Abstract
The use of plant systems as factories for recombinant protein production became a prominent alternative for pharmaceutical industries due to their high potential for protein accumulation. In the last decades, the application of plants for protein production has gained more attention, as plants represent an economic strategy that leads to high levels of purified and active proteins for the pharmaceutical sector. Currently, FDA approval of the first generation of recombinant proteins produced in carrot cells, taliglucerase alfa, demonstrated that plant cells have a significant capacity to express complex proteins for therapeutic use. Although plant systems still have technical and economic barriers that require improvements in future years, the optimization of upstream and downstream components affecting protein accumulation is considered a key feature in the development of new pharmaceutical proteins. Therefore, an improvement of critical features, including transcription, translation and post-translational modifications, in plant cells could make plant systems a safe and economical alternative for biopharmaceutical production. Hence, in this review, the most recent advances influencing the upstream and downstream processes involved in recombinant protein accumulation in plant cells are described. We also discuss how plant systems are becoming the benchmark for the production of several biopharmaceuticals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant molecular pharming is the term related to the ability of plant materials to produce therapeutic proteins (Habibi et al. 2017). The emergence of molecular pharming as a reliable and novel production technology in recent decades is the result of years of work, opening new paths to minimizing the technical problems involved in yeast, bacterial, and mammalian platforms. Moreover, the identification and characterization of a promising regulatory pathway for the large-scale production of biopharmaceuticals could strongly contribute to the benefits of this system (Fischer et al. 2012). Molecular pharming technology presents several advantages, including (a) the production of low-cost biomass; (b) end-products lacking human toxicity; (c) the accumulation of complex proteins with correct and proper folding; and (d) straightforward methods for protein purification (Moustafa et al. 2015). Furthermore, molecular pharming offers a flexible, scalable and diverse alternative method for producing new, patent-protected biopharmaceuticals and biosimilars, expanding product opportunities based on rapidly growing ‘biobetter’ molecule markets (Zimran et al. 2011).
Although the plant-based pharmaceutical industry is still in an early stage of development, many biopharmaceuticals are already in the preclinical and clinical development pipeline. For instance, the commercial development of taligluceraseα (Protalix®, Israel; http://protalix.com/), used for the treatment of Gaucher’s disease, is a significant breakthrough in molecular pharming. Monoclonal antibodies, such as palivizumab and rituximab, moss-GBA (glucerase), moss-aGal (agalsidase) and other biosimilars, are next-generation plant-made recombinant proteins that represent the beneficial attributes of plant cell culture as a promising resource of complex protein production (Grabowski et al. 2014; Niederkrüger et al. 2014). Moreover, the insulin produced in safflower (SemBioSys Genetics, Canada; http://www.sembiosys.com) and the HIV-neutralizing monoclonal antibody produced in tobacco (Pharma-Planta; Germany; http://www.pharma-planta.net) were developed based on transgenic plant manufacturing.
Medicago Inc. (Québec, Canada; http://www.medicago.com) is now working on a phase II clinical trial with influenza VLP (H5) accumulated in Nicotiana benthamiana through agroinfiltration technology. Table 1 summarizes some of the plant-based vaccines for human and animal diseases.
Although plant platforms have many advantages in the production of biopharmaceuticals, future investigations will be required to invigorate the final product and to overcome the significant challenges and risks associated with large-scale production of biopharmaceuticals. For instance, regulatory unreliability and global concerns regarding the intrinsic yield of recombinant proteins are some of the barriers encountered by molecular pharming users. Hence, the identification and characterization of factors influencing protein accumulation levels are necessary. The yield may be divided into endogenous and exogenous factors that regulate protein accumulation in plants. Protein accumulation can be regulated by several steps, including (a) genetic elements (transcription, translation and post-translation) (Shinmyo and Kato 2010); (b) epigenetic factors inducing gene expression; and (c) environmental factors. Specific host platforms and the subcellular targeting of proteins to specific compartments are also considered critical parameters that contribute to the increase of recombinant protein yield (Fig. 1). Therefore, visualization and optimization of these features could be pre-eminent in mediating translational activity and boosting the amount of end-products within plant systems (Silverman et al. 2013). In this review, we first describe the upstream and downstream features affecting recombinant plant production and new perspectives on the optimization and improvement of these parameters are also discussed.
Expression cassette collection
An expression cassette is known as an important construct for high-level production of recombinant proteins in a host plant. Expression cassettes can be classified according to the number of cistrons (recombinant genes) within the corresponding mRNA. Monocistronic cassettes are suitable for expressing single proteins with synthesis driven by their endogenous regulatory sequences (Al-Rubeai 2011). They can either be provided by separated plasmids or be cloned into a single vector, leading to a higher prospect for the synergistic transfection of all genes of interest (Al-Rubeai 2011). In the case of single vectors, more than one protein can be expressed in the same cassette, although sequence length can be a restricting factor for complete integration into the plant genome, which means that an increasing number of linked transgenes leads to a lower probability of integration. The expression of all these factors can randomly result in genetic rearrangements and fragmentations that eliminate one or more transgenes from this process (Naqvi et al. 2010).
Bicistronic cassettes commonly use viral-derived internal ribosome entry sites (IRES) (Houdebine and Attal 1999; Lopez-Lastra et al. 2005) to bypass the 5′-cap-dependent translation process through a ribosomal skip mechanism. However, this approach is not widely used because the ORF expression level downstream of the IRES is usually decreased (Hennecke et al. 2001) Nevertheless, polycistronic cassettes can be used in an operon-like structure (Osbourn and Field 2009). Polycistronic cassettes are efficient and very useful for metabolic engineering purposes in plants, such as for secondary metabolite expression via the addition of whole heterologous metabolic pathways (Mozes-Koch et al. 2012). Cassettes carry out the body of required elements to increase the transcription rate (Clark and Pazdernik 2013) (Fig. 2).
The selection of a suitable promoter is an important factor for boosting gene expression via binding and interaction with trans-actin factors (Moustafa et al. 2015). Expression vectors can harbour either endogenous/homologous promoters or exogenous/heterologous promoters in relation to the host species. The plant promoter database (http://ppdb.agr.gifu-u.ac.jp/ppdb/cgi-bin/index.cgi) provides variety of core promoter structure and regulatory element groups for Physcomitrella patens, Oryza sativa, Arabidopsis thaliana, and Populus trichocarpa (poplar) (Hieno et al. 2014).
However, some heterologous promoters are commonly used in plants (Twyman et al. 2013). For example, the CaMV35S (Cauliflower Mosaic Virus 35S RNA subunit) vector is highly compatible with the transcription machinery of dicots and is commonly used for compact expression cassettes; however, CaMV35S activity may be decreased in some tissue and cells, such as newly developed tissues (Biłas et al. 2016). In this case, the region between nucleotides −90 and 208 acts as an enhancer and plays a key role in increasing the promoter activity. Act-1 and Ubi-1 are other examples of promoters that show more compatibility with the transcription machinery of monocots (Kang et al. 2008). The efficiency of polyubiquitin promoters PvUbi1 and PvUbi2 derived from switchgrass showed several fold higher constitutive expression when it was compared to the efficiency of the CaMV35S and OsAct1 promoters (Mann et al. 2011). Additionally, non-classical promoters were used for both monocots and dicots, such as the CmYLCV (Cestrum Yellow Leaf Curling Virus) promoter (Stavolone et al. 2003) and the pPLEX series promoters (Schünmann et al. 2003), respectively.
The efficiency of several commonly inducible (such as alcA, PR-1 and ACE1) and tissue-specific promoters (such as 2S albumin promoter, arc5-I promoter, CHS and RB7) has been reported in plants (Twyman et al. 2013). Recently, a β-oestradiol-inducible promoter has been used to create the TRANSPLANTA collection in A. thaliana (Coego et al. 2014) and to establish a stable gene expression system in P. patens (Kubo et al. 2013) for identification of the biological functions of transcription factors (TFs), while TFs can regulate gene expression in all organisms. Moreover, the efficacy of synthetic cis-acting motifs in transgenic tomato has been investigated, and it was compared with the efficiency of native CaMV35S and DECaMV35S promoters (Koul et al. 2012). Interestingly, the synthetic cis-acting modules led to increased transgene expression in tomato in comparison to the native promoters.
In contrast to the chemically inducible system developed to control transgene expression, a physical approach is considered safer and more applicable in terms of spatiotemporal resolution and the toxic effects of gene expression (Kang et al. 1999; Amirsadeghi et al. 2007; Muller et al. 2014). Thus, the use of chemically inducible transgene expression in plant cell cultures seems to be undesirable. In comparison to a chemical inducer, light was considered a compatible source for the bioproduction of recombinant proteins with high temporal resolution. In this context, a red light-switchable promoter has been developed to control transgene expression in the moss P. patens (Muller et al. 2014).
Additionally, multimeric protein subunits or different independent proteins can be expressed and assembled in planta using a single ORF (sORF) through linkage to the 2A peptide from foot-and-mouth virus (Luke et al. 2015) or inteins (interspacing polypeptide blocks of functional protein parts) (Evans et al. 2005). The 2A strategy is largely used for producing important proteins, such as a multi-epitope and low-cost candidate vaccine against cysticercosis using the so-called Helios2A polyprotein system (Monreal-Escalante et al. 2015). Although the 2A peptide is known to trigger the auto-cleavage and release of separate subunits, this mechanism occurs before the proteins enter the endoplasmic reticulum (ER). Otherwise, inteins ensure that the entire polypeptide is targeted to the ER and that autocleavage occurs in the lumen to guarantee a balanced protein expression level in an equimolar ratio (Kunes et al. 2009). Once inside the ER, exteins—protein subunit blocks flanking inteins—can be joined via protein splicing through a mechanism that is desirable for protein multimerization (Hauptmann et al. 2013), for instance, or they can be separately released (O’Brien et al. 2010). Interestingly, this last approach is very suitable for expressing recombinant antibodies, but has only been performed for mammalian cells (Gion et al. 2013).
Thus, considering all these possibilities, cassettes can also be shuffled according to their order in the vector backbone, or they can be rearranged in different orientations for expression (in tandem—the 5′ and 3′ ends are kept in the same orientation related to the other cassette—or sandwich—the 5′ and 3′ ends of each cassette are inverted to each other in a reversed orientation) (Al-Rubeai 2011). However, regardless of the vector setup, it is important to ensure that the coding DNA sequence (CDS) of interest has efficient control elements for translation initiation. In this way, it is advisable to provide an appropriate ribosome-binding site (RBS) for plant machinery via the addition of a eukaryote-derived RBS (Kozak sequence—consensus: GCCRCCATG) immediately upstream of the initiation codon (Kozak 1999; Mohammadzadeh et al. 2015) in the case of nuclear genes, or to adapt the CDS and its surrounding elements when performing chloroplast transformation. Most plastid mRNAs harbour Shine-Dalgarno (SD)-like sequences with a similar role, even though their distances from the initiation codon are not conserved. Furthermore, the initiation codons (e.g., AUG, GUG) can vary for plastid transgenes (Cardi et al. 2010).
Codon usage
Codon bias is a crucial step in regulating synthetic gene expression in a plant platform as it can influence numerous processes associated with protein production (e.g., RNA processing, protein translation and protein folding). Through evolutionary mechanisms, plant hosts developed similar substitutions of rare codons with favourable ones to reach a desired nucleotide distribution, as rare codons can inhibit protein translation (Gould et al. 2014). Gene expression based on codon usage can be predicted using diverse metrics, which have been reviewed by (Lindgreen 2012). Additionally, the codon usage database (http://www.kazusa.or.jp/codon/) released values on the number of times each codon is used per 1000 codons and the total number of times each codon is known to be used.
Codon usage optimization in plants was reviewed (Serres-Giardi et al. 2012), and it was demonstrated that rare codons and AU-rich destabilizing sequences may result in mRNA decline, reducing recombinant protein expression in plants (Laguía-Becher et al. 2010). The correlation between GC content, codon usage, and gene expression has also been reported in plants (Palidwor et al. 2010). These findings show the counterintuitive effect of GC content on determining the codon usage. Similar to these findings, the dominant effect of GC-biased genes on nucleotide distribution was reported in many seed plants (Serres-Giardi et al. 2012). Moreover, the influence of GC bias in codon context has been recognized for a set of codons but not for individual codons.
Codon usage optimization boosts gene expression and influences the amount of transgene expression more than 1000 fold (Gustafsson et al. 2004). In this context, (Franklin et al. 2002; Gisby et al. 2011) demonstrated significant increases in expression (75- to 80-fold) after codon optimization. In a recent study, (Kwon et al. 2016) the importance of codon usage optimization was shown with increasing levels of gene expression (4.9- to 7.1-fold or 22.5- to 28.1-fold) in lettuce and tobacco chloroplasts, respectively. The expression of recombinant protein was very low when heterologous genes were transferred into Chlamydomonas reinhardtii chloroplasts without codon usage optimization (Ishikura et al. 1999). In this way, there are various methods to evaluate the effect of codon usage on gene expression (Box 1) and to provide the best tools for codon optimization. The codon adaptation index (CAI) was used to estimate the expression level of heterologous genes. Genome-specific CAI must be used for optimal protein production as the nuclear, mitochondrial and chloroplast genomes may show different codon biases. For instance, a comparative study on the codon bias patterns of chloroplasts and their host nuclear genes demonstrated that the GC content of entire genes and the three-codon positions were higher in nuclear genes than in chloroplast genes, demonstrating different genomic organization and mutation pressures in nuclear and chloroplast genes (Liu and Xue 2005). Codon biases can boost expression efficiency by influencing translation rates and decreasing susceptibility to gene silencing (Heitzer et al. 2007).
Previous reports stated that the translational elongation step is affected by codon bias optimization (Irwin et al. 1995). Moreover, many works provided evidence of codon bias optimization on translation efficiency in prokaryotes and eukaryotes (Duret 2002; Mueller et al. 2010; Coleman et al. 2011). These findings indicate that codon context effects are significantly correlated with the abundance of tRNA isoacceptor molecules on the ribosome surface. Moreover, post-translation modifications might be affected by codon bias as silent mutations within the transcript, resulting in unwanted protein instability and misfolding (Brest et al. 2011). Nevertheless, it is important to observe that these complex correlations are still not fully recognized and that our knowledge of the effects of codon sequence changes during translation and post-translational modification are somewhat limited. Moreover, we should keep in mind that the optimization of codon usage is not the only factor in obtaining high level expression of recombinant proteins, as various factors affect recombinant protein levels.
Characterization of untranslated regions (UTRs)
A recent study reported that the transcript sequence or the region close to the start codon AUG can be crucial for translation efficiency (Kim et al. 2014). Hence, the sequence located 21 bp upstream of the start codon was identified as a significant feature for determining translation efficiency in A. thaliana. However, how this region can affect translation efficiency was not fully determined, although it was previously shown that mRNA folding of the sequence near the initiation codon might strongly influence translation efficiency (Plotkin and Kudla 2011). Moreover, this conserved region in plants is required for translational initiation factors to recruit ribosome subunits for start codon recognition (Simon and Miller 2013).
5′ UTR introns are other sequences that can influence gene expression by boosting the steady-state ratio of mRNA and by correlating with polyadenylation factors in plants (Rose 2008; Morello et al. 2011; Rose et al. 2011). Although introns are not part of translated regions and should be removed by splicing processes, in certain cases, 5′ UTR introns act as transcriptional enhancers to influence gene expression. In this context, the 5′ UTR intron of the rice rubi3 gene was shown to boost gene expression up to 29-fold in transgenic rice cells (Lu et al. 2008). Additionally, the effect of 5′ introns on stimulating gene expression in seeds has been confirmed, as the maize Adh1 intron increased the production of the reporter gene (Callis et al. 1987).
Genes harbouring a 5′ UTR intron of actin (act) genes from P. patens can also increase the production of human vascular endothelial growth factor by influencing the activity of upstream promoter regions (Weise et al. 2006). In this way, the regulatory properties of riboswitches as non-coding and conserved elements located in the untranslated regions have been studied in plants (Cheah et al. 2007; Croft et al. 2007), and they indicate that riboswitches can regulate gene expression via splicing and alternative 3′ end processing of mRNAs (Wachter et al. 2007). How this conserved element affects splicing in plants was reviewed by (Bocobza and Aharoni 2014).
Another example of a 5′ UTR intron used in Nicotiana tabacum was demonstrated by (Herz et al. 2005). They developed novel types of plastid transformation vectors harbouring 5′ UTR to increase expression levels. The expression levels were strongly increased when the 5′-UTR of phage 7 gene 10 was used. Therefore, the improvement of expression cassette design based on the 5′ UTR intron region may increase the likelihood of producing recombinant proteins at economically feasible levels for commercial applications. Understanding the expression-promoting mechanism of the 5′ UTR intron region of Act genes can open new possibilities for vector design based on a moss-derived expression system in the near future.
RNA secondary structure
Among various features affecting gene translation, mRNA secondary structure plays a key role in the process (Kim et al. 2010; Sun et al. 2012). Its negative outcome on translation can reduce protein yields, making mRNA secondary structure a decisive factor for the regulation of gene expression (Gaspar et al. 2013). mRNAs are the most complex group of cellular RNAs, and they originate not only from transcription itself but also from numerous modification reactions, such as precursor mRNA (pre-mRNA) splicing, capping, polyadenylation, and 3′ end processing. Furthermore, the association of mRNAs with protein complexes and factors results in the regulation of mRNA translation and metabolism (Wachter 2014). Therefore, screening the functional capabilities of RNA folding might identify sequence features that contribute to gene regulation in plant molecular pharming. Based on high-throughput structure mapping analysis, coupled with transcriptome data from different RNAs, it is clear that the abundance of mRNA transcripts, transcript half-life in the cytoplasm and the probability of the secondary structure that is formed in the transcript can affect the regulation of gene expression since the formation of a stable structure in an RNA strand can increasingly affect the expression quality of a targeted protein (Farrell 2007).
A global view on protein expression could provide insights into translation regulation mechanisms occurring at the level of initiation and in early stages of elongation. Previous works have revealed the dramatic effect of variable mRNA structural stability on coding portions (Ullrich et al. 2015). The stable structure next to the start codon might inhibit initiation and elongation by providing a longer pause during ribosome movement, which consequently, can affect the regulation of co-translational protein folding (Xie 2015).
In this context, measurements of ribosome movement along mRNA set a correlation between ribosome translation, codon usage and mRNA secondary structure (Mao et al. 2014). Notably, when adjacent ribosomes are close together, the mRNA secondary structure between them will be weakened and will then disappear; as a result, different ribosomes might encounter the structure with different folding strengths at the same site.
It is noteworthy that the significant competition between transcripts to bind ribosomes is similar to the competition of mRNAs, which bind initiation factors with high affinity to synthesize as much protein as mRNAs with a lower binding affinity. In this context, the formation of secondary structure can be preclusive. For instance, the deterrent effect of mRNA secondary structures, which suppress mRNA scanning via ribosomes when they form in 5′ leader sequences, has been demonstrated for plants (Farrell 2007). Some important characteristics of mRNA structure, such as the thermodynamic stability of the hairpin and its position, can affect the degree of translation inhibition. In this case, a highly stable hairpin in front of an AUG codon can efficiently repress translation, while hairpin with low stability that is downstream of an initiation codon can increase translation. One of the systematic studies reported in plant systems unveiled the correlation between mRNA secondary structures and protein expression (Wang and Wessler 2001). The formation of an RNA hairpin into the 5′ leader sequence of the Zea mays Lc gene, which is involved in the anthocyanin biosynthetic pathway, has been reported to suppress translation since the creation of mutation and deletion within the RNA hairpin increased the amount of protein production by enhancing the ribosome’s ability to load onto the mRNA. There are convincing reports showing that mutations and deletions within hairpins decreased hairpin stability and increased protein expression (Wang and Wessler 2001).
Moreover, the combined effect of mRNA secondary structure and codon usage in highly translated mRNAs causes a short ribosomal distance in structural regions, eliminating the structures during translation, which leads to a high elongation rate. RNA structural motifs can alter the stability of RNA backbones by exhibiting more regions for ribosome–RNA interactions. For example, the identification of the glmS (glutamine-fructose-6-phosphate amidotransferase) ribozyme (Winkler et al. 2004) and an allosteric self-splicing intron (Lee et al. 2010) as domains within mRNAs affect gene regulation by ribozyme-containing mRNA domains. Hence, riboswitches have regulatory properties. Interestingly, these domains facilitate mRNAs to adjust gene expression without associating with regulatory factors (Penchovsky and Stoilova 2013).
Previously, forms of a plant riboswitch, thiamin pyrophosphate (TPP), were characterized as ligands and were found to regulate thiamin biosynthesis in plants and algae (Cheah et al. 2007; Croft et al. 2007). Riboswitches control gene expression in plants by the splicing and alternative 3′ end processing of mRNAs (Wachter et al. 2007). How the structural rearrangement of the TPP aptamer affects splicing in plants was previously reviewed by Bocobza and Aharoni (2014). Their findings indicate that ribosome stepping and mRNA unwinding are force-dependent because the mechanistic nature of this force relies on ribosomal distance. Counterintuitively, the portended correlation between strong mRNA folding and translating ribosomes has been proposed, and the stronger folding of more abundant mRNAs results in the slower evolution of more highly expressed genes and proteins. Therefore, it unveils the impact of natural selection at the mRNA level in constraining protein evolution (Park et al. 2013). However, no systematic study has been reported in plant systems that unveils the correlation between mRNA secondary structures and the higher expression levels of recombinant proteins.
Subcellular targeting
The overexpression of a targeted gene via cellular compartments has gained more attention in recent years. However, in addition to the optimization of the exogenous mechanism involved in recombinant protein production, endogenous factors related to housekeeping genes and essential metabolism are features limiting the increased yield of recombinant proteins. This limitation could influence the accumulation of recombinant proteins that require post-translational modification in the ER through the activation of the unfolded protein response (UPR) (Thomas and Walmsley 2015).
Furthermore, directing recombinant proteins to subcellular organelles creates an environment low in proteases and helps to increase protein production and recovery (Schillberg et al. 1999; Fischer and Emans 2000). In addition to the ER and oil bodies, proteins can be targeted to the vacuoles, apoplast, and plastids, and they can even be directed to a hydroponic medium in plant roots (Horvath et al. 2000; Doran 2006).
Cytoplasm accumulation
Usually, cytoplasm targeting is an unfavourable strategy for the accumulation of proteins due to several reasons, including the presence of chemical reduction–oxidation reactions that result in the production of unfolded proteins; the secretion of proteases; the effectiveness of the Ubiquitin Proteasome Pathway (UPP), which is responsible for the identification and degradation of unfolded proteins; and the lack of post-translational modifications for correct folding, assembly or/and stability of recombinant proteins (Benchabane et al. 2008). Thus, different strategies can be applied to circumvent these challenges. An alternative could be the co-expression of protease inhibitors to minimize protein degradation during storage in the cytoplasm (Egelkrout et al. 2012). For example, a tomato cathepsin D inhibitor (CDI) expressed in potato leaves showed an average increase of 35–40% in leaf protein content for intrinsic and transgenic proteins in the cytosol (Goulet et al. 2010). The use of fused proteins or other “tags” can also assist in protein recovery and stability. In some cases, they can increase protein accumulation in the cytosol and resistance to proteolysis (Amin et al. 2004). Moreover, recombinant protein accumulation in subcellular organelles, such as the ER, chloroplast and oil bodies, is an alternative strategy to circumvent the lability of cytoplasm targeting (Alvarez et al. 2010; Giddings et al. 2000; Torrent et al. 2009).
Organelle accumulation
The ER is an ideal region to increase protein accumulation and improve protein assembly, as well as to control glycosylation (Aebi 2013). The ER is a region with a low quantity of proteases, and the presence of a high concentration of chaperones can assist recombinant proteins in post-translational folding and stability (Nuttall et al. 2002). Previous studies demonstrated that the apoplast targeting of recombinant antibody fragments, when retained in the ER, yielded 3.8 µg/g of protein in O. sativa cells. The same antibody fragment, when produced in tobacco cells, corresponded to 0.064% of the total soluble protein (Table 1) (Fischer et al. 1999; Torres et al. 1999). However, ER addressing is not recommended for proteins that require downstream modifications in the Golgi, vacuoles and chloroplast (Doran 2006).
Furthermore, the accumulation of antibodies directed to the ER using (SE)/(H)/(K)DEL signal peptides increased 2- to 10-fold compared to proteins lacking retention signals (Conrad and Fiedler 1998). In addition, the use of N-terminal γ-zein proline-rich sequences to target proteins to the ER and protein bodies (PBs) increased the stable accumulation of proteins in seeds (Torrent et al. 2009).
The use of apoplasts to store different human recombinant proteins expressed in tobacco plants—such as human serum albumin and human granulocyte–macrophage colony-stimulating factor (hGM-CSF)—significantly increased protein yield (Table 1) (Sijmons et al. 1990; James et al. 2000; Ramirez et al. 2000). In some cases, however, targeting an organelle for protein accumulation does not imply that different tissues or organs will store the same concentration of recombinant protein. For example, when the silk-like protein DP1B was directed to vacuoles, transgenic Arabidopsis seed cells presented higher concentration levels of the recombinant protein than leaf cells (Yang et al. 2005).
The chloroplast is another compartment used for the production of recombinant proteins, as it provides several advantages during protein accumulation, including the production of a large copy number of inserted genes and the absence of gene silencing (Michelet et al. 2011). The mechanism of gene expression in the chloroplast is regulated at the transcriptional and post-transcriptional levels (Stern et al. 2010). It was previously reported that this mechanism could be controlled by chloroplast-produced signals (Michelet et al. 2011). The chloroplast genome is assembled as operons that are transcribed as polycistronic transcriptional units and it shows prokaryotic and eukaryotic properties, as the control of chloroplast gene expression, including transcription, post-transcriptional processing, translation, and post-translational modifications, is similar to that of prokaryotic and/or eukaryotic systems (del Campo 2009). Moreover, chloroplasts offer a location with a low content of proteases, and they perform transcription, translation post-transcriptional processing and post-translational modifications. These organelles also offer high transgene stability over continuous generations, making them a secure bio-containment system, as the genes are not transmitted via pollen (Dufourmantel et al. 2006; Gray et al. 2009).
Many recombinant proteins have already been targeted to chloroplasts in order to increase protein accumulation and stability, such as human growth hormone (Staub et al. 2000), human serum albumin (Fernandez-San Millan et al. 2003), cholera and tetanus toxin fragments (Daniell et al. 2001; Tregoning et al. 2003), and a thermostable xylanase (Leelavathi et al. 2003). In addition, increased levels of a recombinant cellulose from Thermobifida fusca expressed in tobacco corresponded to 10.7% of the total soluble protein due to accumulation in the cell chloroplasts (Gray et al. 2009). In a recent work, the expression and development of several antigens and vaccines were reported for algae chloroplasts (Specht and Mayfield 2014). The green microalgae chloroplast provides a unique space for the assembly, folding and post-translational modifications of transgenic proteins (Fletcher et al. 2007; Chebolu and Daniell 2009; Specht et al. 2010) through cis-acting units, including 5′- and 3′ UTRs and promoters that affect transcription, mRNA stability and translation (Michelet et al. 2011).
Post-transcriptional gene silencing (PTGS) is considered a key parameter in low-level protein accumulation. Therefore, PTGS suppression might lead to boosts in protein production. In addition, targeting proteins to various cellular compartments, coupled with an agroinfection approach, is a new strategy to increase protein accumulation. A novel technology for accumulating recombinant protein has been established in Nicotiana benthamiana using a suppressor of PTGS and specific sub-cellular localization (Azhakanandam et al. 2007). Amplicon-plus Targeting Technology (APTT) demonstrated remarkable efficacy for increasing the accumulation levels in chloroplasts by creating fusions between the recombinant protein and different targeting peptides. APTT can overcome existing troubles regarding agroinfection systems, making them economically feasible and promising large-scale production of recombinant protein in a short period of time.
Host system
The selection of a suitable host plant and the consideration of its effect on the efficiency of recombinant protein accumulation are important key strategies for boosting intrinsic yield. There are economic issues affecting the selection of plant hosts as suitable benchmarks for the production of recombinant proteins. These economic factors include storage property, scalability and transportation, the cost of downstream processing, the potential of a short-time scale and edibility (Obembe et al. 2011). Additionally, efficient transformation and regeneration may contribute to the selection of a host plant as an amenable resource for recombinant protein production. However, it is not possible to set a perfect single system with all economic features, as every system has its own advantages and disadvantages that need to be considered as signposts for selecting the best system for protein production. As currently reviewed (Thomas and Walmsley 2015), there is a significant correlation between host benchmarks and endogenous molecular mechanisms, such as protein folding, glycosylation profiles, and chaperone pools. Moreover, the endogenous proteolytic activity of the host system might severely affect protein stability during expression, extraction, and harvesting (Pillay et al. 2014). Hence, modification of the host system can significantly increase the accumulation of recombinant proteins in plant systems (Egelkrout et al. 2012). In this context, cellular and molecular factors can contribute to the accumulation of recombinant proteins, which is correlated with the rate of synthesis and degradation of the desired protein. Understanding the association between flux (explained as the rate limiting step) and metabolic pathway, as well as identifying the limiting reaction event during protein accumulation in the host system, can increasingly influence protein production (Morandini 2013).
Furthermore, the replacement of the host plant pathway with a non-host plant pathway through metabolic engineering might facilitate increased recombinant protein production. For example, the accumulation of bacterial polyesters (polyhydroxyalkanoates) was reported by introducing the microbial pathway into plants (Poirier 2001). In another similar work, bacterial genes related to the generation of butanetriol, a useful precursor for the synthesis of several drugs, was applied into A. thaliana (Abdel-Ghany et al. 2013). However, toxic side effects on plant growth produced by metabolic engineering are considered limiting factors for the accumulation of these metabolites in plant systems (Keasling 2012; Zingaro and Papoutsakis 2012). The toxic side effects could be decreased by the identification of intermediate metabolites involved in toxicity and their direction to specific organelles (Bornke and Broer 2010).
Leafy crop-based expression
Leafy crops, including tobacco, lettuce, and alfalfa, are well established in the commercialization of biopharmaceuticals due to the many expectations regarding their biomass efficiency and capability at a massive scale. For example, the ability of transgenic tobacco (N. tabacum) to produce 1-100 tons of biomass per hectare per year makes this plant a reliable platform for the production of a high concentration of biopharmaceuticals. Moreover, easy genetic transformation and regeneration are favoured by using tobacco as a laboratory model in terms of molecular pharming. Therefore, both scalability and successful gene expression history make tobacco a pioneer for the production of various biopharmaceuticals (Twyman et al. 2003). In this context, Kentucky BioProcessing (USA) uses its proprietary stable nuclear platform in tobacco to produce an oral subunit vaccine for Norwalk virus (NoroVAXX) (http://www.kentuckybioprocessing.com). Moreover, the oral delivery of bioencapsulated transmucosal carrier cholera toxin B subunit (CTB) fused with green fluorescent protein (GFP) expressed in tobacco chloroplast genome could be used to tackle brain failure, especially in terms of the blood–brain barrier (BBB) and blood–retinal barrier (BRB), using a mouse model (Kohli et al. 2014). The results of this investigation showed that the oral administration of CTB-GFP resulted in binding to intestinal GM1 receptors and the release of fused protein (CTB) into the circulatory system. In this context, the long-term stabilization of plant-based protein reduced the high cost of oral delivery of neurotherapeutic proteins in terms of the BBB and BRB. A drawback of tobacco is its inherent production of a large number of toxic compounds, which interfere with downstream processing, as well as its protein instability due to secreted proteolysis enzymes (Rosales-Mendoza et al. 2010; Obembe et al. 2011; Stoger et al. 2014). It is worth bearing in mind that these drawbacks decrease the value of leafy crops for the production of recombinant proteins. In this context, many researchers have focused on ways to minimize these adverse effects and boost the capacity of leafy crops for the production of recombinant proteins.
Seed-based expression system
Seed-based expression systems provide a good platform for the production of recombinant proteins at massive and minute levels. (Stoger et al. 2014). In both cases, the high cost of process development, such as bioreactor-based production, would be reduced. Moreover, this system could resolve problems related to leafy crops, including proteolytic degradation, protein instability and decreased activity of recombinant proteins due to long-term storage (Nochi et al. 2007; Faye and Gomord 2010). Most importantly, seeds can be used to create master and working cell culture platforms (Paul et al. 2013). As an example, Ventria Bioscience (Fort Collins, USA; http://www.ventria.com/) developed the large-scale production of human serum albumin based on rice seeds. The worldwide demand for this recombinant protein is approximately 500 tons per year (Chen et al. 2013b).
A seed-based expression system is also an excellent platform for the production of orally administered vaccines and antigens. Orally delivered vaccines contribute to the reduction of immunogenicity via systemic humoral and cellular immune responses in the gut and mucosal surface (Woodrow et al. 2012). Seed-based oral immunotherapy presents a cost-effective system for the production of T-cell epitope peptides or recombinant hypoallergens. Compared to a purified vaccine, an orally administered antigen produced in transgenic seed plants shows more resistance to gastrointestinal enzymes, due to the bioencapsulation of the vaccine via plant cell barriers, such as protein bodies (Takaiwa 2009). Several studies have demonstrated the benefits of encapsulation, including increased resistance to enzymatic digestion and stronger immune response (Chikwamba et al. 2003; Takagi et al. 2010; Takaiwa 2011; Suzuki et al. 2012). Additionally, the delivery of orally administered vaccines (attenuated vaccines) may reduce the possibility of reversion to virulence, guaranteeing a high immune response of the vaccine (Stoger et al. 2014). Therefore, the ability of transgenic rice seed to produce T cell epitope peptides of Cry j I and Cry j for the induction of a high mucosal immune response to allergen pollen in mice is a good example of a drug orally administered by plant seeds (Takagi et al. 2005).
Moreover, the expression and accumulation of an orally administered vaccine in plant seeds facilitate the delivery of autoantigens to mucosal surfaces while maintaining a high immune response (Lakshmi et al. 2013; Kohli et al. 2014). Thus, rice, barley and maize seeds have been explored as commercial benchmarks for recombinant protein production. Given the numerous advantages of seed-based expression, Pharma-Planta Consortium (http://www.pharma-planta.net/) and ProdiGene Inc. (USA; http://www.prodigene.com/) adopted and developed the commercial production of HIV microbicide and bovine trypsin using the maize seed platform. Ventria Bioscience established an Expressed-Tec system in rice seeds for the production of various biopharmaceuticals, including lactoferrin and lysozyme, transferrin, and human albumin (Tang et al. 2010; Zhang et al. 2010). An Icelandic biotechnology company (http://www.orfgenetics.com/), ORF Genetics, uses its proprietary ORFeus platform in the endosperm of barely seed to accumulate a range of cytokines and human growth hormones.
Fruit and vegetable expression systems
Fruits and vegetables are the last categories of transgenic host plants that have contributed to the production of vaccines. The capability of oral vaccine delivery is recognized as a benefit of this system over conventional production. This ability not only removes the required purification step for recombinant protein but also enables the distribution of the product in the absence of a cold chain process (Paul et al. 2013; Stoger et al. 2014).
Currently, Protalix Biotherapeutics released some oral therapeutic enzymes into clinical trials using lyophilized carrot cells, including a modified version of the recombinant alpha-Galactosidase-A protein (PRX 102) in phases 1/2 of clinical trials; PRX-106 (Oral antiTNF), which has completed phase I of a clinical trial; PRX-105, a biodefence drug, in phase 1; and PRX-112 (Oral glucocerebrosidase for Gaucher’s Disease), which has completed phase I (Wolfson 2013).
Due to the benefits of molecular pharming for the production of recombinant proteins in terms of stability and scalability, the EU Pharma-Planta consortium provided a new project establishing the commercial production of two HIV antibodies, 2G12 and 2F5, using maize and tobacco platforms (Rademacher et al. 2008; Paul et al. 2011). This project resulted in the GMP-compliant development of recombinant protein 2G12 using transgenic tobacco and launched to a phase I clinical trial, indicating the success of molecular pharming in microbicide protein production. Nevertheless, the accessibility of molecular pharming products to underdeveloped countries and their connection to the global health network requires a cost-efficient production and development infrastructure, which can be offered by transgenic plants.
Moss-based expression system
Recently, a moss-based expression system has been exploited for the accumulation of high-value biopharmaceuticals. The moss P. patens is a reliable system for the production of recombinant proteins, as stable genetic transformation and endogenous gene disruption are easily achieved. The latter is beneficial for knocking out genes involved in the addition of a non-human glycogen structure onto the protein sequence, enabling moss to express humanized glycoprotein (Reski et al. 2015). Moreover, protein secretion and product stability in the medium add versatility to this system, as proteolytic degradation has not been reported so far. Thus, protein homogeneity and purification costs are advantages of this system. Recently, several human proteins were expressed using a moss system, including asialo-erythropoietin (AEPO) (Parsons et al. 2012), placental secreted alkaline phosphatase (SEAP) (Gitzinger et al. 2009), complement factor H (FH) (Buttner-Mainik et al. 2011), epidermal growth factor (EGF), hepatocyte growth factor (HGF) (Niederkrüger et al. 2014), and multi-epitope fusion protein from human immunodeficiency virus (Poly HIV) (Orellana-Escobedo et al. 2015). The German company Greenovation Biotech GmbH (http://www.greenovation.com/) developed a highly stable and high-yielding strain of P. patens as a benchmark for the establishment of several recombinant proteins involved in orphan diseases, such as Fabry’s Disease, Gaucher’s Disease, atypical haemolytic uremic syndrome (aHUS), and Pompe Disease.
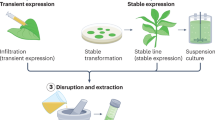
Transient expression-based systems: agroinfiltration and agroinfection
Transient gene expression is an efficient, cost-effective and time-saving strategy for yielding high amounts of recombinant proteins, as genetic transformation is a slow process, requiring months or years to generate transgenic plants due to regeneration protocols (Hefferon 2012). In addition, it is a genome integration-independent strategy, and consequently, it is not affected by position effects, existing in a stable transformation once the expression vector remains as an episomal DNA molecule. Moreover, transient gene expression can be detected within 3 h after plasmid delivery, reaching an expression threshold after 18–48 h and remaining transcriptionally active for approximately 10 days. Besides these features, transient expression can contribute to overcoming concerns on biosafety issues (Komarova et al. 2010).
In plants, transient gene expression is usually accomplished using Agrobacterium tumefaciens in agroinfiltration experiments by infiltrating bacterial cell suspensions into leaf cells with the consequent delivery of T-DNA to the host cells (Circelli et al. 2010). Furthermore, an A. tumefaciens approach can be coupled with replicating plant virus genomes in a virus-based replicon system, whose advantages rely on viral properties: viruses are small, can be easily manipulated, have a simple infection process and are able to replicate at high levels. These properties make them ideal vectors for heterologous expression and a suitable alternative to stable transgenic systems (Lico et al. 2008).
In some reports using viral-based systems, high-level expressions were achieved for different biopharmaceuticals, such as full-size mAbs and VLP (Virus-like particle) vaccines, showing a much higher yield from 0.5 to 0.8 mg per gram of fresh-leaf biomass (Giritch et al. 2006; Huang et al. 2010; Pogue et al. 2010). To achieve this, viral genomes were cloned as full-length complementary DNA (cDNA) into the desired expression vector through gene replacement, gene insertion or gene fusion (Ronald 2007). Hence, replicon systems belong to two possible categories: independent-virus (inoculated as virions or isolated viral genomes, and the infection activity is triggered by cell-to-cell and systemic movement spreading) or minimal-virus (viral vectors lacking cell-to-cell movement capacity, which allows the efficient expression of larger recombinant proteins). In general, replicon systems are derived mainly from RNA virus genomes (e.g., Potexvirus, Tobamovirus, Comovirus, Potyvirus, Tobravirus, Closterovirus, and Sobemovirus) (Pogue et al. 2010).
Minimal-virus infection is usually coupled with agroinfiltration in an Agrobacterium-mediated delivery system defined as agroinfection, which is practical technique because it does not require any delay associated with systemic spreading. Moreover, it is able to trigger high-level replication and, consequently, very large amounts of proteins in a shorter period than independent-virus infection (Pogue et al. 2010). Furthermore, due to the method, most of the infiltrated area provides synchronous transgene expression once at least 96% of the cells become infiltrated (Komarova et al. 2010).
Some plant species (e.g., A. thaliana, Nicotiana spp.) are also able to trigger PTGS against replicon transgenes, resulting in an accumulation failure of transcripts as a consequence of their sequence-specific targeting and destruction (Mallory et al. 2002). Accordingly, to maintain a very high level of replication, PTGS can be overcome through the co-expression of viral or non-viral silencing suppressors (e.g., Tombusvirus P19 protein; Potyvirus P1/HC-Pro proteins; pectin methylesterase inhibitors; and Pol II-directed short noncoding RNAs), which enhance mRNA stability and improve heterologous expression (Komarova et al. 2010).
During the last years, multi-component viral vectors were commonly used for these purposes, and DNA systems were developed and shown to be feasible and efficient for driving protein expression (Huang et al. 2009). These Geminivirus-based systems have already been used for VLP-based vaccine and monoclonal antibody production (Huang et al. 2010). Once the recombinant protein is produced, the leaves must be harvested for protein extraction and complete purification as required for biopharmaceutical production.
A recent significant development in the area of agroinfiltration is using “deconstructed” viral vectors. In this new type of vector, unnecessary viral genome components for the function of plasmid expression are removed, which leads to the assembly of larger transgenes while keeping viral replication and transcription (Chen et al. 2013a). The MagnICON system represents an efficient and robust gene-transferring technology for the transient expression of biopharmaceuticals in plant platforms. Using this system, the need for plasmid delivery based on complicated methods of generating RNA is eliminated. The MagnICON system provides an efficient system without functional infectious proteins because of the deletion of the CP gene, whereas the yield and speed of viral system are maintained. Moreover, this technology is able to integrate the posttranslational processing of plant systems for the production of complex proteins (Chen et al. 2013a). Numerous proteins have been expressed by this system, and the high-level production of desired proteins demonstrated up to 1 mg per gram of fresh weight in a short period of time (7–10 days after agroinfiltration) (Giritch et al. 2006; Lai et al. 2010; Phoolcharoen et al. 2011; He et al. 2012; Lai and Chen 2012). Currently, with the help of this system, the well-documented biopharmaceutical ZMapp™, which is composed of three humanized monoclonal antibodies, is manufactured in Nicotiana for the treatment of Ebola infections (http://mappbio.com/). The development of this system will efficiently contribute to using plant transient expression systems as a robust and prominent platform for the commercial production of pharmaceuticals.
Conclusion
Plant benchmarks have been engineered as expression vehicles for the production of different recombinant proteins. The secretion of produced proteins and their simple purification process provide an economical alternative to other systems. Moreover, plant-produced proteins are safer than those derived from other benchmarks. The development of plant systems for high protein accumulation is a priority as a large-scale concept. Several features, from transcription to translation, regulate protein production, and they need to be considered before beginning the steps of protein production. Currently, successful optimization resulted in good protein secretion at high levels (>30% TSP), and there are valuable databases and online software that can screen and evaluate many characteristics influencing protein accumulation. Different approaches can be selected, combined, and used as strategies to improve protein expression levels in plants. Among them, numerous parameters could be altered, which are related to the genetic elements that constitute the expression vector used to drive heterologous protein expression and their orientation within the backbone. There are also several molecular strategies for controlling glycosylation patterns to obtain the desired glycoforms, and these strategies vary from molecular tools for gene silencing to subcellular targeting. The latter can also be used for storage purposes to optimize and enhance protein accumulation, which, when coupled to viral-based replicon systems, might overexpress proteins at high levels.
References
Abdel-Ghany SE, Day I, Heuberger AL, Broeckling CD, Reddy ASN (2013) Metabolic engineering of Arabidopsis for butanetriol production using bacterial genes. Metab Eng 20:109–120. doi:10.1016/j.ymben.2013.10.003
Aebi M (2013) N-linked protein glycosylation in the ER. Biophys Acta 1833(11):2430–2437 doi:10.1016/j.bbamcr.2013.04.001
Al-Rubeai M (2011) Antibody expression and production. Springer Netherlands, Dordrecht
Alvarez ML, Topal E, Martin F, Cardineau GA (2010) Higher accumulation of F1-V fusion recombinant protein in plants after induction of protein body formation. Plant Mol Biol 72(1–2):75–89. doi:10.1007/s11103-009-9552-4
Amin N, Liu AD, Ramer S, Aehle W, Meijer D, Metin M, Wong S, Gualfetti P, Schellenberger V (2004) Construction of stabilized proteins by combinatorial consensus mutagenesis. Protein Eng Des Sel 17(11):787–793. doi:10.1093/protein/gzh091
Amirsadeghi S, McDonald AE, Vanlerberghe GC (2007) A glucocorticoid-inducible gene expression system can cause growth defects in tobacco. Planta 226(2):453–463. doi:10.1007/s00425-007-0495-1
Atsmon J, Brill-Almon E, Nadri-Shay C, Chertkoff R, Alon S, Shaikevich D, Volokhov I, Haim KY, Bartfeld D, Shulman A, Ruderfer I, Ben-Moshe T, Shilovitzky O, Soreq H, Shaaltiel Y (2015) Preclinical and first-in-human evaluation of PRX-105, a PEGylated, plant-derived, recombinant human acetylcholinesterase-R. Toxicol Appl Pharmacol 287(3):202–209. doi:10.1016/j.taap.2015.06.004
Azhakanandam K, Weissinger SM, Nicholson JS, Qu R, Weissinger AK (2007) Amplicon-plus targeting technology (APTT) for rapid production of a highly unstable vaccine protein in tobacco plants. Plant Mol Biol 63(3):393–404. doi:10.1007/s11103-006-9096-9
Baur A, Reski R, Gorr G (2005) Enhanced recovery of a secreted recombinant human growth factor using stabilizing additives and by co-expression of human serum albumin in the moss Physcomitrella patens. Plant Biotechnol J 3(3):331–340. doi:10.1111/j.1467-7652.2005.00127.x
Benchabane M, Goulet C, Rivard D, Faye L, Gomord V, Michaud D (2008) Preventing unintended proteolysis in plant protein biofactories. Plant Biotechnol J 6(7):633–648. doi:10.1111/j.1467-7652.2008.00344.x
Biłas R, Szafran K, Hnatuszko-Konka K, Kononowicz AK (2016) Cis-regulatory elements used to control gene expression in plants. PCTOC 127(2):269–287. doi:10.1007/s11240-016-1057-7
Bocobza SE, Aharoni A (2014) Small molecules that interact with RNA: riboswitch-based gene control and its involvement in metabolic regulation in plants and algae. Plant J 79(4):693–703. doi:10.1111/tpj.12540
Bornke F, Broer I (2010) Tailoring plant metabolism for the production of novel polymers and platform chemicals. Curr Opin Plant Biol 13(3):354–362. doi:10.1016/j.pbi.2010.01.005
Brest P, Lapaquette P, Souidi M, Lebrigand K, Cesaro A, Vouret-Craviari V, Mari B, Barbry P, Mosnier JF, Hebuterne X, Harel-Bellan A, Mograbi B, Darfeuille-Michaud A, Hofman P (2011) A synonymous variant in IRGM alters a binding site for miR-196 and causes deregulation of IRGM-dependent xenophagy in Crohn’s disease. Nat Genet 43(3):242–245. doi:10.1038/ng.762
Buttner-Mainik A, Parsons J, Jerome H, Hartmann A, Lamer S, Schaaf A, Schlosser A, Zipfel PF, Reski R, Decker EL (2011) Production of biologically active recombinant human factor H in Physcomitrella. Plant Biotechnol J 9(3):373–383. doi:10.1111/j.1467-7652.2010.00552.x
Callis J, Fromm M, Walbot V (1987) Introns increase gene expression in cultured maize cells. Genes Dev 1(10):1183–1200
Cheah MT, Wachter A, Sudarsan N, Breaker RR (2007) Control of alternative RNA splicing and gene expression by eukaryotic riboswitches. Nature 447(7143):497–500. http://www.nature.com/nature/journal/v447/n7143/suppinfo/nature05769_S1.html
Chebolu S, Daniell H (2009) Chloroplast-derived vaccine antigens and biopharmaceuticals: expression, folding, assembly and functionality. Curr Top Microbiol Immunol 332:33–54. doi:10.1007/978-3-540-70868-1_3
Chen Q, Lai H, Hurtado J, Stahnke J, Leuzinger K, Dent M (2013a) Agroinfiltration as an effective and scalable strategy of gene delivery for production of pharmaceutical proteins. Adv Tech Biol Med 1(1):103. doi:10.4172/atbm.1000103
Chen Z, He Y, Shi B, Yang D (2013b) Human serum albumin from recombinant DNA technology: challenges and strategies. Biochim Biophys Acta 1830(12):5515–5525. doi:10.1016/j.bbagen.2013.04.037
Chikwamba RK, Scott MP, Mejia LB, Mason HS, Wang K (2003) Localization of a bacterial protein in starch granules of transgenic maize kernels. Proc Natl Acad Sci USA 100(19):11127–11132. doi:10.1073/pnas.1836901100
Circelli P, Donini M, Villani ME, Benvenuto E, Marusic C (2010) Efficient Agrobacterium-based transient expression system for the production of biopharmaceuticals in plants. Bioeng Bugs 1(3):221–224 doi:10.4161/bbug.1.3.11722
Coego A, Brizuela E, Castillejo P, Ruíz S, Koncz C, del Pozo JC, Piñeiro M, Jarillo JA, Paz-Ares J, León J, The TC (2014) The TRANSPLANTA collection of Arabidopsis lines: a resource for functional analysis of transcription factors based on their conditional overexpression. Plant J 77(6):944–953. doi:10.1111/tpj.12443
Cardi T, Lenzi P, Maliga P (2010) Chloroplasts as expression platforms for plant-produced vaccines. Expert Rev Vaccines 9(8):893–911. doi:10.1586/erv.10.78
Clark DP, Pazdernik NJ (2013) Regulation of transcription in eukaryotes molecular biology, 2nd edn, Chap. e17. Academic Press, Boston, pp e374–e378
Coleman JR, Papamichail D, Yano M, Garcia-Suarez Mdel M, Pirofski LA (2011) Designed reduction of Streptococcus pneumoniae pathogenicity via synthetic changes in virulence factor codon-pair bias. J Infect Dis 203(9):1264–1273. doi:10.1093/infdis/jir010
Conrad U, Fiedler U (1998) Compartment-specific accumulation of recombinant immunoglobulins in plant cells: an essential tool for antibody production and immunomodulation of physiological functions and pathogen activity. Plant Mol Biol 38(1–2):101–109
Croft MT, Moulin M, Webb ME, Smith AG (2007) Thiamine biosynthesis in algae is regulated by riboswitches. Proc Natl Acad Sci USA 104(52):20770–20775 doi:10.1073/pnas.0705786105
da Cunha NB, Murad A, Vianna G, Rech E (2014) Recombinant biosynthesis of functional human growth hormone and coagulation factor IX in transgenic soybean seeds. BMC Proceed 8(4):P112 doi:10.1186/1753-6561-8-s4-p112
Daniell H, Lee SB, Panchal T, Wiebe PO (2001) Expression of the native cholera toxin B subunit gene and assembly as functional oligomers in transgenic tobacco chloroplasts. J Mol Biol 311(5):1001–1009. doi:10.1006/jmbi.2001.4921
del Campo EM (2009) Post-transcriptional control of chloroplast gene expression. Gene Regul Syst Biol 3:31–47
Doran PM (2006) Foreign protein degradation and instability in plants and plant tissue cultures. Trends Biotechnol 24(9):426–432. doi:10.1016/j.tibtech.2006.06.012
dos Reis M, Savva R, Wernisch L (2004) Solving the riddle of codon usage preferences: a test for translational selection. Nucleic Acids Res 32(17):5036–5044. doi:10.1093/nar/gkh834
Dufourmantel N, Tissot G, Garcon F, Pelissier B, Dubald M (2006) Stability of soybean recombinant plastome over six generations. Transgenic Res 15(3):305–311. doi:10.1007/s11248-005-5262-0
Duret L (2002) Evolution of synonymous codon usage in metazoans. Curr Opin Genet Dev 12(6):640–649
Egelkrout E, Rajan V, Howard JA (2012) Overproduction of recombinant proteins in plants. Plant Sci 184:83–101. doi:10.1016/j.plantsci.2011.12.005
Evans TC Jr, Xu MQ, Pradhan S (2005) Protein splicing elements and plants: from transgene containment to protein purification. Annu Rev Plant Biol 56:375–392. doi:10.1146/annurev.arplant.56.032604.144242
Evron T, Geyer BC, Cherni I, Muralidharan M, Kilbourne J, Fletcher SP, Soreq H, Mor TS (2007) Plant-derived human acetylcholinesterase-R provides protection from lethal organophosphate poisoning and its chronic aftermath. The FASEB J 21(11):2961–2969. doi:10.1096/fj.07-8112com
Farrell RE (2007) The regulation of gene expression in plants and animals. In: Bassett CL (ed) Regulation of gene expression in plants: the role of transcript structure and processing. Springer, Boston, pp 1–38
Faye L, Gomord V (2010) Success stories in molecular farming-a brief overview. Plant Biotechnol J 8(5):525–528. doi:10.1111/j.1467-7652.2010.00521.x
Fernandez-San Millan A, Mingo-Castel A, Miller M, Daniell H (2003) A chloroplast transgenic approach to hyper-express and purify human serum albumin, a protein highly susceptible to proteolytic degradation. Plant Biotechnol J 1(2):71–79. doi:10.1046/j.1467-7652.2003.00008.x
Fischer R, Emans N (2000) Molecular farming of pharmaceutical proteins. Transgenic Res 9(4–5):279–299 (discussion 277)
Fischer R, Schumann D, Zimmermann S, Drossard J, Sack M, Schillberg S (1999) Expression and characterization of bispecific single-chain Fv fragments produced in transgenic plants. Eur J Biochem 262(3):810–816
Fischer R, Schillberg S, Hellwig S, Twyman RM, Drossard J (2012) GMP issues for recombinant plant-derived pharmaceutical proteins. Biotechnol Adv 30(2):434–439. doi:10.1016/j.biotechadv.2011.08.007
Fletcher SP, Muto M, Mayfield SP (2007) Optimization of recombinant protein expression in the chloroplasts of green algae. Adv Exp Med Biol 616:90–98. doi:10.1007/978-0-387-75532-8_8
Franklin S, Ngo B, Efuet E, Mayfield SP (2002) Development of a GFP reporter gene for Chlamydomonas reinhardtii chloroplast. Plant J 30(6):733–744
Gaspar P, Moura G, Santos MAS, Oliveira JL (2013) mRNA secondary structure optimization using a correlated stem–loop prediction. Nucleic Acids Res. doi:10.1093/nar/gks1473
Giddings G, Allison G, Brooks D, Carter A (2000) Transgenic plants as factories for biopharmaceuticals. Nat Biotechnol 18(11):1151–1155. doi:10.1038/81132
Gion WR, Davis-Taber RA, Regier DA, Fung E, Medina L, Santora LC, Bose S, Ivanov AV, Perilli-Palmer BA, Chumsae CM, Matuck JG, Kunes YZ, Carson GR (2013) Expression of antibodies using single open reading frame (sORF) vector design: demonstration of manufacturing feasibility. mAbs 5(4):595–607. doi:10.4161/mabs.25161
Giritch A, Marillonnet S, Engler C, van Eldik G, Botterman J, Klimyuk V, Gleba Y (2006) Rapid high-yield expression of full-size IgG antibodies in plants coinfected with noncompeting viral vectors. Proc Natl Acad Sci USA 103(40):14701–14706. doi:10.1073/pnas.0606631103
Gisby MF, Mellors P, Madesis P, Ellin M, Laverty H, O’Kane S, Ferguson MW, Day A (2011) A synthetic gene increases TGFbeta3 accumulation by 75-fold in tobacco chloroplasts enabling rapid purification and folding into a biologically active molecule. Plant Biotechnol J 9(5):618–628. doi:10.1111/j.1467-7652.2011.00619.x
Gitzinger M, Parsons J, Reski R, Fussenegger M (2009) Functional cross-kingdom conservation of mammalian and moss (Physcomitrella patens) transcription, translation and secretion machineries. Plant Biotechnol J 7(1):73–86. doi:10.1111/j.1467-7652.2008.00376.x
Gould N, Hendy O, Papamichail D (2014) Computational tools and algorithms for designing customized synthetic genes. Front Bioeng Biotechnol 2:41. doi:10.3389/fbioe.2014.00041
Goulet C, Benchabane M, Anguenot R, Brunelle F, Khalf M, Michaud D (2010) A companion protease inhibitor for the protection of cytosol-targeted recombinant proteins in plants. Plant Biotechnol J 8(2):142–154. doi:10.1111/j.1467-7652.2009.00470.x
Grabowski GA, Golembo M, Shaaltiel Y (2014) Taliglucerase alfa: an enzyme replacement therapy using plant cell expression technology. Mol Genet Metab 112(1):1–8. doi:10.1016/j.ymgme.2014.02.011
Gray BN, Ahner BA, Hanson MR (2009) High-level bacterial cellulase accumulation in chloroplast-transformed tobacco mediated by downstream box fusions. Biotechnol Bioeng 102(4):1045–1054. doi:10.1002/bit.22156
Greer AL (2015) Early vaccine availability represents an important public health advance for the control of pandemic influenza. BMC Res Notes 8(1):191. doi:10.1186/s13104-015-1157-1
Gustafsson C, Govindarajan S, Minshull J (2004) Codon bias and heterologous protein expression. Trends Biotechnol 22(7):346–353. doi:10.1016/j.tibtech.2004.04.006
Habibi P, Grossi de Sa MF, Makhzoum A, Malik S, da Silva ALL, Hefferon K, Soccol CR (2017) Bioengineering hairy roots: phytoremediation, secondary metabolism, molecular pharming, plant-plant interactions and biofuels. In: Lichtfouse E (ed) Sustainable agriculture reviews. Springer, Cham, pp 213–251
Hauptmann V, Weichert N, Menzel M, Knoch D, Paege N, Scheller J, Spohn U, Conrad U, Gils M (2013) Native-sized spider silk proteins synthesized in planta via intein-based multimerization. Transgenic Res 22(2):369–377. doi:10.1007/s11248-012-9655-6
He J, Lai H, Brock C, Chen Q (2012) A novel system for rapid and cost-effective production of detection and diagnostic reagents of West Nile virus in plants. J Biomed Biotechnol 2012:106783. doi:10.1155/2012/106783
Hefferon KL (2012) Plant virus expression vectors set the stage as production platforms for biopharmaceutical proteins. Virology 433(1):1–6. doi:10.1016/j.virol.2012.06.012
Heitzer M, Eckert A, Fuhrmann M, Griesbeck C (2007) Influence of codon bias on the expression of foreign genes in microalgae. Adv Exp Med Biol 616:46–53. doi:10.1007/978-0-387-75532-8_5
Hennecke M, Kwissa M, Metzger K, Oumard A, Kroger A, Schirmbeck R, Reimann J, Hauser H (2001) Composition and arrangement of genes define the strength of IRES-driven translation in bicistronic mRNAs. Nucleic Acids Res 29(16):3327–3334
Herz S, Fussl M, Steiger S, Koop HU (2005) Development of novel types of plastid transformation vectors and evaluation of factors controlling expression. Transgenic Res 14(6):969–982. doi:10.1007/s11248-005-2542-7
Hieno A, Naznin HA, Hyakumachi M, Sakurai T, Tokizawa M, Koyama H, Sato N, Nishiyama T, Hasebe M, Zimmer AD, Lang D, Reski R, Rensing SA, Obokata J, Yamamoto YY (2014) ppdb: plant promoter database version 3.0. Nucleic Acids Res 42(database issue):D1188–D1192. doi:10.1093/nar/gkt1027
Horvath H, Huang J, Wong O, Kohl E, Okita T, Kannangara CG, von Wettstein D (2000) The production of recombinant proteins in transgenic barley grains. Proc Natl Acad Sci USA 97(4):1914–1919. doi:10.1073/pnas.030527497
Houdebine LM, Attal J (1999) Internal ribosome entry sites (IRESs): reality and use. Transgenic Res 8(3):157–177
Huang Z, Chen Q, Hjelm B, Arntzen C, Mason H (2009) A DNA replicon system for rapid high-level production of virus-like particles in plants. Biotechnol Bioeng 103(4):706–714. doi:10.1002/bit.22299
Huang Z, Phoolcharoen W, Lai H, Piensook K, Cardineau G, Zeitlin L, Whaley KJ, Arntzen CJ, Mason HS, Chen Q (2010) High-level rapid production of full-size monoclonal antibodies in plants by a single-vector DNA replicon system. Biotechnol Bioeng 106(1):9–17. doi:10.1002/bit.22652
Ikemura T (1981) Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes: a proposal for a synonymous codon choice that is optimal for the E. coli translational system. J Mol Biol 151(3):389–409
Irwin B, Heck JD, Hatfield GW (1995) Codon pair utilization biases influence translational elongation step times. J Biol Chem 270(39):22801–22806
Ishikura K, Takaoka Y, Kato K, Sekine M, Yoshida K, Shinmyo A (1999) Expression of a foreign gene in Chlamydomonas reinhardtii chloroplast. J Biosci Bioeng 87(3):307–314. doi:10.1016/S1389-1723(99)80037-1
James EA, Wang C, Wang Z, Reeves R, Shin JH, Magnuson NS, Lee JM (2000) Production and characterization of biologically active human GM-CSF secreted by genetically modified plant cells. Protein Expr Purif 19(1):131–138. doi:10.1006/prep.2000.1232
Kang HG, Fang Y, Singh KB (1999) A glucocorticoid-inducible transcription system causes severe growth defects in Arabidopsis and induces defense-related genes. Plant J 20(1):127–133
Kang MS, Prasanta KS, Baisakh N, Priyadarshan PM (2008) Crop breeding methodologies: classic and modern. In: Kang MS, Priyadarshan PM (eds) Breeding major food staples. Wiley, Hoboken, pp 5–40
Keasling JD (2012) Synthetic biology and the development of tools for metabolic engineering. Metab Eng 14(3):189–195. doi:10.1016/j.ymben.2012.01.004
Kim M-Y, Yang M-S, Kim T-G (2009) Expression of dengue virus E glycoprotein domain III in non-nicotine transgenic tobacco plants. Biotechnol Bioprocess Eng 14(6):725–730 doi:10.1007/s12257-009-3011-6
Kim HJ, Lee SJ, Kim HJ (2010) Optimizing the secondary structure of human papillomavirus type 16 L1 mRNA enhances L1 protein expression in Saccharomyces cerevisiae. J Biotechnol 150(1):31–36. doi:10.1016/j.jbiotec.2010.07.032
Kim Y, Lee G, Jeon E, Sohn EJ, Lee Y, Kang H, Lee DW, Kim DH, Hwang I (2014) The immediate upstream region of the 5′-UTR from the AUG start codon has a pronounced effect on the translational efficiency in Arabidopsis thaliana. Nucleic Acids Res 42(1):485–498. doi:10.1093/nar/gkt864
Kircheis R, Halanek N, Koller I, Jost W, Schuster M, Gorr G, Hajszan K, Nechansky A (2012) Correlation of ADCC activity with cytokine release induced by the stably expressed, glyco-engineered humanized Lewis Y-specific monoclonal antibody MB314. mAbs 4(4):532–541. doi:10.4161/mabs.20577
Kohli N, Westerveld DR, Ayache AC, Verma A, Shil P, Prasad T, Zhu P, Chan SL, Li Q, Daniell H (2014) Oral delivery of bioencapsulated proteins across blood-brain and blood-retinal barriers. Mol Ther 22(3):535–546. doi:10.1038/mt.2013.273
Komarova TV, Baschieri S, Donini M, Marusic C, Benvenuto E, Dorokhov YL (2010) Transient expression systems for plant-derived biopharmaceuticals. Expert Rev Vaccines 9(8):859–876. doi:10.1586/erv.10.85
Koul B, Yadav R, Sanyal I, Sawant S, Sharma V, Amla DV (2012) Cis-acting motifs in artificially synthesized expression cassette leads to enhanced transgene expression in tomato (Solanum lycopersicum L.). PPB 61:131–141 doi:10.1016/j.plaphy.2012.09.014
Kozak M (1999) Initiation of translation in prokaryotes and eukaryotes. Gene 234(2):187–208
Kubo M, Imai A, Nishiyama T, Ishikawa M, Sato Y, Kurata T, Hiwatashi Y, Reski R, Hasebe M (2013) System for stable β-estradiol-inducible gene expression in the moss Physcomitrella patens. PLoS ONE 8(9):e77356. doi:10.1371/journal.pone.0077356
Kunes YZ, Gion WR, Fung E, Salfeld JG, Zhu RR, Sakorafas P, Carson GR (2009) Expression of antibodies using single-open reading frame vector design and polyprotein processing from mammalian cells. Biotechnol Prog 25(3):735–744. doi:10.1002/btpr.182
Kwon K-C, Chan H-T, León IR, Williams-Carrier R, Barkan A, Daniell H (2016) Codon optimization to enhance expression yields insights into chloroplast translation. Plant Physiol 172(1):62–77. doi:10.1104/pp.16.00981
Laguía-Becher M, Martín V, Kraemer M, Corigliano M, Yacono ML, Goldman A, Clemente M (2010) Effect of codon optimization and subcellular targeting on Toxoplasma gondii antigen SAG1 expression in tobacco leaves to use in subcutaneous and oral immunization in mice. BMC Biotechnol 10(1):1–14. doi:10.1186/1472-6750-10-52
Lai H, Chen Q (2012) Bioprocessing of plant-derived virus-like particles of Norwalk virus capsid protein under current good manufacture practice regulations. Plant Cell Rep 31(3):573–584. doi:10.1007/s00299-011-1196-6
Lai H, Engle M, Fuchs A, Keller T, Johnson S, Gorlatov S, Diamond MS, Chen Q (2010) Monoclonal antibody produced in plants efficiently treats West Nile virus infection in mice. Proc Natl Acad Sci USA 107(6):2419–2424. doi:10.1073/pnas.0914503107
Lakshmi PS, Verma D, Yang X, Lloyd B, Daniell H (2013) Low cost tuberculosis vaccine antigens in capsules: expression in chloroplasts, bio-encapsulation, stability and functional evaluation in vitro. PLoS ONE 8(1):e54708. doi:10.1371/journal.pone.0054708
Lee ER, Baker JL, Weinberg Z, Sudarsan N, Breaker RR (2010) An allosteric self-splicing ribozyme triggered by a bacterial second messenger. Science 329(5993):845–848. doi:10.1126/science.1190713
Leelavathi S, Gupta N, Maiti S, Ghosh A, Reddy VS (2003) Overproduction of an alkali- and thermo-stable xylanase in tobacco chloroplasts and efficient recovery of the enzyme. Mol Breed 11(1):59–67. doi:10.1023/A:1022168321380
Li H, Yang J, Chen Y, Guan L, Du L, Guo Y, Wang W, Wang L, Li H, Jiang C, Li X (2014) Expression of a functional recombinant oleosin-human hyaluronidase hPH-20 fusion in Arabidopsis thaliana. Protein Expr Purif 103:23–27. doi:10.1016/j.pep.2014.03.007
Lico C, Chen Q, Santi L (2008) Viral vectors for production of recombinant proteins in plants. J Cell Physiol 216(2):366–377. doi:10.1002/jcp.21423
Lindgreen S (2012) Codon evolution: mechanisms and models. Trends Evol Biol 4(1):8. doi:10.4081/eb.2012.e8
Liu Q, Xue Q (2005) Comparative studies on codon usage pattern of chloroplasts and their host nuclear genes in four plant species. J Genet 84(1):55–62
Lopez-Lastra M, Rivas A, Barria MI (2005) Protein synthesis in eukaryotes: the growing biological relevance of cap-independent translation initiation. Biol Res 38(2–3):121–146
Lu J, Sivamani E, Azhakanandam K, Samadder P, Li X, Qu R (2008) Gene expression enhancement mediated by the 5′ UTR intron of the rice rubi3 gene varied remarkably among tissues in transgenic rice plants. Mol Genet Genom 279(6):563–572. doi:10.1007/s00438-008-0333-6
Luke GA, Roulston C, Tilsner J. Ryan MD (2015) Growing uses of 2A in plant biotechnology. In: Ekinci D (ed) Biotechnology. InTech Croatia, Rijeka, pp 165–193
Mallory AC, Parks G, Endres MW, Baulcombe D, Bowman LH, Pruss GJ, Vance VB (2002) The amplicon-plus system for high-level expression of transgenes in plants. Nat Biotechnol 20(6):622–625. doi:10.1038/nbt0602-622
Mann DGJ, King ZR, Liu W, Joyce BL, Percifield RJ, Hawkins JS, LaFayette PR, Artelt BJ, Burris JN, Mazarei M, Bennetzen JL, Parrott WA, Stewart CN (2011) Switchgrass (Panicum virgatum L.) polyubiquitin gene (PvUbi1 and PvUbi2) promoters for use in plant transformation. BMC Biotechnol 11:74–74. doi:10.1186/1472-6750-11-74
Mao Y, Liu H, Liu Y, Tao S (2014) Deciphering the rules by which dynamics of mRNA secondary structure affect translation efficiency in Saccharomyces cerevisiae. Nucleic Acids Res. doi:10.1093/nar/gku159
Michelet L, Lefebvre-Legendre L, Burr SE, Rochaix JD, Goldschmidt-Clermont M (2011) Enhanced chloroplast transgene expression in a nuclear mutant of Chlamydomonas. Plant Biotechnol J 9(5):565–574. doi:10.1111/j.1467-7652.2010.00564.x
Mohammadzadeh S, Roohvand F, Ajdary S, Ehsani P, Hatef Salmanian A (2015) Heterologous expression of hepatitis C virus core protein in oil seeds of Brassica napus L. Jundishapur. J Microbiol 8(11):e25462. doi:10.5812/jjm.25462
Monreal-Escalante E, Banuelos-Hernandez B, Hernandez M, Fragoso G, Garate T, Sciutto E, Rosales-Mendoza S (2015) Expression of multiple taenia solium immunogens in plant cells through a ribosomal skip mechanism. Mol Biotechnol 57(7):635–643. doi:10.1007/s12033-015-9853-6
Morandini P (2013) Control limits for accumulation of plant metabolites: brute force is no substitute for understanding. Plant Biotechnol J 11(2):253–67. doi:10.1111/pbi.12035
Morello L, Gianì S, Troina F, Breviario D (2011) Testing the IMEter on rice introns and other aspects of intron-mediated enhancement of gene expression. J Exp Bot 62(2):533–544. doi:10.1093/jxb/erq273
Moustafa K, Makhzoum A, Tremouillaux-Guiller J (2015) Molecular farming on rescue of pharma industry for next generations. Crit Rev Biotechnol. doi:10.3109/07388551.2015.1049934
Mozes-Koch R, Gover O, Tanne E, Peretz Y, Maori E, Chernin L, Sela I (2012) Expression of an entire bacterial operon in plants. Plant Physiol 158(4):1883–1892. doi:10.1104/pp.111.186197
Mueller S, Coleman JR, Papamichail D, Ward CB, Nimnual A, Futcher B, Skiena S, Wimmer E (2010) Live attenuated influenza virus vaccines by computer-aided rational design. Nat Biotechnol 28(7):723–726. doi:10.1038/nbt0.1636
Muller K, Siegel D, Rodriguez Jahnke F, Gerrer K, Wend S, Decker EL, Reski R, Weber W, Zurbriggen MD (2014) A red light-controlled synthetic gene expression switch for plant systems. Mol Biosyst 10(7):1679–1688. doi:10.1039/c3mb70579j
Naqvi S, Farre G, Sanahuja G, Capell T, Zhu C, Christou P (2010) When more is better: multigene engineering in plants. Trends Plant Sci 15(1):48–56. doi:10.1016/j.tplants.2009.09.010
Niederkrüger H, Dabrowska-Schlepp P, Schaaf A (2014) Suspension culture of plant cells under phototrophic conditions industrial scale suspension culture of living cells. Wiley, New York, pp 259–292
Nochi T, Takagi H, Yuki Y, Yang L, Masumura T, Mejima M, Nakanishi U, Matsumura A, Uozumi A, Hiroi T, Morita S, Tanaka K, Takaiwa F, Kiyono H (2007) Rice-based mucosal vaccine as a global strategy for cold-chain- and needle-free vaccination. Proc Natl Acad Sci USA 104(26):10986–10991. doi:10.1073/pnas.0703766104
Nuttall J, Vine N, Hadlington JL, Drake P, Frigerio L, Ma JK (2002) ER-resident chaperone interactions with recombinant antibodies in transgenic plants. Eur J Biochem 269(24):6042–6051
O’Brien KM, Schufreider AK, McGill MA, O’Brien KM, Reitter JN, Mills KV (2010) Mechanism of protein splicing of the Pyrococcus abyssi lon protease intein. Biochem Biophys Res Commun 403(3–4):457–461. doi:10.1016/j.bbrc.2010.11.055
O’Keefe BR, Murad AM, Vianna GR, Ramessar K, Saucedo CJ, Wilson J, Buckheit KW, da Cunha NB, Araújo ACG, Lacorte CC, Madeira L, McMahon JB, Rech EL (2015) Engineering soya bean seeds as a scalable platform to produce cyanovirin-N, a non-ARV microbicide against HIV. Plant Biotechnol J 13(7):884–892. doi:10.1111/pbi.12309
Obembe OO, Popoola JO, Leelavathi S, Reddy SV (2011) Advances in plant molecular farming. Biotechnol Adv 29(2):210–222. doi:10.1016/j.biotechadv.2010.11.004
Orellana-Escobedo L, Rosales-Mendoza S, Romero-Maldonado A, Parsons J, Decker EL, Monreal-Escalante E, Moreno-Fierros L, Reski R (2015) An Env-derived multi-epitope HIV chimeric protein produced in the moss Physcomitrella patens is immunogenic in mice. Plant Cell Rep 34(3):425–433. doi:10.1007/s00299-014-1720-6
Osbourn AE, Field B (2009) Operons. Cell Mol Life Sci 66(23):3755–3775. doi:10.1007/s00018-009-0114-3
Palidwor GA, Perkins TJ, Xia X (2010) A general model of codon bias due to GC mutational bias. PLoS ONE 5(10):e13431. doi:10.1371/journal.pone.0013431
Park C, Chen X, Yang J-R, Zhang J (2013) Differential requirements for mRNA folding partially explain why highly expressed proteins evolve slowly. Proc Natl Acad Sci USA 110(8):E678–E686. doi:10.1073/pnas.1218066110
Parsons J, Altmann F, Arrenberg CK, Koprivova A, Beike AK, Stemmer C, Gorr G, Reski R, Decker EL (2012) Moss-based production of asialo-erythropoietin devoid of Lewis A and other plant-typical carbohydrate determinants. Plant Biotechnol J 10(7):851–861. doi:10.1111/j.1467-7652.2012.00704.x
Paul M, van Dolleweerd C, Drake PM, Reljic R, Thangaraj H, Barbi T, Stylianou E, Pepponi I, Both L, Hehle V, Madeira L, Inchakalody V, Ho S, Guerra T, Ma JK (2011) Molecular pharming: future targets and aspirations. Hum Vaccin 7(3):375–382
Paul MJ, Teh AY, Twyman RM, Ma JK (2013) Target product selection: where can molecular pharming make the difference? Curr Pharm Des 19(31):5478–5485
Penchovsky R, Stoilova CC (2013) Riboswitch-based antibacterial drug discovery using high-throughput screening methods. Expert Opin Drug Discov 8(1):65–82. doi:10.1517/17460441.2013.740455
Phoolcharoen W, Bhoo SH, Lai H, Ma J, Arntzen CJ, Chen Q, Mason HS (2011) Expression of an immunogenic Ebola immune complex in Nicotiana benthamiana. Plant Biotechnol J 9(7):807–816. doi:10.1111/j.1467-7652.2011.00593.x
Pillay P, Schluter U, van Wyk S, Kunert KJ, Vorster BJ (2014) Proteolysis of recombinant proteins in bioengineered plant cells. Bioengineered 5(1):15–20. doi:10.4161/bioe.25158
Plotkin JB, Kudla G (2011) Synonymous but not the same: the causes and consequences of codon bias. Nat Rev Genet 12(1):32–42
Pogue GP, Vojdani F, Palmer KE, Hiatt E, Hume S, Phelps J, Long L, Bohorova N, Kim D, Pauly M, Velasco J, Whaley K, Zeitlin L, Garger SJ, White E, Bai Y, Haydon H, Bratcher B (2010) Production of pharmaceutical-grade recombinant aprotinin and a monoclonal antibody product using plant-based transient expression systems. Plant Biotechnol J 8(5):638–654
Poirier Y (2001) Production of polyesters in transgenic plants. In: Babel W, Steinbüchel A (eds) Biopolyesters. Springer, Berlin, pp 209–240
Rademacher T, Sack M, Arcalis E, Stadlmann J, Balzer S, Altmann F, Quendler H, Stiegler G, Kunert R, Fischer R, Stoger E (2008) Recombinant antibody 2G12 produced in maize endosperm efficiently neutralizes HIV-1 and contains predominantly single-GlcNAc N-glycans. Plant Biotechnol J 6(2):189–201. doi:10.1111/j.1467-7652.2007.00306.x
Ramirez N, Lorenzo D, Palenzuela D, Herrera L, Ayala M, Fuentes A, Perez M, Gavilondo J, Oramas P (2000) Single-chain antibody fragments specific to the hepatitis B surface antigen, produced in recombinant tobacco cell cultures. Biotechnol Lett 22(15):1233–1236. doi:10.1023/A:1005605214703
Reski R, Parsons J, Decker EL (2015) Moss-made pharmaceuticals: from bench to bedside. Plant Biotechnol J 13(8):1191–1198. doi:10.1111/pbi.12401
Rigano MM, Alvarez ML, Pinkhasov J, Jin Y, Sala F, Arntzen CJ, Walmsley AM (2004) Production of a fusion protein consisting of the enterotoxigenic Escherichia coli heat-labile toxin B subunit and a tuberculosis antigen in Arabidopsis thaliana. Plant Cell Rep 22(7):502–508. doi:10.1007/s00299-003-0718-2
Ronald PC (2007) Plant-pathogen interactions: methods and protocols, vol 354. Human Press, Totowa
Rosales-Mendoza S, Soria-Guerra RE, Moreno-Fierros L, Alpuche-Solis AG, Martinez-Gonzalez L, Korban SS (2010) Expression of an immunogenic F1-V fusion protein in lettuce as a plant-based vaccine against plague. Planta 232(2):409–416. doi:10.1007/s00425-010-1176-z
Rose AB (2008) Intron-mediated regulation of gene expression. Curr Top Microbiol Immunol 326:277–290
Rose AB, Emami S, Bradnam K, Korf I (2011) Evidence for a DNA-based mechanism of intron-mediated enhancement. Front Plant Sci 2:98. doi:10.3389/fpls.2011.00098
Schillberg S, Zimmermann S, Voss A, Fischer R (1999) Apoplastic and cytosolic expression of full-size antibodies and antibody fragments in Nicotiana tabacum. Transgenic Res 8(4):255–263. doi:10.1023/A:1008937011213
Schünmann PHD, Surin B, Waterhouse PM (2003) A suite of novel promoters and terminators for plant biotechnology. II. The pPLEX series for use in monocots. Funct Plant Biol 30(4):453–460. doi:10.1071/FP02167
Serres-Giardi L, Belkhir K, David J, Glemin S (2012) Patterns and evolution of nucleotide landscapes in seed plants. Plant Cell 24(4):1379–1397. doi:10.1105/tpc.111.093674
Sharp PM, Li WH (1987) The codon adaptation index: a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res 15(3):1281–1295
Sharp PM, Tuohy TM, Mosurski KR (1986) Codon usage in yeast: cluster analysis clearly differentiates highly and lowly expressed genes. Nucleic Acids Res 14(13):5125–5143
Shinmyo A, Kato K (2010) Molecular farming: production of drugs and vaccines in higher plants. J Antibiot 63(8):431–433
Sijmons PC, Dekker BM, Schrammeijer B, Verwoerd TC, van den Elzen PJ, Hoekema A (1990) Production of correctly processed human serum albumin in transgenic plants. Nat Biotechnol 8(3):217–221
Silverman IM, Li F, Gregory BD (2013) Genomic era analyses of RNA secondary structure and RNA-binding proteins reveal their significance to post-transcriptional regulation in plants. Plant Sci 205–206:55–62. doi:10.1016/j.plantsci.2013.01.009
Simon AE, Miller WA (2013) 3′ cap-independent translation enhancers of plant viruses. Annu Rev Microbiol 67:21–42. doi:10.1146/annurev-micro-092412-155609
Specht EA, Mayfield SP (2014) Algae-based oral recombinant vaccines. Front Microbiol 5:60. doi:10.3389/fmicb.2014.00060
Specht E, Miyake-Stoner S, Mayfield S (2010) Micro-algae come of age as a platform for recombinant protein production. Biotechnol Lett 32(10):1373–1383. doi:10.1007/s10529-010-0326-5
Staub JM, Garcia B, Graves J, Hajdukiewicz PT, Hunter P, Nehra N, Paradkar V, Schlittler M, Carroll JA, Spatola L, Ward D, Ye G, Russell DA (2000) High-yield production of a human therapeutic protein in tobacco chloroplasts. Nat Biotechnol 18(3):333–338. doi:10.1038/73796
Stavolone L, Kononova M, Pauli S, Ragozzino A, de Haan P, Milligan S, Lawton K, Hohn T (2003) Cestrum yellow leaf curling virus (CmYLCV) promoter: a new strong constitutive promoter for heterologous gene expression in a wide variety of crops. Plant Mol Biol 53(5):663–673. doi:10.1023/B:PLAN.0000019110.95420.bb
Stern DB, Goldschmidt-Clermont M, Hanson MR (2010) Chloroplast RNA metabolism. Annu Rev Plant Biol 61:125–155. doi:10.1146/annurev-arplant-042809-112242
Stoger E, Fischer R, Moloney M, Ma JK (2014) Plant molecular pharming for the treatment of chronic and infectious diseases. Annu Rev Plant Biol 65:743–768. doi:10.1146/annurev-arplant-050213-035850
Sun Y, Ye SH, Lu HW (2012) Study of RNA secondary structure prediction algorithms. In: Advanced materials research, vol 393. Trans Tech Publications, Zurich, pp 955–960
Sunil Kumar GB, Ganapathi TR, Srinivas L, Revathi CJ, Bapat VA (2006) Expression of hepatitis B surface antigen in potato hairy roots. Plant Sci 170(5):918–925. doi:10.1016/j.plantsci.2005.12.015
Suzuki H, Saito R, Tomita M (2004) The ‘weighted sum of relative entropy’: a new index for synonymous codon usage bias. Gene 335:19–23. doi:10.1016/j.gene.2004.03.001
Suzuki K, Yang L, Takaiwa F (2012) Transgenic rice accumulating modified cedar pollen allergen Cry j 2 derivatives. J Biosci Bioeng 113(2):249–251. doi:10.1016/j.jbiosc.2011.10.005
Tacket CO, Pasetti MF, Edelman R, Howard JA, Streatfield S (2004) Immunogenicity of recombinant LT-B delivered orally to humans in transgenic corn. Vaccine 22(31–32):4385–4389. doi:10.1016/j.vaccine.2004.01.073
Takagi H, Hiroi T, Yang L, Tada Y, Yuki Y, Takamura K, Ishimitsu R, Kawauchi H, Kiyono H, Takaiwa F (2005) A rice-based edible vaccine expressing multiple T cell epitopes induces oral tolerance for inhibition of Th2-mediated IgE responses. Proc Natl Acad Sci USA 102(48):17525–17530. doi:10.1073/pnas.0503428102
Takagi H, Hiroi T, Hirose S, Yang L, Takaiwa F (2010) Rice seed ER-derived protein body as an efficient delivery vehicle for oral tolerogenic peptides. Peptides 31(8):1421–1425. doi:10.1016/j.peptides.2010.04.032
Takaiwa F (2009) Allergen-specific immunotherapy with plant-based oral vaccines. Immunotherapy 1(4):517–9. doi:10.2217/imt.09.28
Takaiwa F (2011) Seed-based oral vaccines as allergen-specific immunotherapies. Hum Vaccin 7(3):357–366
Tang L, Cui T, Wu JJ, Liu-Mares W, Huang N, Li J (2010) A rice-derived recombinant human lactoferrin stimulates fibroblast proliferation, migration, and sustains cell survival. Wound Repair Regen 18(1):123–131. doi:10.1111/j.1524-475X.2009.00563.x
Thomas DR, Walmsley AM (2015) The effect of the unfolded protein response on the production of recombinant proteins in plants. Plant Cell Rep 34(2):179–187. doi:10.1007/s00299-014-1680-x
Thuenemann EC, Meyers AE, Verwey J, Rybicki EP, Lomonossoff GP (2013) A method for rapid production of heteromultimeric protein complexes in plants: assembly of protective bluetongue virus-like particles. Plant Biotechnol J 11(7):839–846. doi:10.1111/pbi.12076
Torrent M, Llompart B, Lasserre-Ramassamy S, Llop-Tous I, Bastida M, Marzabal P, Westerholm-Parvinen A, Saloheimo M, Heifetz PB, Ludevid MD (2009) Eukaryotic protein production in designed storage organelles. BMC Biol 7:5. doi:10.1186/1741-7007-7-5
Torres E, Vaquero C, Nicholson L, Sack M, Stoger E, Drossard J, Christou P, Fischer R, Fischer R, Perrin Y (1999) Rice cell culture as an alternative production system for functional diagnostic and therapeutic antibodies. Transgenic Res 8(6):441–449. doi:10.1023/A:1008969031219
Tregoning JS, Nixon P, Kuroda H, Svab Z, Clare S, Bowe F, Fairweather N, Ytterberg J, van Wijk KJ, Dougan G, Maliga P (2003) Expression of tetanus toxin fragment C in tobacco chloroplasts. Nucleic Acids Res 31(4):1174–1179. doi:10.1093/nar/gkg221
Twyman RM, Schillberg S, Fischer R (2013) Optimizing the yield of recombinant pharmaceutical proteins in plants. Curr Pharm Des 19(31):5486–5494
Twyman RM, Stoger E, Schillberg S, Christou P, Fischer R (2003) Molecular farming in plants: host systems and expression technology. Trends Biotechnol 21(12):570–578. doi:10.1016/j.tibtech.2003.10.002
Ullrich KK, Hiss M, Rensing SA (2015) Means to optimize protein expression in transgenic plants. Curr Opin Biotechnol 32:61–67. doi:10.1016/j.copbio.2014.11.011
Vamvaka E, Arcalis E, Ramessar K, Evans A, O’Keefe BR, Shattock RJ, Medina V, Stöger E, Christou P, Capell T (2016a) Rice endosperm is cost-effective for the production of recombinant griffithsin with potent activity against HIV. Plant Biotechnol J 14(6):1427–1437. doi:10.1111/pbi.12507
Vamvaka E, Twyman RM, Murad AM, Melnik S, Teh AY-H, Arcalis E, Altmann F, Stoger E, Rech E, Ma JKC, Christou P, Capell T (2016b) Rice endosperm produces an underglycosylated and potent form of the HIV-neutralizing monoclonal antibody 2G12. Plant Biotechnol J 14(1):97–108. doi:10.1111/pbi.12360
Wachter A (2014) Gene regulation by structured mRNA elements. Trends Genet 30(5):172–181. doi:10.1016/j.tig.2014.03.001
Wachter A, Tunc-Ozdemir M, Grove BC, Green PJ, Shintani DK, Breaker RR (2007) Riboswitch control of gene expression in plants by splicing and alternative 3′ end processing of mRNAs. Plant Cell 19(11):3437–3450. doi:10.1105/tpc.107.053645
Wan XF, Xu D, Kleinhofs A, Zhou J (2004) Quantitative relationship between synonymous codon usage bias and GC composition across unicellular genomes. BMC Evol Biol 4:19. doi:10.1186/1471-2148-4-19
Wang L, Wessler SR (2001) Role of mRNA secondary structure in translational repression of the maize transcriptional activator Lc(1,2). Plant Physiol 125(3):1380–1387
Wang DM, Zhu JB, Peng M, Zhou P (2008) Induction of a protective antibody response to FMDV in mice following oral immunization with transgenic Stylosanthes spp. as a feedstuff additive. Transgenic Res 17(6):1163–1170. doi:10.1007/s11248-008-9188-1
Weise A, Rodriguez-Franco M, Timm B, Hermann M, Link S, Jost W, Gorr G (2006) Use of Physcomitrella patens actin 5′ regions for high transgene expression: importance of 5′ introns. Adv Appl Microbiol 70(3):337–345. doi:10.1007/s00253-005-0087-6
Winkler WC, Nahvi A, Roth A, Collins JA, Breaker RR (2004) Control of gene expression by a natural metabolite-responsive ribozyme. Nature 428(6980):281–286
Wolfson W (2013) Grow your own: protalix biotherapeutics produces drugs in carrot cells. Chemistry Biology 20(8):969–970. doi:10.1016/j.chembiol.2013.08.003
Woodrow KA, Bennett KM, Lo DD (2012) Mucosal vaccine design and delivery. Annu Rev Biomed Eng 14:17–46. doi:10.1146/annurev-bioeng-071811-150054
Xie P (2015) Dwell-time distribution, long pausing and arrest of single-ribosome translation through the mRNA duplex. Int J Mol Sci 16(10):23723–23744. doi:10.3390/ijms161023723
Yang J, Barr LA, Fahnestock SR, Liu ZB (2005) High yield recombinant silk-like protein production in transgenic plants through protein targeting. Transgenic Res 14(3):313–324
Yang J, Guan L, Guo Y, Du L, Wang F, Wang Y, Zhen L, Wang Q, Zou D, Chen W, Yu L, Li H, Li X (2015) Expression of biologically recombinant human acidic fibroblast growth factor in Arabidopsis thaliana seeds via oleosin fusion technology. Gene 566(1):89–94. doi:10.1016/j.gene.2015.04.036
Youm JW, Jeon JH, Kim H, Kim YH, Ko K, Joung H, Kim H (2008) Transgenic tomatoes expressing human beta-amyloid for use as a vaccine against Alzheimer’s disease. Biotechnol Lett 30(10):1839–1845. doi:10.1007/s10529-008-9759-5
Zhang D, Nandi S, Bryan P, Pettit S, Nguyen D, Santos MA, Huang N (2010) Expression, purification, and characterization of recombinant human transferrin from rice (Oryza sativa L.). Protein Expr Purif 74(1):69–79. doi:10.1016/j.pep.2010.04.019
Zimran A, Brill-Almon E, Chertkoff R, Petakov M, Blanco-Favela F, Munoz ET, Solorio-Meza SE, Amato D, Duran G, Giona F, Heitner R, Rosenbaum H, Giraldo P, Mehta A, Park G, Phillips M, Elstein D, Altarescu G, Szleifer M, Hashmueli S, Aviezer D (2011) Pivotal trial with plant cell-expressed recombinant glucocerebrosidase, taliglucerase alfa, a novel enzyme replacement therapy for Gaucher disease. Blood 118(22):5767–5773. doi:10.1182/blood-2011-07-366955
Zingaro KA, Papoutsakis ET (2012) Toward a semisynthetic stress response system to engineer microbial solvent tolerance. MBio. doi:10.1128/mBio.00308-12
Acknowledgements
The authors thank EMBRAPA, CNPq, CAPES, and FAP-DF.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that the research review was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Communicated by Sergio Rosales-Mendoza.
Rights and permissions
About this article
Cite this article
Habibi, P., Prado, G.S., Pelegrini, P.B. et al. Optimization of inside and outside factors to improve recombinant protein yield in plant. Plant Cell Tiss Organ Cult 130, 449–467 (2017). https://doi.org/10.1007/s11240-017-1240-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-017-1240-5