containing medium enhanced embryogenic callus, embryos in Phoenix dactylifera (Al-Khayri 2001). It was found presently that Morel vitamins induced direct embryos in nodal explants whereas additional PGRs requirement was needed for indirect embryogenic callus initiation and enhancement in internodal explants.
Generally, in vitro somatic embryogenesis was enhanced by the addition of PGRs such as cytokinins and auxins alone or in combinations (Ji et al. 2011). Zygotic or immature embryo of the terrestrial orchid species requires cytokinins for their germination and proliferation (Masanori 2002). Hence, the nodal explant cultured on Mitra medium contains Morel vitamins and supplemented with different types of cytokinins and auxins. TDZ plays effective role in combination with NAA for the production of direct embryos and embryogenic callus in A. elatus. Other PGRs like 2iP and 2,4-D failed to produce direct embryos and embryogenic callus. The similar results were observed in many orchid species like Dendrobium sps., Phalaenopsis aphrodite, and Malaxis densiflora (Feng and Chen 2014; Juntada et al. 2015; Mahendran and Bai 2015). Types and concentration of exogenous hormones play a vital role in indirect somatic embryogenesis (Shajahan et al. 2016). Several studies in orchid species suggest TDZ combined with additives like PEP and CW gave desirable effects and promoted embryogenic callus mediated embryo formation (Rachmawati et al. 2015).
In orchids and other monocotyledons like graminaceous species, somatic embryo development undergoes globular to mature embryos (Gray 1996). Light and scanning electron microscopy are the essential tool to analyse morphology and development phases of somatic embryos (Moyo et al. 2015). In the present study, the developmental phases of direct and indirect somatic embryos of A. elatus analysed of through light and SEM microscopy. Several morpho-histological changes were observed like bipolar structure, cell separation with prominent nucleus and intracellular accumulation of storage substances. These are the identical features of true somatic embryos and their developmental phase mostly depends on responsiveness of explant, media, PGRs, illumination, and other intrinsic conditions. The same results have been observed in Phalaenopsis sps. (Ishii et al. 1998), Oncidium sps. (Chen and Chang 2000), Coelogyne cristata (Naing et al. 2011), Vanilla planifolia (Kodja et al. 2015) and Curcuma longa (Soundar Raju et al. 2015).
Hardening and ex vitro acclimatization
Orchid cultivation is one of the most economically significant practices (Teixeira Da Silva 2013). They are largely produced via tissue culture for raising numerous plantlets for commercial application and conservation aspects. Eco-sensitivity and difficulty in adaptation of regenerated plants at ex vitro conditions leads to great loss of plantlets and reduces the profit. Hence, plant species grown in vitro require a step-by-step adaptation process in order to ensure their survival when transferred to ex vitro (Alatar 2015).
At present, hardening and acclimatization process was carried out with histological evidence to establish plantlets at ex vitro. In vitro condition, carbon source, low range of light intensity, high humidity, and constant temperature causes hyper hydricity in addition to histological modification in stomata and roots (Mokhtarzadeh et al. 2013; Isah 2015). In this condition transfer of plantlets to ex vitro condition surely affect their survival in nature by desiccation of water content, tissues are highly susceptible to disease and pathogen attack because of young leaves with immature cuticle layer scattered with dysfunctional stomata. Several reports suggest that propagating medium with a high carbon source, internal abscisic acid level and high temperature, carbon dioxide concentration in the environment affect stomatal movements (Brearley et al. 2014; Kaur 2015). In nature, carbon dioxide act as antitranspirants which affects stomatal movements as well as retarded photosynthesis. Apart from this, immature root development affects absorption of water and roots are easily susceptible to pathogenic soil mycroflora which affects root cells and growth of plants (McAdam and Brodribb 2014). Previous documentation suggests that the age of plantlets, leaf maturity, and accumulation of photosynthetic pigments are associating in vitro to ex vitro plant establishment (Brearley et al. 2014; Shekhawat and Manokari 2016).
In order to overcome this problem in this species, plantlets initially cultured on solid basal medium without sucrose and hormones to enhance the photoautotrophic and physiological function of root and stomata. Primary and secondary hardening helps to protect plants from disease-causing fungus, mites and strengthen leaf lamina, regulate the physiological function of stomata and initiate photosynthesis. After 3 months of a time period, 95% of plants successfully acclimatized in poly tunnel chamber with developed anatomical structures, photosynthetic pigments, and roots. This survival rate is higher when compared to previous acclimatization success of jewel orchids like Haemaria discolor, Anoectochilus regalis and A. roxiburghii (Gangaprasad et al. 2000; Shiau et al. 2005; Zhang et al. 2015). This cost effective findings may be useful to commercially important orchid species cultivation to get good quality of plants and increase their vase life.
Determination of genetic homogeneity using ISSRs
The somatic embryogenesis technique is time expensive for many orchid species because of long developmental phases, may lead to somaclonal variation among the regenerates (Dey et al. 2015). Conversely, it is quick in certain methods like meristem culture and callus-mediated through regeneration (Saha et al. 2016). This controversy may be due to the quick response of explant, favourable nutrient availability in the substratum or cells tolerates methylation of DNA (Shajahan et al. 2016).
In the present study, the in vitro raised plants of A. elatus morphologically resembled to that of the parental plants. ISSRs revealed the plants developed from DSE and IDSE possessing 94.22 (DSE) & 93.05% (IDSE) homogeneity and 5.77 (DSE) 6.90% (IDSE) variability. However, 100% of genetic homogeneity was obtained from somatic embryogenesis derived plants (both direct and indirect means) from Citrullus lanatus (Vinoth and Ravindran 2016), Abutilon indicum (Seth et al. 2016), and Bacopa monnieri (Khilwani et al. 2016). Their reports suggest that the somaclonal variations caused by factors like illumination, culture substratum, PGRs, repeated division of cells, long term maintenance of in vitro culture and other laboratory conditions do not affect their mass production of plantlets due to short span of dedifferentiation followed by quick regeneration. The outcome of the present result endorses the in vitro regenerated plants produced via somatic embryogenesis are contain less soma-clonal variation because of any one of the above-mentioned mandatory factors specifically TDZ. Since, several studies suggest that TDZ can induce morphological abnormalities because of it multi-dimensional function and chemical nature which may lead genetic modification (Huetteman and Preece 1993; Guo et al. 2011; Sherif et al. 2012). Although, several reports suggest that above 90% of similarity between parental and in vitro raised plant is inevitable and consider as safe zone of in vitro regenerates from somaclonal variation (Zhang et al. 2010; Bhattacharyya et al. 2014; Viehmannova et al. 2014). Hence, in vitro plants regenerated from these techniques will be used for the industrial oriented commercial supply of stock materials as well as germplasm conservation.
Conclusion
In conclusion, this is the first report on direct and indirect somatic embryogenesis via plant regeneration in the genus Anoectochilus and the species A. elatus which is an important ‘Jewel Orchid’. Mitra medium augmented with Morel vitamins, PGRs like TDZ (4.54 µM), NAA (2.69 µM) and organic elicitors such as PEP (50 mg/L), CW (5%) alone as well as combination are crucial for improving embryogenesis and further plant regeneration at in vitro. Direct somatic embryogenesis from nodal culture produced the inadequate number of somatic embryos and flourished limited plantlets as compared to embryos derived from internodes (IDSE). Among the two different embryogenesis protocol, IDSE from callus phase is suitable for this proposed plant species. The histology study through light microscopy and SEM structural justifications revealed the origin of the somatic embryos from aseptic cultures and further confirms the bipolar structure of the PLBs, which are valid somatic embryos. Improved effective practices have been established to reduce plantlets mortality during acclimatization ex vitro. In addition, ISSRs primers authenticated the in vitro plants derived from both DSE and IDSE showed the low risk of genetic instability that of the mother plant. In future, this technique will be employed to this species, which targeting synseed production, cryopreservation of germplasm, somatic cell fusion to form viable hybrids, gene conversion and finally to increase the populations in nature.
Abbreviations
- 2,4-D:
-
2,4-diclorophenoxyacetic acid
- 2iP:
-
N6-(2-isopentenyl) adenine
- BA:
-
N6-benzyleadenine
- CW:
-
Coconut water
- DSE:
-
Direct somatic embryogenesis
- IDSE:
-
Indirect somatic embryogenesis
- ISSRs:
-
Inter simple sequence repeats
- NAA:
-
∞-naphthaleneacetic acid
- PEP:
-
Peptone
- PGRs:
-
Plant growth regulators
- PLBs:
-
Protocorm like bodies
- SEM:
-
Scanning electron microscopy
- TDZ:
-
Thidiazuron
References
Abrahamian P, Kantharajah A (2011) Effect of vitamins on in vitro organogenesis of plant. Am J Plant Sci 2:669–674
Alatar AA (2015) Thidiazuron induced efficient in vitro multiplication and ex vitro conservation of Rauvolfia serpentine—a potent antihypertensive drug producing plant. Biotechnol Biotechnol Equip 29:489–497
Al-Khayri JM (2001) Optimization of biotin and thiamine requirements for somatic embryogenesis of date palm (Phoenix dactylifera L.). In Vitro Cell Dev Biol Plant 37:453–456
Baskaran P, Kumari A, Van Staden J (2015) Embryogenesis and synthetic seed production in Mondia whitei. Plant Cell Tissue Organ C 121:205–214
Bhattacharjee A, Chowdhery HJ (2012) Jewel Orchids of India- an overview. International Seminar on “Multidisciplinary approaches in Angiosperm Systematics”. pp 554–556
Bhattacharyya P, Kumaria S (2014) Molecular characterization of Dendrobium nobile Lindl., an endangered medicinal orchid, based on randomly amplified polymorphic DNA. Plant Syst Evol 301:201–210
Bhattacharyya P, Kumaria S, Diengdoh R, Tandon P (2014) Genetic stability and phytochemical analysis of the in vitro regenerate plants of Dendrobium nobile Lindl., an endangered medicinal orchid. Meta Gene 2:489–504
Brearley TA, Vaidya BN, Joshee N (2014) Cytokinin, carbon source, and acclimatization requirements for in vitro propagation of Scutellaria barbata D. Don and Scutellaria racemosa Pers. Am J Plant Sci 5:36–62
Chen JT, Chang WC (2000) Efficient plant regeneration through somatic embryogenesis from callus cultures of Oncidium (Orchidaceae). Plant Sci 160:87–93
Chen JT, Chang WC (2001) Effects of auxins and cytokinins on direct somatic embryogenesis on leaf explants of Oncidium ‘Gower Ramesy’. Plant Growth Regul 34:229–232
Christenhusz MJ, Byng JW (2016) The number of known plants species in the world and its annual increase. Phytotaxa 261:201–217
Dangi B, Khurana-Kaul V, Kothari SL, Kachhwaha S (2014) Micropropagation of Terminalia bellerica from nodal explants of mature tree and assessment of genetic fidelity using ISSR and RAPD markers. Physiol Mol Biol Plants 20:509–516
Dey T, Saha S, Ghosh PD (2015) Somaclonal variation among somatic embryo derived plants, evaluation of agronomically important somaclones and detection of genetic changes by RAPD in Cymbopogon winterianus. S Afr J Bot 96:112–121
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Feng JH, Chen JT (2014) A novel in vitro protocol for inducing direct somatic embryogenesis in Phalaenopsis aphrodite without taking explants. Scientific World J. https://doi.org/10.1155/2014/263642
Gangaprasad A, Latha PG, Seeni S (2000) Micropropagation of terrestrial orchids, Anoectochilus sikkimensis and Anoectochilus regalis. Indian J Exp Biol 38:149–154
George EF (1996) Plant tissue culture procedure—background. In: George EF, Hall MA, De Klerk G (eds) Plant propagation by tissue culture. (3rd edn) Springer, Basingstoke
Gray DJ (1996) Non-zygotic embryogenesis. In: Trigiano RN, Gray DJ (eds) Plant tissue culture concepts and laboratory exercise. CRC Press, New York, pp 133–147
Guo B, Abbasi BH, Zeb A, Xu LL, Wei YH (2011) Thidiazuron a multi-dimensional plant growth regulator. Afr J Biotechnol 10:8984–9000
Hossain MM, Kant R, Van PT, Winarto B, Zeng S, Teixeira da Silva JA (2013) The application of biotechnology to orchids. Crit Rev Plant Sci 32:69–139
Huetteman CA, Preece JE (1993) Thidiazuron a potent cytokinin for woody plant tissue culture. Plant Cell Tissue Organ C 33:105–119
Isah T (2015) Adjustments to in vitro culture conditions and associated anomalies in plants. Acta Biol Cracoviensia Ser Bot 57:9–28
Ishii Y, Takamura T, Goi M, Tanaka M (1998) Callus induction and somatic embryogenesis of Phalaenopsis. Plant Cell Rep 17:446–450
Jalal JS, Jayanthi J (2012) Endemic orchids of peninsular India: a review. J Threatened Taxa 4:3415–3425
Ji A, Geng X, Zhang Y, Yang H, Wu G (2011) Advances in somatic embryogenesis research of horticultural plants. Am J Plant Sci 2:727–732
Juntada K, Taboonmee S, Meetum P, Poomjae S, Chiangmai (2015) Somatic embryogenesis induction from protocorm-like bodies and leaf segments of Dendrobium Sonia ‘Earsakul’. Silpakorn U Sci Tech J 9:9–19
Kaur RP (2015) Photoautotrophic micropropagation an emerging new vista in micropropagation—a review. Agric Rev 36:198–207
Ket NV, Hahn EJ, Park SY, Chakrabarty D, Paek KY (2004) Micropropagation of an endangered orchid Anoectochilus formosanus. Biol Plant 48:339–344
Khilwani B, Kaur A, Ranjan R, Kumar A (2016) Direct somatic embryogenesis and encapsulation of somatic embryos for in vitro conservation of Bacopa monnieri (L.) Wettst. Plant Cell Tissue Organ C 127:433–442
Kodja H, Noirot M, Khoyratty SS, Limbada H, Verpoorte R, Palama LT (2015) Biochemical characterization of embryogenic calli of Vanilla planifolia in response to two years of thidiazuron treatment. Plant Physiol Biochem 96:337–344
Lee YI, Hsu ST, Yeung EC (2013) Orchid protocorm-like bodies are somatic embryos. Am J Bot 100:2121–2131
Ma SS (1991) Somatic embryogenesis and plant regeneration from cell suspension culture of banana. In: Proceedings of symposium on tissue culture of horticultural crops, Taipei, Taiwan, 8–9 March, pp 181–188
Mahendran G, Bai VN (2015) Direct somatic embryogenesis of Malaxis densiflora (A. Rich.) Kuntze. J Genet Eng Biotechnol 14:77–81
Maridass M, Hussain MI, Raju G (2008) Phytochemical survey of orchids in the Tirunelveli hills of South India. Ethnobot Leafl 12:705–712
Masanori T (2002) The cytokinin preference for immature embryo culture of some terrestrial orchids. Comb Proc Int Plant Propag Soc 52:331–334
McAdam SA, Brodribb TJ (2014) Separating active and passive influences on stomatal control of transpiration. Plant Physiol 164:1578–1586
Milella L, Martelli G, Salava J, Fernandez E, Ovesna J, Greco I (2011) Total phenolic content, RAPDs, AFLPs and morphological traits for the analysis of variability in Smallanthus sonchifolius. Genet Resour Crop Evol 58:545–551
Misra S (2007) Orchids of India—a glimpse. Bishen Singh Mahendra Pal Singh, Dehradun (ISBN: 978-81-211-0618-4)
Mitra GC, Prasad RN, Choudhury RA (1976) Inorganic salts and differentiation of protocorms in seed callus of orchid correlative changes in its free amino acid content. Indian J Exp Biol 14:350–351
Mokhtarzadeh S, Hajyzadeh M, Ahmad HA, Khawar KM (2013) The problems in acclimatisation of in vitro multiplied plants of Lavandula angustifolia Miller under field conditions. Acta Hortic (ISHS) 988:71–76
Morel G, Wetmore RH (1951) Tissue culture of monocotyledons. Am J Bot 38:138–140
Moyo M, Aremu AO, Van Staden J (2015) Insights into the multifaceted application of microscopic techniques in plant tissue culture systems. Planta 242:773–790
Mukerji KG, Chamola BP, Sharma AK (2000) Glimpse in Botany. APH Publishing Corporation, New Delhi. (ISBN: 81-7648-204-8)
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Naing AH, Chung JD, Lim KB (2011) Plant regeneration through indirect somatic embryogenesis in Coelogyne cristata Orchid. Am J Plant Sci 2:262–267
Parthibhan S, Rao MV, Kumar TS (2015) In vitro regeneration from protocorms in Dendrobium aqueum Lindley—an imperiled orchid. J Genet Eng Biotechnol 13:227–233
Pikulthong V, Teerakathiti T, Thamchaipenet A, Peyachoknagul S (2016) Development of somatic embryos for genetic transformation in Curcuma longa L. and Curcuma manga Valeton & Zijp. Agric Nat Resour 50:276–285
Rachmawati F, Winarto B, Mattjik AN, Wiendi AMN, Furwito A (2015) Shoot tips derived-somatic embryogenesis in mass propagation of Dendrobium Indonesia Raya ‘Ina’. Emir J Food Agric 27:1–10
Saha S, Adhikari S, Dey T, Ghosh P (2016) RAPD and ISSR based evaluation of genetic stability of microprpagated plantlets of Morus alba L. variety S-1. Meta Gene 7:7–15
Sarkar MK (2012) Management strategies for endemic and threatened medicinal plants in India- a geoinformatic approach with special reference to Kalakad Mundanthurai Tiger Reserve, Southern Western Ghats of Tamil Nadu, India, vol 1, Department of Environment, Government of Tamil Nadu, Chennai
Seth S, Rath CS, Rout SC, Rout GR, Panigrahi J (2016) Somatic embryogenesis in Abutilon indicum (L.) Sweet and assessment of genetic homogeneity using SCoT markers. Plant Biosyst 0:1–11
Shajahan A, Raju CS, Thilip C, Varutharaju K, Faizal K, Mehaboob VM, Aslam A (2016) Direct and indirect somatic embryogenesis in mango ginger (Curcuma amada Roxb.). In: Loyola-Vargas VM, Ochoa-Alejo N (eds) Somatic embryogenesis: fundamental aspects and applications, Springer, Cham, pp 367–379
Shekhawat MS, Manokari M (2016) In vitro regeneration frequency, micro-morphological studies and ex vitro rooting of Hemidesmus indicus (L.) R. Br.: a multi-potent endangered climber. Indian J Plant Physiol 21:151–160
Sherif NA, Franklin Benjamin JH, Muthukrishnan S, Senthil Kumar T, Rao MV (2012) Regeneration of plantlets from nodal and shoot tip explants of Anoectochilus elatus Lindley, an endangered terrestrial orchid. Afr J Biotechnol 11:7549–7553
Sherif NA, Senthil Kumar T, Rao MV (2016) In vitro regeneration by callus of Anoectochilus elatus Lindley, an endangered terrestrial jewel orchid. In Vitro Cell Dev Biol Plant 52:72–80
Shiau YJ, Nalawade SM, Hsai CN, Tsay HS (2005) Propagation of Haemaria discolor via in vitro seed germination. Biol Plant 49:341–346
Soundar Raju C, Aslam A, Shajahan A (2015) High frequency direct somatic embryogenesis and plant regeneration from leaf base explants of turmeric (Curcuma longa L.). Plant Cell Tissue Organ C 122:79–87
Strosse H, Schoofs H, Panis B, Andre E, Reyniers K, Swennen R (2006) Development of embryonic cell suspension from shoot meristematic tissue in Banana and plantains (Musa Spp.). Plant Sci 170:104–112
Takagi H, Sugawara S, Saito T, Tasaki H, Lu Y, Guan K, Han DS, Godo T, Nakano M (2011) Plant regeneration via direct and indirect adventitious shoot formation and chromosome doubled somaclonal variation in Titanotrichum oldhamii (Hemsl.) Solereder. Plant Biotechnol Rep 5:187–195
Teixeira Da Silva JA (2013) Orchids: advances in tissue culture, genetics, phytochemistry and transgenic biotechnology. Floric Ornam Biotechnol 7:1–52
Viehmannova I, Bortlova Z, Vitamvas J, Cepkova HP, Eliasova K, Svobodova E, Travnickova M (2014) Assessment of somaclonal variation in somatic embryo derived plants of yacon [Smallanthus sonchifolius (Poepp. And Endl.) H. Robinson] using inter simple sequence repeat analysis and flow cytometry. Electron J Biotechnol 17:102–106
Vinoth A, Ravindhran R (2016) Efficient plant regeneration of watermelon (Citrullus lanatus Thunb.) via somatic embryogenesis and assessment of genetic fidelity using ISSR markers. In Vitro Cell Dev Biol Plant 52:107–115
Zhang F, Lv Y, Dong H, Guo S (2010) Analysis of genetic stability through inter simple sequence repeats molecular markers in microprpagated plantlets of Anoectochilus formosanus Hayata, Medicinal Plant. Bio Pharm Bull 33:384–388
Zhang A, Wang H, Shao Q, Xu M, Zhang W, Li M (2015) Large scale in vitro propagation of Anoectochilus roxburghii for commercial application: pharmaceutical important and ornamental plant. Ind Crops Prod 70:158–162
Acknowledgements
The corresponding author is grateful to the University Grant Commission (UGC), Delhi, India for providing Emeritus Fellowship. All the authors are grateful to Dr. P. Palani, Assistant Professor, Centre for Advanced Studies in Botany, University of Madras, Guindy Campus, Chennai 600 025, Tamil Nadu, India, for valuable help in scanning electron microscopic observation.
Author information
Authors and Affiliations
Contributions
NAS, JHFB, TSK and MVR are equally contributed to bringing out the manuscript in a successful manner.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Communicated by Sergio J. Ochatt.
Rights and permissions
About this article
Cite this article
Sherif, N.A., Franklin Benjamin, J.H., Senthil Kumar, T. et al. Somatic embryogenesis, acclimatization and genetic homogeneity assessment of regenerated plantlets of Anoectochilus elatus Lindl., an endangered terrestrial jewel orchid. Plant Cell Tiss Organ Cult 132, 303–316 (2018). https://doi.org/10.1007/s11240-017-1330-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-017-1330-4