Abstract
A simple and effective somatic embryogenic system was established for watermelon (Citrullus lanatus) cv. ‘Arka Manik’. Embryogenic callus was obtained from leaf explants of 20-d-old in vitro-grown seedlings cultured on embryogenic callus induction medium. The highest frequency of embryogenic callus induction (96.8%) occurred on Murashige and Skoog (MS) medium supplemented with 2.44 μM 2,4-dichlorophenoxyacetic acid (2,4-D) and 2.27 μM thidiazuron (TDZ). Transfer of embryogenic calluses with proembryogenic masses to embryo maturation medium led to the asynchronous development of somatic embryos (SEs), which progressed from the globular stage to the cotyledonary stage. The maximum number of SEs/explant (16.1 ± 0.24) was obtained on MS medium supplemented with 2.44 μM 2,4-D, 2.27 μM TDZ, and 30 g L−1 sucrose. Plantlet conversion from cotyledonary-stage SEs was tested on different strengths of MS medium (quarter-, half-, and full-strength) lacking plant growth regulators. The highest frequencies of germination (91.5%) and survivability (82.1%) of plantlets were achieved on full-strength MS medium. Transverse sections of embryogenic callus revealed SE development from callus cells near the epidermis. Secondary SEs occasionally formed from globular-shaped primary embryos. Genetic fidelity of mother plants and ex vitro plants was confirmed by inter-simple sequence repeat (ISSR) markers. The present study is the first report on the use of molecular markers in in vitro culture of watermelon. The developed protocol facilitates rapid production of true-to-type watermelon plants by somatic embryogenesis and thus could serve to generate effective target material for genetic transformation protocols.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Watermelon (Citrullus lanatus Thunb.) is an economically important fruit crop of the family Cucurbitaceae. It is widely grown in the tropics and subtropics, including most parts of Southeast Asia, Africa, the Caribbean, and the southern United States. Among the watermelon producers of the world, India ranks sixth with an annual production of 400,000 mt (FAO 2013). Watermelon fruits are a rich source of vitamins A, C, and B6 and mineral nutrients such as potassium, iron, and calcium (Anonymous 1992). Watermelon also contains a high amount of lycopene (23.0 to 72.0 mg/g wet weight), a carotenoid molecule with significant antioxidant activity (Fraser and Bramley 2004; Hall 2004). The fleshy fruits with high water content (∼91%) are valuable alternative sources of water in desert areas.
Watermelon breeders worldwide focus on developing cultivars with improved resistance to environmental stress and enhanced nutritional quality by the use of biotechnology (Compton et al. 2004). Transgenic watermelons with improved resistance to biotic and abiotic stress have been mostly raised through adventitious shoot regeneration (Choi et al. 1994; Ellul et al. 2003; Wang et al. 2003; Akashi et al. 2005; Park et al. 2005; Huang et al. 2011; Lin et al. 2012). Agrobacterium-mediated transformation of watermelon using cotyledon explants is limited by the production of shoot escapes, as the cotyledons are moderately resistant to kanamycin (Gaba et al. 2004). In addition, a larger proportion of shoots obtained by direct regeneration were hyperhydric than when obtained through somatic embryogenesis (Choi et al. 1994; Ellul et al. 2003; Park et al. 2005; Cho et al. 2008; Vinoth and Ravindhran 2015). Hence, somatic embryogenesis as an alternative propagation technology could accelerate the genetic improvement of watermelon.
Somatic embryogenesis is an effective method for clonal propagation of economically important plant species. It helps in the production of true-to-type plants (Stasolla and Yeung 2003) and synthetic seeds production (Maruyama et al. 2003). Differentiation of embryonic cells from somatic cells also enables the study of cell differentiation in plants (Quiroz-Figueroa et al. 2006). Establishment of somatic embryos (SEs) provides a steady supply of material for gene transformation (Parimalan et al. 2010). In cucurbit crops, somatic embryogenesis facilitates crop improvement by micropropagation of triploid plants, male-sterile genotypes, and interspecific hybrids (Fassuliotis and Nelson 1988).
The explant type is a crucial factor in somatic embryogenesis. Leaf and cotyledon explants were found to be highly competent for the induction of SEs (Kintzios et al. 2002; Vengadesan et al. 2005; Thiruvengadam et al. 2006). There are well-established somatic embryogenic systems for bitter gourd (Momordica charantia; Thiruvengadam et al. 2006; Paul et al. 2009), cucumber (Cucumis sativus; Kuijpers et al. 1996; Elmeer and Hennerty 2008), melon (Cucumis melo; Nakagawa et al. 2001), Styrian pumpkin (Cucurbita pepo subsp. pepo var. styriaca; Urbanek et al. 2004), and squash (Cucurbita pepo; Kintzios et al. 2002). Until now, very few reports have been available on somatic embryogenesis of watermelon (Compton and Gray 1993).
Plantlets regenerated under in vitro conditions, either by direct or indirect differentiation, are prone to somaclonal variation (Larkin and Scowcroft 1981; Matthes et al. 2001; Kanita and Kothari 2002). Assessment of genetic uniformity of tissue-cultured plantlets using molecular markers is thus essential to ascertain the true-to-type nature of the plantlets. PCR-based markers (random amplified polymorphic DNA [RAPD], inter-simple sequence repeat [ISSR]) are highly efficient in determining the genetic stability of in vitro-regenerated plantlets in many crop species (Joshi and Dhawan 2007; Huang et al. 2009). Both of these markers are simple, cost-effective, and highly discriminative, while ISSR is preferable for its reproducibility (Reddy et al. 2002). ISSRs have successfully established the genetic integrity of Calliandra tweedii (Benth.) plantlets derived from SEs (Heikrujam et al. 2014), Rauvolfia serpentina plantlets grown from synthetic seeds (Faisal et al. 2012), and micropropagated Gerbera jamesonii (Bolus) (Bhatia et al. 2009). Therefore, the present study was aimed to establish an efficient protocol for somatic embryogenesis in watermelon and to assess the clonal fidelity of ex vitro plants by ISSR markers.
Materials and Methods
Seed germination.
Seeds of watermelon (C. lanatus) cultivar ‘Arka Manik’ were obtained from the Indian Institute of Horticultural Research, Bangalore, India. Manually de-coated seeds were surface disinfected for 10 min in 1% (v/v) sodium hypochlorite solution (available chlorine 4% w/v approximate; Qualigens, Mumbai, India) containing 1 mL of Tween-20 per 100 mL and rinsed five times with autoclaved, double-distilled water. The seeds were blot dried on sterile filter paper and then placed on Murashige and Skoog (MS; Murashige and Skoog 1962) medium supplemented with 30 g L−1 sucrose and 8 g L−1 agar (HiMedia®, Mumbai, India). The pH of the medium was adjusted to 5.8 ± 0.02 prior to autoclaving at 121°C/100 kPa for 20 min. The seeds were incubated at 25 ± 1°C under a 16-h photoperiod with a light intensity of 50 μmol m−2 s−1 supplied with cool-white fluorescent lamps (Philips, Chennai, India).
Embryogenic callus induction.
Leaf explants (∼25 mm2) from 20-d-old seedlings germinated on basal MS medium were cultured with the adaxial side down on embryogenic callus induction medium (ECIM) containing MS medium supplemented with 2.44–12.19 μM 2,4-dichlorophenoxyacetic acid (2,4-D) or 2.68–13.42 μM α-naphthaleneacetic acid (NAA) alone or in combination with 2.27 μM thidiazuron (TDZ) or 2.22 μM 6-benzylaminopurine (BAP) (HiMedia®) and gelled with 8 g L−1 agar. Induction of embryogenic callus was observed for 3 wk. Embryogenic callus induction frequency ([explants with proembryogenic masses]/[total number of explants] × 100) was averaged with six replicates of eight explants per vessel. Each experiment was conducted three times. Cultures were maintained in glass bottles (15-cm height × 10-cm diameter) with non-vented lids, sealed with cling wrap (Sivasakthi Systems, Chennai, India), and incubated at 25 ± 1°C under complete darkness. These conditions were also used for the development of SEs.
Somatic embryo development, germination, and survivability.
Embryogenic calluses (3 wk old) obtained from ECIM containing 2.44 μM 2,4-D plus 2.27 μM TDZ were cultured on embryo maturation medium (EMM) containing MS medium supplemented with (1) 2.44 μM 2,4-D plus 0.45–4.54 μM TDZ and 30 g L−1 sucrose, (2) 2.44 μM 2,4-D plus 0.44–4.44 μM BAP and 30 g L−1 sucrose, or (3) 2.44 μM 2,4-D plus 2.27 μM TDZ and 10–50 g L−1 sucrose gelled with 8 g L−1 agar. SEs of different developmental stages (globular, heart, torpedo, and cotyledonary stage) were observed after 3 wk. Mean number of SEs/explant was averaged from six replicates of five explants per vessel. Each experiment was conducted three times.
Cotyledonary-stage SEs obtained from MS medium supplemented with 2.44 μM 2,4-D plus 2.27 μM TDZ and 30 g L−1 sucrose were transferred to MS (quarter-, half-, or full-strength) medium without plant growth regulators (PGRs) and gelled with 8 g L−1 agar for germination. Each treatment was assessed based on the responses of 25 replicates, and each experiment was conducted three times. Cultures were maintained in glass bottles (15-cm height × 10-cm diameter) with non-vented lids, sealed with cling wrap, and incubated at 25 ± 1°C under a 16-h photoperiod with a light intensity of 50 μmol m−2 s−1 supplied with cool-white fluorescent lamps (Philips). After 2 wk of culture on germination medium, plantlets were removed from the culture medium, and agar was washed off gently and thoroughly under running tap water. Plantlets were then transferred to paper cups (6-cm diameter, 170 g m−2 [GSM] thick; Mukesh Plastics, Chennai, India) containing sterilized red soil and sand (1:1 [v/v]). The potted plants were then covered with transparent polythene bags (8-cm wide × 12-cm tall, 0.5-mm thick; Mukesh Plastics) to maintain high humidity (85% RH) and grown for 2 wk with a light intensity of 50 μmol m−2 s−1 and 16-h photoperiod. Acclimatized plants with three to five new leaves were transferred to HDPE grow bags (30-cm wide × 35-cm tall; SK Organic Farms, Chennai, India) containing a mixture of red soil, sand, and organic manure (1:1:1 [v/v/v]) and grown to maturity under greenhouse conditions. The survival percentage of ex vitro plants was recorded after 6 wk.
Histological investigations.
Embryogenic calluses with SEs (6 wk old) were fixed in FAA (1:1:18 [v/v/v] formaldehyde/glacial acetic acid/70% [v/v] ethanol) solution, maintained at 28 ± 2°C for 1 wk, and then transferred to 70% ethanol for storage until required for analysis. The samples were stained with methylene blue, then dehydrated in an increasing ethanol series (35%, 50%, 75%, 85%, 95%, and absolute ethanol), and embedded in paraffin. Transverse sections (5-μm thick) were made with a paraffin-compatible microtome (Weswox Optik MT-1090A; Weswox Optik, Haryana, India). The sections were dewaxed with xylene three times and finally covered with neutral balsam. Sections were photographed using a trinocular light microscope (Olympus CH20i; Olympus Corporation, Tokyo, Japan).
Genetic fidelity analysis using ISSR markers.
Genomic DNA was isolated from the leaves of the mother plants (8-wk-old in vitro germinated seedlings) and ex vitro plants (6 wk old) using a CTAB method (Doyle and Doyle 1990). Six 3′-anchored ISSR primers (Eurofins Genomics, Bangalore, India) were used for genetic fidelity analysis. ISSR reactions were set up in a volume of 10 μL containing 1 μL genomic DNA (100 ng), 5 μL 2× master mix (Ampliqon, Odense M, Denmark), 0.8 μL of 10 pmol ISSR primers, and 3.2 μL nuclease-free water (Invitrogen Bioservices, Bangalore, India). PCR amplifications comprised an initial denaturation step at 94°C for 5 min, 30 cycles of denaturation at 94°C for 1 min, annealing at 48.6–52°C for 1 min, and extension at 72°C for 2 min, followed by a final extension at 72°C for 7 min. Amplifications were performed on a thermocycler (ProflexTM PCR system; Applied Biosystems, Waltham, MA) and PCR products were electrophoresed in 1.2% [w/v] agarose gel in 1× TAE buffer. PCR amplicons were photographed using a gel documentation system (VisiDoc-It™ Imaging System; UVP, Upland, CA). The sizes of the amplicons were estimated by comparing with a lambda DNA HindIII digest ladder.
Statistical analysis.
A completely randomized design was used. Quadratic regression analysis was performed using IBM SPSS Statistics version 20 (http://www-01.ibm.com/software/analytics/spss/pro-ducts/statistics/) to identify a functional relationship between the response and treatment levels. A high R 2 coefficient indicates that much of the variability is described by the model. For ISSR analysis, only consistent, well-resolved scorable bands were manually scored on the basis of their presence (1) or absence (0) in the gel. NTSYS version 2.1 software (Rohlf 2000) was used to obtain the distance matrix and cluster analysis of the data set. Genetic integrity of ex vitro plants was measured by Jaccard’s similarity coefficient (Jaccard 1908). The similarity matrix was subjected to a cluster analysis using UPGMA (unweighted pair group method with arithmetic mean).
Results and Discussion
Embryogenic callus induction.
Leaf explants expanded and curved during the initial period, forming yellow, compact callus from the cut end. Small proembryogenic masses (PEM) were observed after 3 wk on ECIM (Fig. 1a ). The optimal concentrations of auxins and cytokinins, either alone or in combination, for induction of embryogenic callus were determined (Fig. 2). 2,4-D alone produced embryogenic callus at a higher frequency than NAA alone, with a maximum frequency (83.3%) at 2.44 μM 2,4-D. Combinations of TDZ with 2,4-D or NAA increased the embryogenic callus induction frequency, and the highest frequency (96.8%) was obtained on MS medium containing 2.44 μM 2,4-D plus 2.27 μM TDZ. Addition of 2.22 μM BAP to MS medium containing 2,4-D decreased the frequency of callus induction relative to 2,4-D alone, while only a slight increase was observed when BAP was added to NAA-containing medium. From these results, it is clear that TDZ induced more embryogenic callus than BAP. In a similar fashion, cotyledons from immature seeds of four watermelon cultivars exhibited the best embryogenic response on MS medium containing 10 or 20 μM 2,4-D and 0.5 μM TDZ; however, the maximum frequency of explants with SEs reached only 30% (Compton and Gray 1993). Embryogenic potential of callus was maintained for another 6 wk by subculturing onto the same ECIM at 3-wk intervals. Prolonged subculture on ECIM led to browning of callus, thus reducing its embryogenic potency.
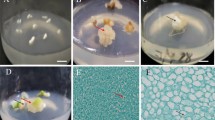
Somatic embryogenesis and regeneration from leaf explants of Citrullus lanatus Thunb. cv. ‘Arka Manik’: (a) embryogenic callus with proembryogenic masses (PEM) on embryogenic callus induction medium (ECIM) containing 2.44 μM 2,4-D + 2.27 μM TDZ after 3 wk (arrow indicates PEM); (b) development of somatic embryos (SEs; globular, heart, torpedo, and cotyledonary stage) on embryo maturation medium (EMM) containing 2.44 μM 2,4-D + 2.27 μM TDZ + 30 g L−1 sucrose after 3 wk (arrow indicates SEs); (c) globular SEs; (d) heart-shaped SE; (e) torpedo-stage SE; (f) cotyledonary SE with prominent shoot apex and root axis; (g) fused SEs with hypocotyls attached on EMM containing 2.44 μM 2,4-D + 3.33 μM BAP + 30 g L−1 sucrose; (h) germination of cotyledonary-stage SEs on full-strength MS medium; (i) SE-derived plantlet; (j) ex vitro plants. Bars = 5 mm (a, b); 2 mm (c–g); 10 mm (h–j).
Effect of auxins and cytokinins on embryogenic callus induction from leaf explants of Citrullus lanatus Thunb. cv. ‘Arka Manik’. MS medium was supplemented with 2,4-D (a) or NAA (b) alone or with 2.27 μM TDZ or 2.22 μM BAP. Lines represent trends fitted using quadratic regression analysis (P < 0.05): (a) 2,4-D (■) = −0.121 (PGR2) − 7.201 (PGR) + 89.6, R 2 = 0.984; 2,4-D + TDZ (♦) = −0.735 (PGR2) − 4.075 (PGR) + 102.0, R 2 = 0.997; 2,4-D + BAP (▲) = −1.942 (PGR2) + 4.837 (PGR) + 56.22, R 2 = 0.935. (b) NAA (■) = −3.771 (PGR2) + 20.60 (PGR) + 14.52, R 2 = 0.931; NAA + TDZ (▲) = −6 (PGR2) + 34.58 (PGR) + 8.78, R 2 = 0.907; NAA + BAP (▲) = −3.228 (PGR2) + 21.67 (PGR) + 0.48, R 2 = 0.645. Data were recorded after 3 wk of culture on embryogenic callus induction medium (ECIM). Values represent mean of six replicates with eight explants per replicate. BAP 6-benzylaminopurine, NAA α-naphthaleneacetic acid, PGR plant growth regulator, TDZ thidiazuron.
Somatic embryo development, germination, and survival.
Maturation of SEs through various developmental stages (globular, heart, torpedo, and cotyledonary stage) occurred on EMM containing 2.44 μM 2,4-D plus various concentrations of either TDZ or BAP (Figs. 1b–f and 3). All media contained 30 g L−1sucrose. TDZ supplementation of EMM favored production of more SEs per explant, with the highest number of SEs (all stages combined) obtained on MS medium supplemented with 2.44 μM 2,4-D and 2.27 μM TDZ (16.1 ± 0.24) (Fig. 3). Increases in the concentration of 2,4-D in MS medium containing 2.27 μM TDZ resulted in the proliferation of non-embryogenic cells (data not shown). A higher frequency of somatic embryogenesis was also obtained from cotyledon explants of muskmelon (Cucumis melo) by substituting BAP with TDZ (Gray et al. 1993). In the present study, BAP was less effective than TDZ in the transformation of PEM to SEs and resulted in abnormalities, e.g., fused hypocotyls, abnormal cotyledons, or absence of roots (Fig. 1g ). These abnormalities led to the poor conversion of SEs into plantlets, as also reported by Debeaujon and Branchard (1993).
Effect of cytokinin concentration on the development of somatic embryos (SEs) on MS medium supplemented with 2.44 μM 2,4-D and TDZ (a) or BAP (b). All media contained 30 g L−1sucrose. Lines represent trends fitted using quadratic regression analysis (P < 0.05): (a) globular (■) = −1.314 (TDZ2) + 8.211 (TDZ) + 2.24, R 2 = 0.818; heart (♦) = −0.862 (TDZ2) + 5.719 (TDZ) + 1.17, R 2 = 0.807; torpedo (▲) = −0.850 (TDZ2) + 5.241 (TDZ) − 0.758, R 2 = 0.689; cotyledonary (●) = −0.611 (TDZ2) + 3.696 (TDZ) − 0.578, R 2 = 0.793. (b) globular (■) = −0.562 (BAP2) + 3.397 (BAP) + 0.54, R 2 = 0.954; heart (♦) = −0.214 (BAP2) + 1.185 (BAP) + 1.01, R 2 = 0.767; torpedo (▲) = −0.083 (BAP2) + 0.486 (BAP) + 0.68, R 2 = 0.796; cotyledonary (●) = −0.107 (BAP2) + 0.690 (BAP) + 0.01, R 2 = 0.793. Data recorded after 3 wk of culture on embryo maturation medium (EMM). Values represent mean of six replicates with five explants per replicate. 2,4-D 2,4-dichlorophenoxyacetic acid, BAP 6-benzylaminopurine, TDZ thidiazuron.
Sugars play a vital role in the maturation of SEs by providing energy and carbon and by maintaining osmotic potential. Significant differences in the number of SEs were observed with the addition of 10–50 g L−1 sucrose to EMM containing 2.44 μM 2,4-D and 2.27 μM TDZ (Fig. 4). The maximum number of SEs was obtained on EMM containing 30 g L−1 sucrose. The lowest sucrose concentration (10 g L−1) failed to produce cotyledonary-stage embryos. In Cucumis sativus, sucrose was more effective for maturation of SEs than other carbon sources, with the greatest production of SEs in liquid MS medium containing 30 g L−1 sucrose (Vengadesan et al. 2005). Germination of cotyledonary-stage SEs into plantlets occurred within 2 wk on MS medium devoid of PGRs, containing different strengths of basal salts and vitamins, and containing 30 g L−1 sucrose (Fig. 1h , i ). The percentage of germination and survivability of SEs decreased proportionally with the reduction in strength of MS medium (Fig. 5). The highest frequencies of germination (91.5%) and survivability (82.1%) were achieved on full-strength MS medium. A previous report by Compton and Gray (1993) also described the germination of watermelon SEs in MS medium lacking PGRs. Plantlets with well-developed shoot and root systems were successfully transferred to greenhouse conditions (Fig. 1j ).
Effect of sucrose concentration on the development of somatic embryos (SEs) on MS medium supplemented with 2.44 μM 2,4-D and 2.27 μM TDZ. Lines represent trends fitted using quadratic regression analysis (P < 0.05): globular (■) = −1.759 (Suc2) + 10.89 (Suc) − 3.994, R 2 = 0.667; heart (♦) = −1.377 (Suc2) + 8.578 (Suc) − 4.704, R 2 = 0.687; torpedo (▲) = −1.260 (Suc2) + 7.821 (Suc) − 5.744, R 2 = 0.663; cotyledonary (●) = −0.962 (Suc2) + 6.131 (Suc) − 5.666, R 2 = 0.688. Data recorded after 3 wk of culture on embryo maturation medium (EMM). Values represent mean of six replicates with five explants per replicate. 2,4-D 2,4-dichlorophenoxyacetic acid, Suc sucrose, TDZ thidiazuron.
Effect of MS medium salt concentrations on the germination and survivability of somatic embryos (SEs) of Citrullus lanatus Thunb. cv. ‘Arka Manik’. Data recorded for germination after 2 wk of culture on germination medium and for survival percentage after 6 wk under greenhouse conditions. Values represent mean ± SE of 25 replicates.
Histological investigations.
Histological observations revealed that SEs developed from parenchyma-like callus cells near the epidermis. Meristematic cells from the epidermal surface redifferentiated into embryonic cell clusters, thus forming various stages of SEs (Fig. 6a–c ). Supporting evidence for SEs forming at the periphery of callus exists in a diversity of species such as chickpea (Cicer arietinum; Sagare et al. 1995), pea (Pisum sativum; Loiseau et al. 1998), potato (Solanum tuberosum; Sharma and Millam 2004), and rice (Oryza sativa; Vega et al. 2009). Secondary SEs were also found to emerge from the epidermal surface of primary SEs (Fig. 6d ). In torpedo-shaped embryos, tracheary elements with annular thickenings were observed by staining with safranin (Fig. 6e ). Tracheary elements were found as threads of different diameters, transforming gradually from one diameter to another.
Histological analysis of somatic embryos (SEs) from leaf explants of Citrullus lanatus Thunb. cv. ‘Arka Manik’: (a) transverse section (TS) of embryogenic callus from embryo maturation medium (EMM) with globular SE; (b) TS of heart-shaped SE; (c) TS of torpedo-stage SE; (d) TS of secondary somatic embryogenesis and abnormal SEs (SSe secondary somatic embryo, FSe fused somatic embryo); (e) safranin-stained tracheary elements with annular thickenings in torpedo-stage SE. Bars = 0.5 mm (a–d); 0.1 mm (e).
Genetic fidelity assessment using ISSR markers.
ISSR primers containing (AG)8 and (GA)8 dinucleotide repeats anchored on the 3′-end gave clear and reproducible bands (Fig. 7). The optimum annealing temperature for ISSR primers varied from 48.6 to 52.0°C. A total of 50 distinct and scorable bands were produced by six primers. The number of scorable bands varied from 2 (ISSR-5) to 11 (ISSR-1), with an average of 8.3 bands per primer (Table 1). The amplified products ranged in size from 200 to 2200 bp. The identical banding pattern in ex vitro plants and the mother plants for each of the six primers confirmed the genetic homogeneity of the ex vitro plants. The pairwise value of a similarity matrix based on Jaccard’s coefficient was 1, indicating 100% similarity (data not shown). Similarly, ISSR primers with dinucleotide repeats confirmed the clonal fidelity of in vitro plantlets of various crop species (Bhattacharya et al. 2010; Rai et al. 2012).
Assessment of genetic fidelity of mother plants and ex vitro plants of Citrullus lanatus Thunb. cv. ‘Arka Manik’ using ISSR markers (Table 1). The same seven ex vitro plants are represented in the same order in each of the six gel halves. (a) ISSR primers 1 (left) and 2 (right); (b) ISSR primers 3 (left) and 4 (right); (c) ISSR primers 5 (left) and 6 (right). In each panel, lanes 1 and 9 are mother plants; lanes 2–8 and 10–16 are ex vitro plants; lane M is lambda DNA/HindIII marker.
Conclusions
In the present study, efficient in vitro regeneration of watermelon cv. ‘Arka Manik’ was achieved from leaf explants by somatic embryogenesis. Addition of TDZ into both embryogenic callus induction medium and embryo maturation medium markedly improved somatic embryogenesis. Plantlet formation from SEs was achieved with greater survivability on full-strength MS medium than on lower-strength MS media. Histological analysis displayed the developmental pattern of SEs. Molecular assessment of ex vitro plants using ISSR markers revealed no variability. Hence, this protocol can be used for clonal propagation and genetic manipulation of watermelon cultivars.
References
Akashi K, Morikawa K, Yokota A (2005) Agrobacterium-mediated transformation system for the drought and excess light stress-tolerant wild watermelon (Citrullus lanatus). Plant Biotechnol 22:13–18
Anonymous (1992) The Wealth of India—a dictionary of Indian raw materials and industrial products, Vol. 3. Ca-Ci. Publication and Information Directorate, CSIR, New Delhi, pp 606–609
Bhatia R, Singh KP, Jhang T, Sharma TR (2009) Assessment of clonal fidelity of micropropagated gerbera plants by ISSR markers. Sci Hortic 119:208–211
Bhattacharya S, Bandopadhyay TK, Ghosh PG (2010) Somatic embryogenesis in Cymbopogon pendulus and evaluation of clonal fidelity of regenerants using ISSR marker. Sci Hortic 123:505–513
Cho MA, Moon CY, Liu JR, Choi PS (2008) Agrobacterium-mediated transformation in Citrullus lanatus. Biol Plant 52:365–369
Choi PS, Soh WY, Kim YS, Yoo OJ, Liu JR (1994) Genetic transformation and plant regeneration of watermelon using Agrobacterium tumefaciens. Plant Cell Rep 13:344–348
Compton ME, Gray DJ (1993) Somatic embryogenesis and plant regeneration from immature cotyledons of watermelon. Plant Cell Rep 12:61–65
Compton ME, Gray DJ, Gaba VP (2004) Use of tissue culture and biotechnology for the genetic improvement of watermelon. Plant Cell Tissue Organ Cult 77:231–243
Debeaujon I, Branchard M (1993) Somatic embryogenesis in the Cucurbitaceae. Plant Cell Tissue Organ Cult 34:91–100
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Ellul P, Ríos G, Atarés A, Roig LA, Serrano R, Moreno V (2003) The expression of Saccharomyces cerevisiae HAL1 gene increases salt tolerance in transgenic watermelon [Citrullus lanatus (Thunb.) Matsun. & Nakai.]. Theor Appl Genet 107:462–469
Elmeer KMS, Hennerty MJ (2008) Observations on the combined effects of light, NAA and 2,4-D on somatic embryogenesis of cucumber (Cucumis sativus) hybrids. Plant Cell Tissue Organ Cult 95:381–384
Faisal M, Alatar AA, Ahmad N, Anis M, Hegazy AK (2012) Assessment of genetic fidelity in Rauvolfia serpentina plantlets grown from synthetic (encapsulated) seeds following in vitro storage at 4°C. Molecules 17:5050–5061
FAO (2013) Food and Agricultural Organization. http://faostat3.fao.org. Cited 27 April 2015
Fassuliotis G, Nelson BV (1988) Interspecific hybrids of Cucumis metuliferus × C. anguria obtained through embryo culture and somatic embryogenesis. Euphytica 37:53–60
Fraser PD, Bramley PM (2004) The biosynthesis and nutritional uses of carotenoids. Prog Lipid Res 43:228–265
Gaba V, Zelcer A, Gal-On A (2004) Cucurbit biotechnology—the importance of virus resistance. In Vitro Cell Dev Biol—Plant 40:346–358
Gray DJ, McColley DW, Compton ME (1993) High-frequency somatic embryogenesis from quiescent seed cotyledons of Cucumis melo cultivars. J Am Soc Hortic Sci 118:425–432
Hall CV (2004) Watermelons as food in the 22 century. In: Nath P, Gaddagimath PB, Dutta OP (eds) Food security and vegetables: a global perspective. Dr. Prem Nath Agricultural Science Foundation, Bangalore, India, pp 135–148
Heikrujam M, Kumar D, Kumar S, Gupta SC, Agrawal V (2014) High efficiency cyclic production of secondary somatic embryos and ISSR based assessment of genetic fidelity among the emblings in Calliandra tweedii (Benth.). Sci Hortic 177:63–70
Huang WJ, Ning GG, Liu GF, Bao MZ (2009) Determination of genetic stability of long-term micropropagated plantlets of Platanus acerifolia using ISSR markers. Biol Plant 53:159–163
Huang YC, Chiang CH, Li CM, Yu TA (2011) Transgenic watermelon lines expressing the nucleocapsid gene of Watermelon silver mottle virus and the role of thiamine in reducing hyperhydricity in regenerated shoots. Plant Cell Tissue Organ Cult 106:21–29
Jaccard P (1908) Nouvelles recherches sur la distribution rurale. Bull Soc Vaudoise Sci Nat 44:223–270
Joshi P, Dhawan V (2007) Assessment of genetic fidelity of micropropagated Swertia chirayita plantlets by ISSR marker assay. Biol Plant 51:22–26
Kanita A, Kothari SI (2002) High efficiency adventitious shoot bud formation and plant regeneration from leaf explants of Dianthus chinensis L. Sci Hortic 96:205–212
Kintzios S, Sereti E, Bluchos P, Drossopoulos JB, Kitsaki CK, Liopa-Tsakalidis A (2002) Growth regulator pretreatment improves somatic embryogenesis from leaves of squash (Cucurbita pepo L.) and melon (Cucumis melo L.). Plant Cell Rep 21:1–8
Kuijpers AM, Bouman H, de Klerk GJ (1996) Increase of embryogenic callus formation in cucumber by initial culture on high concentration of 2,4-dichlorophenoxyacetic acid. Plant Cell Tissue Organ Cult 46:81–83
Larkin PJ, Scowcroft WR (1981) Somaclonal variation—a novel source of variability from cell cultures for plant improvement. Theor Appl Genet 60:197–214
Lin CY, Ku HM, Chiang YH, Ho HY, Yu TA, Jan FJ (2012) Development of transgenic watermelon resistant to Cucumber mosaic virus and Watermelon mosaic virus by using a single chimeric transgene construct. Transgenic Res 21:983–993
Loiseau J, Michaux-Ferrière N, Le Deunff Y (1998) Histology of somatic embryogenesis in pea. Plant Physiol Biochem 36:683–687
Maruyama E, Hosoi Y, Ishii K (2003) Somatic embryo culture for propagation, artificial seed production, and conservation of sawara cypress (Chamaecyparis pisifera Sieb. et Zucc.). J For Res 8:1–8
Matthes M, Singh R, Cheah SC, Karp A (2001) Variation in oil palm (Elaeis guineensis Jacq.) tissue culture-derived regenerants revealed by AFLPs with methylation-sensitive enzymes. Theor Appl Genet 102:971–979
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nakagawa H, Saijyo T, Yamauchi N, Shigyo M, Kako S, Ito A (2001) Effects of sugars and abscisic acid on somatic embryogenesis from melon (Cucumis melo L.) expanded cotyledon. Sci Hortic 29:85–92
Parimalan R, Akshatha V, Giridhar P, Ravishankar GA (2010) Somatic embryogenesis and Agrobacterium-mediated transformation in Bixa orellana (L.). Plant Cell Tissue Organ Cult 105:317–328
Park SM, Lee JS, Jegal S, Jeon BY, Jung M, Park YS, Han SL, Shin YS, Her NH, Lee JH, Lee MY, Ryu KH, Yang SG, Harn CH (2005) Transgenic watermelon rootstock resistant to CGMMV (cucumber green mottle mosaic virus) infection. Plant Cell Rep 24:350–356
Paul A, Mitter K, Raychaudhuri SS (2009) Effect of polyamines on in vitro somatic embryogenesis in Momordica charantia (L.). Plant Cell Tissue Organ Cult 97:303–311
Quiroz-Figueroa FR, Rojas-Herrera R, Galaz-Avalos RM, Loyola-Vargas VM (2006) Embryo production through somatic embryogenesis can be used to study cell differentiation in plants. Plant Cell Tissue Organ Cult 86:285–301
Rai MK, Phulwaria M, Harish GAK, Shekhawat NS, Jaiswal U (2012) Genetic homogeneity of guava plants derived from somatic embryogenesis using SSR and ISSR markers. Plant Cell Tissue Organ Cult 111:259–264
Reddy MP, Sarla N, Siddiq EA (2002) Inter simple sequence repeat (ISSR) polymorphism and its application in plant breeding. Euphytica 128:9–17
Rohlf FJ (2000) NTSYS-PC: Numerical Taxonomy and Multivariate Analysis System, version 2.1. Exeter Software, Setauket
Sagare AP, Suhasini K, Krishnamurthy KV (1995) Histology of somatic embryo initiation and development in chickpea (Cicer arietinum L.). Plant Sci 109:87–93
Sharma SK, Millam S (2004) Somatic embryogenesis in Solanum tuberosum L: a histological examination of key developmental stages. Plant Cell Rep 23:115–119
Stasolla C, Yeung EC (2003) Recent advances in conifer somatic embryogenesis: improving somatic embryo quality. Plant Cell Tissue Organ Cult 74:15–35
Thiruvengadam M, Mohamed SV, Yang CH, Jayabalan N (2006) Development of an embryogenic suspension culture of bitter melon (Momordica charantia L.). Sci Hortic 109:123–129
Urbanek A, Zechmann B, Müller M (2004) Plant regeneration via somatic embryogenesis in Styrian pumpkin: cytological and biochemical investigations. Plant Cell Tissue Organ Cult 79:329–340
Vega R, Vásquez N, Espinoza AM, Gatica AM, Valdez-Melara M (2009) Histology of somatic embryogenesis in rice (Oryza sativa cv. 5272). Rev Biol Trop 57:141–150
Vengadesan G, Selvaraj N, Anand RP, Gaba V, Ganapathi A (2005) Ontogeny of somatic embryos in cucumber (Cucumis sativus L.). In Vitro Cell Dev Biol—Plant 41:789–793
Vinoth A, Ravindhran R (2015) Reduced hyperhydricity in watermelon shoot cultures using silver ions. In Vitro Cell Dev Biol—Plant 51:258–264
Wang HZ, Zhao PJ, Xu JC, Zhao H, Zhang HS (2003) Virus resistance in transgenic watermelon plants containing a WMV-2 coat protein gene. J Genet Genom 30:70–75
Acknowledgments
The authors are thankful to the management of Loyola College, Chennai, for providing the laboratory facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Jeffrey Adelberg
Rights and permissions
About this article
Cite this article
Vinoth, A., Ravindhran, R. Efficient plant regeneration of watermelon (Citrullus lanatus Thunb.) via somatic embryogenesis and assessment of genetic fidelity using ISSR markers. In Vitro Cell.Dev.Biol.-Plant 52, 107–115 (2016). https://doi.org/10.1007/s11627-015-9731-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-015-9731-8