Abstract
Cadmium (Cd) is a toxic heavy metal naturally present in soils. It causes environmental pollution because of the increase of mining and industrial activities in the twentieth century. Although plants do not require Cd, they can accumulate high levels of this metal. The response of plants to Cd pollution involves several signaling pathways involving stress responsive transcription factors. Among these transcription factors, it has been reported that DREB (drought-responsive element binding) factors are involved in plant response to abiotic constraints. In this context, in previous studies, we isolated and characterized two DREB genes from potato that were designated StDREB1 and StDREB2. Their overexpression in transgenic potato lines led to enhanced drought and salt stress tolerance in comparison to untransformed plants. These DREB transcription factors play crucial regulatory roles in different stress responsive pathways in potato. The goal of this report is to study the response of these transgenic potato plants overexpressing StDREB1 and StDREB2 to Cd stress. Therefore, transgenic plants and control plants were cultivated in vitro in MS (Murashige and Skoog) medium supplemented with 50–150 µM CdCl2. After 7 and 14 days of treatment, plant tissues were used to evaluate a number of physiological parameters. Transgenic plants submitted to Cd stress, showed lower decrease in growth, chlorpophyll and carotenoid content than wild-type (WT) plants. Similarly, almost no loss of water content was measured in BF3, SP1 and SPM lines. All transgenic plants exhibited lower H2O2 content and higher antioxidant enzyme expression than WT. A higher proline content was also measured in these plants after Cd treatment in comparison to WT. All these data suggest that overexpression of the StDREB genes improves tolerance of potato plants to Cd by improving plant growth, proline and antioxidant production leading to low oxidative stress damage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Potato (Solanum tuberosum) belongs to the fourth largest food crop in the world that is a staple in many diets. It is a source of highly digestible carbohydrates, nutritional proteins, vitamins and minerals (potassium, phosphorus, calcium and magnesium; Suttle 2008). Despite its important economic value, potato is sensitive to environmental constraints such as dehydration, salinity and heavy metals.
Nowadays, many reports have been focused on the impact of heavy metals in plants such as cadmium (Cd), lead (Pb), copper (Cu) and mercury (Hg). These metals cause environmental damage (Ellen et al. 1990). Heavy metal inputs derive from commercial fertilizers, agrochemicals, sewage sludge, liming materials and other wastes used as soil amendments, irrigation water and atmospheric deposition (Senesi et al. 1999). Kabata-Pendias and Pendias (2001) reported that the concentrations of heavy metals in plants are associated with their concentrations in soils and depend on plant species and genotypes. Cd is considered the highest toxic metal that causes hazards to human health and plant development (Gonçalves et al. 2012). Sanità di Toppi and Gabrielli (1999) reported that soil can be considered as polluted when the Cd concentration ranges from 0.32 to 1 mM, while the non-polluted soil solutions contain Cd levels varying from 0.04 to 0.32mM (Benavides et al. 2005).
Cd threats the environment especially through zinc mining and smelting, industrial activities and the use of phosphate as fertilizer (Waisberg et al. 2003; Senesi et al. 1999). A number of studies showed that Cd can interact with different metabolic processes. It reduces nutrient uptake and water disponibility (Li et al. 2008) resulting in necrosis and chlorosis of plant leaves, growth inhibition and browning of roots, and deficiencies in some essential elements leading in some cases to plant death (Tkalec et al. 2014). Kabata-Pendias and Pendias (2001) reported that the concentrations of heavy metals in plants are associated with their concentrations in soils and depend on plant species and genotypes. Cd has also been found to cause oxidative damage in plants by increasing the accumulation of ROS (reactive oxygen species), leading to an increase in lipid peroxidation and inactivation of macromolecules and cell structures of plant tissues (Smeets et al. 2005). Furthermore, Cd competes with essential nutrients such as calcium for uptake and disturbs the essential intracellular metal homeostasis (Clarkson and Luttge 1989). It accumulates in plant tissues and cell compartments and hampers the general metabolism of the plant when the Cd concentration is about 1.5–10 mg/l (Turner 1997). In 1997, the Australia New Zealand Food Authority (ANZFA) revised limits for Cd in potatoes and other vegetables. The maximum permitted concentration is 0.1 mg Cd/kg fresh weight, for roots, tubers and leafy vegetables.
A wide range of metal transporters have been identified in plants including the heavy metal ATPases (CPx-type ATPases), the natural resistance-associated macrophage proteins (NRAMPs) and the cation diffusion facilitators (CDFs; Williams et al. 2000), the ZIP family (ZRT, IRT- related proteins; Guerinot 2000) and the cation antiporters (Gaxiola et al. 2002). The NRAMP family of metal transporters has been identified in several plant species (Wei et al. 2009) and they seem to be involved in Fe and Cd uptake and homeostasis (Lanquar et al. 2005).
Plants develop different tolerance mechanisms to cope with Cd toxicity. These are mainly based on the exclusion or sequestration of Cd (Katrin 2014). Such processes result in the suppression of this heavy ion from cellular compartments (Tangahu et al. 2011). Compartmentation of Cd in the roots and in the aerial parts of the plant was also observed in tolerant plants (Tangahu et al. 2011).
These plants can also develop antioxidant defense mechanisms by inducing several detoxification systems including enzymatic antioxidants such as superoxide dismutase, catalase and peroxidase (Gallego et al. 2012; Yan et al. 2015).
Exposure to heavy metal may also allow an increase in ABA (abscissic acid) and ethylene contents, two stress-related hormones involved in several signaling pathways (Maksymiec 2011; Zengin 2006). All these data confirm the complexity of the mechanisms involved in plant response to toxic metals. Therefore, many studies have been undertaken to identify genes involved in response to Cd. They are mainly based on the study of gene transcription after exposure to Cd. In this context, many transcription factors implicated in these stress-response pathways have been characterized, among which the proteins ERF (ethylene-responsive factors) have been identified in Arabidopsis thaliana and A. halleri after Cd stress (Weber et al. 2006). DREB transcription factors can also be regulated by Cd exposure (Ban et al. 2011).
These transcription factors regulate many biological processes such as development, reproduction and hormone responses (Licausi et al. 2010; Rae et al. 2011). They also play vital roles in response to salinity, cold and drought stresses in plants (Sakuma et al. 2002). DREB factors harbor an AP2/ERF domain with 50–70 amino acids that is important for its binding to DRE/CRT sequences (dehydration responsive elements/C-repeat elements; Agarwal et al. 2006) in promoters of stress-inducible genes.
DREB genes were identified in A. thaliana (Sakuma et al. 2002), barley (Choi et al. 2002), rice (Dubouzet et al. 2003), wheat (Jaglo et al. 2001) and potato (Bouaziz et al. 2015; Charfeddine et al. 2015). The CBF/DREB group, that belongs to the A subfamily (Sakuma et al. 2002) of the ERF family, was then divided into five subgroups (Nakano et al. 2006).
The DREB factors were shown to be involved in response to various abiotic stresses as documented in some cases. For example, GmDREBc was induced by dehydration, salinity and ABA treatment whereas GmDREBa/b was upregulated by salinity, cold and dehydration in soybean leaves (Li et al. 2005). The expression of the SbDREB2A gene from Salicornia brachiata was activated by salt, dehydration and heat stresses (Gupta et al. 2010). The expression of StDREB1 and StDREB2 was activated by NaCl, dehydration and cold (Bouaziz et al. 2012, 2013).
Many DREB1-type genes were overexpressed in transgenic plants that improved tolerance to various abiotic stresses in plants (Chen et al. 2012; Sharoni et al. 2011).
In this context, we have previously shown that overexpression of StDREB1 and StDREB2 increased dehydration and salinity tolerance in transgenic potato plants (Bouaziz et al. 2012, 2013). Similarly, the overexpression in rice of AtDREB1A isolated from Arabidopsis improved tolerance to salinity (Datta et al. 2012). Transgenic potato plants overexpressing the DREB1A or DREB1B gene from Arabidopsis showed a significant increase in freezing tolerance (Behnam et al. 2007; Movahedi et al. 2012).
The present study investigated the response of transgenic potato plants overexpressing StDREB1 or StDREB2 to Cd (50 and 150 µM) stress for 28 days. Plant growth and chlorophyll content were evaluated during the Cd treatment. Certain oxidative stress indicators and antioxidant ROS scavenging enzymes were also followed. The results obtained showed an enhanced tolerance of transgenic potato plants expressing StDREB1 or StDREB2 compared to untransformed wild controls. This tolerance was related to enhanced antioxidant activities.
Materials and methods
Plant materials and stress treatments
Transgenic potato plants overexpressing StDREBs genes (Bouaziz et al. 2012, 2013) were used here. The BF3 and BF5 lines overexpress the StDREB1 factor (PGSC0003DMG400026461), while the SP1and SPM lines possess the StDREB2 factor (PGSC0003DMG400007951). Plants from the BF and Spunta (SP) untransformed potato cultivars were also used as controls.
Potato plants cultivated in vitro were propagated in tubes containing 15 ml solid MS basal medium (Murashige and Skoog 1962) using 8 g/l agar supplemented with vitamins (Morel and Wetmore 1951). Plants were cultivated in a growth chamber (16 h light/8 dark, 25 °C and 250 µE m−2 s−1 light intensity). Metallic stress was applied by the transfer of 2-week-old single stem plants harboring 4–5 nodes, to 5 ml aqueous MS medium (pH 6) supplemented with 50 or 150 µM CdCl2 and the culture was pursued for 28 days. During this period, the changes of plant morphology and stem elongation were followed.
Determination of the level of chlorophyll and carotenoid
The chlorophyll and carotenoid levels were evaluated spectrophotometrically (Arnon 1949) on leaves from transgenic and WT plants. The leaf samples were ground in 0.5 mL of 100% acetone, and 1 mL of 80% acetone was then added. The supernatant obtained after centrifugation at 12,000 rpm for 15 min was adjusted to 2 mL with acetone 80%. The content of chlorophyll a, chlorophyll b and carotenoid was calculated by measuring the absorbance at 663, 645 and 450 nm respectively. The total chlorophyll level was determined as described by Arnon (1949).
Determination of relative water content (RWC)
The RWC of transgenic and WT potato plants was evaluated according to Yamasaki and Dillenburg (1999). The fresh weight (FW) of leaves, roots and stems was measured and samples were then dried at 80 °C for 48 h in an oven to obtain the dry weight (DW). The RWC was determined as follow:
Lipid peroxidation evaluation
The oxidative damage was assessed by measuring the malonyldialdehyde (MDA) content in leaves, stems and roots. Samples (50 mg) were ground in trichloroacetic acid (TCA) 0.1% (w/v). After centrifugation at 15,000×g for 10 min, an aliquot of 500 µL supernatant was added to a mixture of 0.5% thiobarbituric acid and 20% TCA solution. The homogenate was heated for 30 min at 90 °C in a water bath and then cooled on ice. The samples were centrifuged at 10,000×g for 5 min, and the absorbance of the supernatant was read at 532 and 600 nm (Hernandez and Almansa 2002). The MDA concentration was calculated using the following formula:
where A, absorbance; extract volume, volume of reaction mixture (ml); slope, determined using a calibration curve; weight, mass of fresh tissue.
Determination of H2O2 content
The H2O2 concentration in both leaves and stems of fresh matter was measured as described by Loreto and Velikova (2001). Approximately 0.1 g of sample was ground at 4 °C in 2 mL TCA 0.1% (w/v). The mixture was centrifuged for 15 min at 12,000×g at 4 °C. Then, 0.5 mL supernatant was taken and 1 mL of 1 M potassium iodide (KI) and 0.5 mL of 10 mM phosphate buffer (KP; pH 7) were added. The H2O2 level was calculated by comparing its absorbance at 390 nm with a standard calibration curve. The H2O2 content is presented as μmol/g FW.
Determination of electrolyte leakage
Leaf samples were cut into discs of 5 mm diameter and placed in test tubes containing 10 ml double distilled water. The tubes were incubated at 32 °C for 2 h and the initial electrical conductivity (EC1) was measured. The tubes were heated at 100 °C in a water bath for 20 min, cooled to 25 °C and the final electrical conductivity of the medium was measured (EC2). The electrolyte leakage (EL) was calculated according to Sairam et al. (2002) using the following formula: EL = (EC1/EC2) × 100 and expressed in % of total electrolytes.
Protein extractions
Fresh leaf, stem and root samples were ground in a potassium phosphate buffer 0.1 M, pH 7 to extract soluble proteins. The homogenate was centrifuged at 13,000×g for 15 min at 4 °C and the supernatants were collected. The protein concentrations were determined according to the method of Bradford (1976) using bovine serum albumin (BSA) as a standard.
Antioxidant enzyme activity assay
The total superoxide dismutase (SOD), catalase (CAT) and gluthatione peroxidase (GPX) activities were determined spectrophotometrically.
The superoxide dismutase activity was assayed according to Dhindsa et al. (1981). The reaction mixture, of 3 ml final volume, contained 50 mM potassium phosphate (pH 7), 0.1 mM EDTA, 9.9 mM methionine, 57 μM NBT (nitroblue tetrazolium) and 2 µM riboflavine. The reaction was initiated by exposing the mixture to white light for 20 min. A blue color developed and was measured at 560 nm. A reaction mixture not exposed to light was used as negative control. The blank corresponds to the reaction mixture without enzyme, exposed to light which developed a maximum color.
One unit of SOD activity was defined as the quantity of enzyme causing 50% inhibition of the photochemical reduction of NBT. The specific activity is expressed as units per mg of protein.
Total catalase activity was determined according to Claiborne (1985). The reaction mixture contained 50 mM potassium phosphate buffer (pH 7), 10 mM H2O2, 1 mM dithiothreitol (DTT) and enzyme extract (25 µl). The CAT activity is expressed as µmol H2O2 decomposed per mg of protein.
Glutathione peroxidase activity was measured according to Floh and Gunzler (1984) by allowing oxidation of glutathione (GSH) by H2O2 in the presence of 5,5′-dithio-bis 2-nitrobenzoic acid (DTNB).
The assay mixture containing 0.1 mM GSH solution in 67 mM phosphate buffer (pH 7.8) was incubated at 25 °C for 5 min. Then, 1.3 mM H2O2 were added and the mixture was incubated for 10 min. The reaction mixture was supplemented with 1 ml 1% TCA and centrifuged at 3000 rpm for 10 min. The supernatant was collected and 2.2 ml of 0.32 M Na2HPO4 and 320 ml of 1 mM DTNB were added.
The GPX activity is expressed as µM GSH/min/mg protein.
Determination of proline content
Leaves and stems from potato plants were harvested every 7 days to determine the proline content. The proline assay was performed according to Bates et al. (1973). Samples were ground in sulphosalicylic acid (3%). After adding glacial acetic acid and ninhydric acid, the mixtures were incubated for 1 h at 100 °C in a water bath. The tubes were then transferred on ice to stop the reaction. A volume of 2 mL toluene was added and the absorbance of the toluene fraction was measured at 520 nm. The concentration of proline was calculated using a calibration curve and is expressed as µmol proline/gFW.
Determination of Cd content
Plant material was collected and leaves, stems and roots were dried at 65 °C for 2 days until a constant mass was reached. Dried plant material was mineralized using nitric acid and the Cd content determined by atomic absorption spectrometry (Analytik Jena ZEEnit700).
RNA extraction and cDNA synthesis
Total RNA was extracted, from leaves of in vitro cultivated potato plants submitted to different concentrations of Cd for 7 days, according to Chomczynski and Sacchi (1987) using the trizol reagent. The samples of 0.1 g were ground in liquid nitrogen and 1 ml of trizol reagent was then added. The homogenate was vigorously shaken and incubated at room temperature for 5 min. Chloroform (200 µl) was added and the mixture was shaken by hand for 15 s. The samples were then incubated for 5 min at room temperature and centrifuged for 15 min at 12,000 rpm at 4 °C. The aqueous phase was transferred to a fresh tube and 0.5 ml of isopropanol was added to precipitate the RNA. The mixture was incubated at room temperature for 10 min and centrifuged at 12,000 rpm for 10 min at 4 °C. The supernatant was discarded and the pellet was washed with 1 ml 75% ethanol and centrifuged at 12,000×g for 5 min at 4 °C. The pellet was dried for 5 min at room temperature and then dissolved in RNase-free water.
The RNA concentration and quality were determined by measuring the absorbance at 260 and 280 nm and the RNA was treated by DNase I. cDNA synthesis was carried out using reverse transcriptase M-MuLV (RT Bio Basic Inc).
Semi-quantitative RT-PCR analysis
RT-PCR analysis of the expression of genes encoding antioxidant enzymes such as SOD, catalase, glutathione peroxidase and NRAMP transporter of Cd was performed. The elongation factor (ef1a) constitutive gene (GenBank ID: AB061263) was used to normalize the amount of template added. All primer sequences are presented in Table 1. PCR amplified products were visualized on ethidium bromide-stained 1.5% agarose gels and quantified using the Gel DocXR Gel Documentation System (Bio-Rad).
Statistical analysis
Data are presented as mean ± standard errors of three parallel measurements. All data were subjected to the ANOVA test (analysis of variance) using the GraphPad Prism Version 5 software and the Newman/Keuls test was used to compare the means. p values <0.05 were considered significantly different.
Results
Overexpression of StDREB genes enhances tolerance to Cd in transgenic potato plants
WT and transgenic potato plants were cultivated in MS medium supplemented with 50 and 150 µM Cd. After 7, 21 and 28 days of treatment, morphological differences between transgenic and WT plants were followed (Fig. 1). Indeed non transgenic SP and BF lines exhibited an important reduction of leaf greenness, while leaves of transgenic plants remained green and were more vigorous than the controls at 150 µM Cd. These data suggest that overexpression of StDREB1 and StDREB2 in transgenic potato plants may improve their tolerance to Cd.
To better understand the response of these transgenic lines to Cd treatment, we measured their above-ground length. Slight but significant growth inhibition was observed for SP and BF WT plants as well as SPM in the presence of Cd (Fig. 2a, c, d). However, similar aboveground lengths were observed for the transgenic linesSP1 in the absence or presence of 50 and 150 µM Cd (Fig. 2b). No significant reduction of aboveground length was observed in BF3 and BF5 transgenic lines (Fig. 2e, f).
The most important process for plant development and biomass production is photosynthesis, in which the chlorophyll pigment plays a crucial role. Therefore, evaluation of the chlorophyll content was performed in potato lines cultivated in Cd supplemented media. A significant loss in chlorophyll content was observed in WT control lines (BF and SP) upon Cd treatment conditions. However, most of the transgenic plants showed a lower decrease of chlorophyll content upon Cd application than WT (Fig. 3a, b). Interestingly the SP1 and SPM lines, overexpressing StDREB2 showed little or no reduction of chlorophyll content when they were submitted to Cd stress (Fig. 3a). Since the decrease in the chlorophyll level is one of the common symptoms of the Cd hazardous effect in plants (Liu et al. 2011), these data revealed that transgenic plants overexpressing StDREB1 or StDREB2 are more tolerant to Cd than non transformed plants. These data are in agreement with the morphological observation since leaf roll and chlorosis, the most visible symptoms of Cd damage (Benavides et al. 2005), were not observed in these lines.
Chlorophyll and carotenoid contents cultivated in the absence or presence of 50 µM or 150 µM CdCl2 for 7 or 21 days. a, c Transgenic plants overexpressing StDREB1 (BF3 and BF5) or WT plants (BF). b, d Transgenic plants overexpressing StDREB2 (SP1 and SPM) or WT (SP) plants. Each value is represented by the mean of three samples ± SD. Means followed by the same letter indicate no significant difference between stressed and control plants (p < 0.05)
Total carotenoid content behaved similarly to chlorophyll (Fig. 3c, d), suggesting that Cd treatment allows a generalized decrease in the concentration of these pigments in WT plants, probably accompanied by diminished photosynthetic capacity. The carotenoid content in the BF3, BF5 and SP1 lines was not affected by the increase of the Cd content. In contrast, the untransformed plants (BF and SP) and the SPM lines overexpressing theStDREB2 gene, revealed a reduction of carotenoid content at both Cd concentrations. These data suggest that heavy metals may affect the biosynthesis of carotenoids.
Estimation of relative water content
The physiological changes in transgenic potato plants submitted to metallic stress were evaluated by comparing the RWC in WT and transgenic lines. The RWC measures the water status of the plant as a physiological consequence of cell water deficiency due to accumulation of Cd. Under control conditions, the relative water content of the WT was more important than that of the different transgenic lines especially in leaves of the SP cultivar (Fig. 4a, b). However, the Cd treatment led to a decrease of RWC in leaves, roots and stems of these lines. In contrast, transgenic plants expressing StDREB2, exhibited a constant RWC in roots when they were submitted to Cd treatment for 21 days. A lower RWC decrease was obtained in StDREB1 transgenic lines compared to WT lines.
The percentage of RWC loss in leaves, stems and roots of potato plants cultivated in the absence or presence of 50 or 150 µM CdCl2 for 7 or21 days. a Transgenic plants overexpressing StDREB2 (SP1 and SPM) or WT (SP) plants. b Transgenic plants overexpressing StDREB1 (BF3 and BF5) or WT (BF) plants. Each value is represented by the mean of three samples ± SD. Means followed by the same letter indicate no significant difference between stressed and control plants (p < 0.05)
Effect of salinity on malondialdehyde (MDA) content
Cd toxicity can involve oxidative damage of lipid membranes (Benavides et al. 2005) which was assessed here by measuring MDA formation. Indeed, evaluating the MDA content and hence, the degree of membrane fatty acid peroxidation, has been used frequently as a tool to evaluate the extent of plant sensitivity and the severity of the oxidative damage (Hernandez et al. 2001). The level of MDA accumulation was determined herein roots, stems and leaves. The results revealed low MDA content in roots of both control and transgenic plants (Fig. 5a, b) submitted to Cd treatment. However, a significant increase in the MDA content was noticed in leaves for both SP and BF WT plants submitted to Cd treatment, indicating an important lipid peroxidation degree. In stems, the highest level of MDA was detected in BF plants after 21 days of treatment. Transgenic lines overexpressing StDREB genes exhibited lower increase in MDA content in leaves and stems than WT plants. These data revealed that StDREB1 and StDREB2 overexpression in potato may enhance plant tolerance to Cd by controlling the oxidative stress response.
MDA contents in leaves, stems and roots of potato plants cultivated in the absence or presence of 50 or 150 µM CdCl2for 7 or 21 days. a Transgenic plants overexpressing StDREB2 (SP1 and SPM) or WT (SP) plants. b Transgenic plants overexpressing StDREB1 (BF3 and BF5) or WT (BF) plants. Each value is represented by the mean of three samples ± SD. Means followed by the same letter indicate no significant difference between stressed and control plants (p < 0.05)
Determination of hydrogen peroxide (H2O2) content
To determine the impact of Cd stess on subsequent oxidative stress generation in plants, we determined the H2O2 production after 7 and 21 days of Cd treatment (Fig. 6a, b). Low H2O2 level was detected in stems and leaves of transgenic lines (SP1 and SPM) submitted to Cd treatment for 21 days. However, H2O2 levels increased at 50 and 150 µM Cd in the WT SP cultivar (Fig. 6a). Only WT plants exhibited differences in H2O2 in response to Cd treatment and these differences were only apparent in leaves of BF plants after 7 days, SP plants after 21 days and stems of SP plants after 21 days. None of the transgenic plants exhibited H2O2 changes in response to Cd (Fig. 6). In leaves, this content did not increase significantly in Cd-treated plants. These results further demonstrate that StDREB1 and StDREB2 overexpression in transgenic potato lines improved their response to oxidative stress generated by Cd contamination.
H2O2 contents in leaves and stems of potato plants cultivated in the absence or presence of 50 or 150 µM CdCl2for 7 or 21 days. a Transgenic plants overexpressing StDREB2 (SP1 and SPM) or WT (SP) plants. b Transgenic plants overexpressing StDREB1 (BF3 and BF5) or WT (BF) plants. Each value is represented by the mean of three samples ± SD. Means followed by the same letter indicate no significant difference between stressed and control plants (p < 0.05)
Measurement of electrolyte leakage
Leaf electrolyte leakage was measured in the transgenic potato plants and the WT plants under Cd treatment (Fig. 7a, b) and showed that the overexpression of the DREB genes increased the tolerance of transgenic plants to Cd exposure.
Relative electrolyte leakage contents in potato leaf tissues cultivated in the absence or presence of 50 or 150 µM CdCl2 for 7 or 21 days. a Transgenic plants overexpressing StDREB2 (SP1 and SPM) or WT (SP) plants. b Transgenic plants overexpressing StDREB1 (BF3 and BF5) or WT (BF) plants. Each value is represented by the mean of three samples ± SD. Means followed by the same letter indicate no significant difference between stressed and control plants (p < 0.05)
The addition of Cd caused a significant increase in electrolyte leakage in leaves of the WT lines, but not of the transgenic lines. Indeed, a reduction of this parameter was observed in the SPM, BF3 and BF5 lines after 7 and 21 days of treatment, whereas, the SP1 line showed a decrease after overexpression reduced membrane damage caused by Cd stress.
Overexpression of StDREB1 and StDREB2 enhances the expression and the activity of antioxidant enzymes
Superoxide dismutase (SOD)
Higher SOD activity was measured in roots than in leaves and stems fall plant lines (Fig. 8a, b). Moreover, this activity was higher in roots of transgenic plants compared to WT plants. The Cd treatment led to a significant increase of SOD in SPM and BF5. An increase of SOD activity was also observed in roots of stressed the SP1 line submitted to 150 µM Cd for 7 days; beyond this period, this activity was stable (Fig. 8a). The BF3 transgenic line showed a significant increase only at 50 µM Cd for 21 days (Fig. 8b).
Analysis of SOD activity in leaves, stems and roots of potato plants cultivated in the absence or presence of 50 or 150 µM CdCl2for 7 or 21 days. a Transgenic plants overexpressing StDREB2 (SP1 and SPM) and WT (SP) plants. b Transgenic plants overexpressing StDREB1(BF3 and BF5) and WT (BF) plants. Semi-quantitative RT-PCR analysis of the expression of the Cu/ZnSOD (c) and FeSOD (d) genes in leaves of WT and transgenic plants. Error bars indicate standard deviation across two biological replicates. Band densities in the gels are expressed in arbitrary units calculated by the analysis Gel DocXR software. Mean values ± SD are presented. The Ef1α constitutive gene was used as a control in RT-PCR amplification
These results may explain the low MDA level in roots.
All SP-derived lines revealed constant and low SOD activity in stems; however, BF plants overexpressing the StDREB1 gene showed an increase of this activity.
In leaves, a very low increase of SOD activity was observed in the SP1, BF3 and BF5 lines after Cd treatment. SPM showed constant SOD activity in all culture conditions (Fig. 8a).
These results confirm that transgenic lines are more tolerant to Cd compared to SP and BF WT lines.
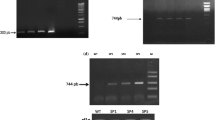
To further understand the effect of StDREB1 overexpression on the expression of the SOD encoding gene, the transcription of a number of SOD genes was investigated. The expression of the Cu/Zn SOD and FeSOD2 genes was assessed (Fig. 8c, d) by semi-quantitative RT-PCR. As observed, the expression level of these genes increased in SP1, SPM, BF3and BF5 transgenic plants submitted to Cd treatment, while it decreased in leaves of SP and BF WT lines.
The mRNA accumulation of the FeSOD2 gene remained stable in BF plants submitted to Cd treatment while plants of the BF3 line showed a stable expression of CuZnSOD in all treatment conditions.
In contrast, SP and BF control plants, showed a decrease in the expression level of the CuZnSOD and FeSOD2 genes when they were submitted to Cd treatment (Fig. 8c).
These data are in agreement with the SOD activities measured in leaves of these plants which showed a decrease in SOD activity in SP and BF and a global increase in the transgenic lines.
Catalase (CAT) and glutathione peroxidase (GPX)
Since, the H2O2 generated by SOD is removed by CAT and GPX, the activity of these two enzymes was evaluated in leaves, stems and roots of all plants submitted to Cd treatment. The results (Fig. 9a, b) showed a significant increase of CAT activity in all tissues of transgenic plants. However, this activity remained constant and low in the untransformed BF line (Fig. 9b). The untransformed SP plants showed a low increase in CAT activity in leaves and stems after 7 and 21 days of treatment respectively (Fig. 9a). In roots, the highest level was obtained in theSP1 line after 7 days and150 µM Cd, and in SP1 and SPM plants after 21 days and 50 µM Cd (Fig. 9a). The SP control plants also showed an increase of CAT activity in roots after 50 µM Cd treatment.
Analysis of CAT activity in leaves, stems and roots of potato cultivated in the absence or presence of 50 or150 µM CdCl2for 7 or21 days. a Transgenic plants overexpressing StDREB2 (SP1 and SPM) or WT (SP) plants. b Transgenic plants overexpressing StDREB1 (BF3 and BF5) or WT (BF) plants. Semi-quantitative RT-PCR analysis of the expression of CAT1 (c), CAT2 (d) and CAT3 (e) genes in leaves of WT and transgenic plants. Error bars indicate standard deviation across two biological replicates. Band densities in the gels are expressed in arbitrary units calculated by the analysis the Gel DocXR software. Mean values ± SD are presented. The Ef1α constitutive gene was used as a control in RT-PCR amplification
To better understand the molecular mechanisms of enhanced CAT activity, the expression of three genes encoding catalase 1, 2 and 3 was evaluated by semi-quantitative RT-PCR analysis in leaves of transgenic and untransformed lines after 7 days of Cd treatment (Fig. 9c). The increase of CAT1 gene expression was visible in theBF3 and BF5 transgenic lines and in the SP untransformed line cultivated in the absence of Cd. At 50 µM, CAT1 transcript accumulation increased in theSP1 transgenic line overexpressing StDREB2 and in BF3 and BF5 overexpressing StDREB1. At 150 µM, high expression levels were observed in all transgenic lines. This increase was much lower in the WT SP and BF lines. The highest level was observed in theBF3 transgenic line at 50 and 150 µM Cd.
No CAT2 gene expression (Fig. 9d) was measured in leaves of SP, SP1 and BF plants in all culture conditions. However, the expression of this gene seemed to be enhanced by Cd stress in the other transgenic lines. The SPM line overexpressing StDREB2 seems to be the only one to express the CAT2 gene in standard conditions.
The expression of the CAT3 gene (Fig. 9e) was observed in leaves of all plant lines cultivated in standard conditions. However, a high increase of the expression levels in transgenic potato plants overexpressing the StDREB1 or StDREB2 transcription factor and submitted to 50 or 150 µM Cd treatment, while, this gene was not expressed in the BF5 transgenic line at 150 µM Cd and in SP.
On the contrary, no expression of CAT3 was observed in WT SP plants at 50 µMCd.
Semi-quantitative RT-PCR showed that CAT2 and CAT3 were not expressed in leaves of potato plants under normal growth conditions.
These RT-PCR analyses corroborated the measured catalase activity and confirmed that the transgenic plants are better adapted to cope with oxidant damage caused by Cd treatment.
The measurement of the GPX activity in leaves, stems and roots of plants cultivated in the absence or presence of Cd was used to evaluate the effectiveness of H2O2 and toxic lipid peroxide removal in the different lines. Very low GPX activity was measured in leaves of BF, BF3, BF5 and SP plants (Fig. 10a, b). The most important GPX activity was observed in the stems of all tested lines. This activity decreased in stems of SP control plants submitted to Cd treatment.
Analysis of GPX activity in leaves, stems and roots of potato plants cultivated in the absence or presence of 50 µM or150 µM CdCl2 for 7 or 21 days. a Transgenic plants overexpressing StDREB2 (SP1 and SPM) or WT (SP) plants. b Transgenic plants overexpressing StDREB1 (BF3 and BF5) or WT (BF) plants. Semi-quantitative RT-PCR analysis of the expression of the GPX gene (c) in leaves of WT and transgenic plants. Error bars indicate standard deviation across two biological replicates. Band densities in the gels are expressed in arbitrary units calculated by the analysis the Gel DocXR software. Mean values ± SD are presented. The Ef1α constitutive gene was used as a control in RT-PCR amplification
Cd treatment caused no significant changes of GPX activity in stems of transgenic plants overexpressing the StDREB2 gene (Fig. 10a).
Similarly, the GPX activity of transgenic lines (BF3 and BF5) overexpressing StDREB1 increased significantly in stems at day 7 of Cd stress (Fig. 10b).
In roots, the GPX activity increased significantly in Cd treated SP1 and SPM on day 15. An increase of GPX activity was also observed in the roots of the BF3 line after 15 days of treatment. However, the BF plants showed an important decrease of GPX activity after 7 and 15 days of Cd exposure.
To further understand the efficiency of GPX to cope with oxidative stress generated by Cd treatment, the analyses of the expression of the gene encoding this enzyme was performed. As shown in Fig. 10c, under Cd treatment, the expression of GPX increased at 50 and at 150 µM Cd in SPM, SP1 and BF5 transgenic plants.
However, no transcription of this gene was noticed in any culture conditions in SP and BF. Similarly, in the BF3 transgenic line, the GPX gene seems not to be activated by Cd stress.
The highest accumulation of the amplicon was observed in SPM lines after 150 µM Cd treatment.
Proline content
Despite its osmoprotectant function, proline also serves as a ROS scavenger, protecting enzymes and membranes against oxidative stress (Szabados et al. 2009). It could be involved in metal chelation (Aghababaei et al. 2014). Bouaziz et al. (2012, 2013) showed that under salinity or dehydration conditions, StDREB1 and StDREB2 overexpression in potato plants enhanced proline osmoprotectant accumulation via increased transcription of the δ1-pyrroline-5-carboxylate synthase (P5CS) target gene. Similarly, the overexpression of Arabidopsis DREB1B in potato increased proline accumulation after drought and freezing conditions (Movahedi et al. 2012).
Proline synthesis has also been widely studied in response to Cd treatment (Aghababaei and Raiesi 2015). Cd was a stronger inducer of proline than Ni, Zn and Cu in rice, the callus of Brassica napus, Armeria moritima, sunflower, Brassica juncea (Hasan et al. 2009) and maize plants (Aghababaei and Raiesi 2015).
Similarly, in the present study, the increase in proline content was much more important in leaves of transgenic plants submitted to Cd treatment than in WT plants (Fig. 11a, b). The proline levels increased also in stems but remained lower than in leaves.
Proline contents in leaves, stems and roots of potato plants cultivated in the absence or presence of 50 or 150 µM CdCl2for 7 or 21 days. a Transgenic plants overexpressing StDREB2 (SP1 and SPM) or WT (SP) plants. b Transgenic plants overexpressing StDREB1 (BF3 and BF5) or WT (BF) plants. Each value is represented by the mean of three samples ± SD. Means followed by the same letter indicate no significant difference between stressed and control plants (p < 0.05)
Indeed, the proline content in the BF5 and SPM transgenic plants carrying the StDREB1 and StDREB2 genes respectively, increased 4 and 5 times at 150 µM Cd after 21 days of Cd treatment in comparison to the standard conditions. In contrast, under Cd treatment, the content of proline in WT plants increased only twofold compared to control conditions mainly in leaves. These data suggest that such accumulation of proline seems to be involved in Cd response, probably via its antioxidant capacity.
Cd accumulation in plant tissues
The accumulation of Cd was determined in roots, stems and leaves of transgenic and WT lines submitted to Cd treatment (Table 2). The accumulation of Cd in plant organs was detected only after treatment with 150 µM Cd. The untransformed WT line (BF) showed the greatest increase in Cd content in all plant tissues mainly in the stems (Table 2). A low level of Cd was translocated to the leaves. The Cd uptake was lower in transgenic plants overexpressing StDREB1compared to non-transgenic plants. These findings suggest that overexpression of the StDREB1 gene activates transcription of target genes involved in the control of absorption and translocation of Cd in the plant. No detectable Cd accumulation was measured in SP and SPM derived transgenic lines (Table 2), suggesting that the Spunta cultivar possesses a different mechanism of Cd translocation in comparison to BF.
RT-PCR analyses of the NRAMP transporter
The uptake and translocation of Cd are performed by a number of transporters such as NRAMP which have been identified in many plant species (Ishimaru et al. 2012).
The NRAMP gene harbors the CRT/DRE element in its promoter sequence. The analysis of the expression of this gene in potato plants overexpressing StDREB genes and WT ones submitted to different concentrations of Cd was assessed. Results (Fig. 12a, b) revealed that the NRAMP gene was upregulated by Cd treatment in transgenic plants. The SPM and BF5 transgenic plants expressed this gene only at 150 µM Cd. The accumulation of the NRAMP transcript was observed in theSP1 line in the presence or the absence of Cd. The highest level was detected at 150 µM Cd (Fig. 12a).
Semi-quantitative RT-PCR analysis of the expression of the NRAMP gene in leaves of WT and transgenic plants submitted to 50 or 150 µM CdCl2 for 7 days. a Transgenic plants overexpressing StDREB2 (SP1 and SPM) or WT (SP) plants. b Transgenic plants overexpressing StDREB1 (BF3 and BF5) or WT (BF) plants. Error bars indicate standard deviation across two biological replicates. Band densities in the gels are expressed in arbitrary units calculated by the analysis the Gel DocXR software. Mean values ± SD are presented. The Ef1α constitutive gene was used as a control in RT-PCR amplification
Moreover, an increase in NRAMP transcription was detected in BF3 at 50 and 150 µM Cd (Fig. 12b). However, no induction of expression of this gene was detected in BF and SP plants cultivated under the same conditions.
Discussion
DREB transcription factors play crucial roles in abiotic stress tolerance of plants. Several reports have demonstrated that DREB transcription factors are involved indifferent abiotic constraints, including salinity, dehydration, cold and oxydative stress (Ma et al. 2014; Rae et al. 2011; Reis et al. 2014; Sharoni et al. 2011). However, there have been few reports on the impact of heavy metal stress on DREB gene expression and on the physiological processes regulated by DREB factors under heavy metal stress conditions. Cong et al. (2008) identified a BjDREB1B gene that is induced by Cd, zinc and nickel, while the AmCBF2 gene of Avicennia marina exhibited a low induction after Cd stress (Peng et al. 2013).
In this report, we investigated the effect of the overexpression of StDREB1 and StDREB2 transcription factors in potato and their response to Cd. Our results show that exposure of plants to 50 and 150 µM Cd results in rapid chlorosis in WT plants, even though, growth reduction was low in these plants. In contrast, the StDREB transgenic plants showed neither growth reduction nor leaf chlorosis in the presence of Cd.
The capacity of plants to cope with heavy metals is related to enhancement of their osmotic pressure (Yan and Tam 2013).
Chlorophyll and carotenoid pigments were used as reliable indicators of heavy metal damage in plants (Gratão et al. 2005). The reduction of chlorophyll content observed in WT plants confirmed the deleterious effect of Cd on plants. These data corroborated those of others showing that Cd is an important inhibitor of photosynthesis (Hasan et al. 2007). The transgenic lines exhibited a lower reduction in chlorophyll content than WT lines. Moreover, the chlorophyll content of the SPM transgenic line remained constant during the Cd treatment. It therefore seems that overexpression of StDREB1 and StDREB2 may activate the transcription of downstream genes that prevent chlorophyll degradation, hence, maintaining normal photosynthesis and enhancing tolerance to Cd (Zhang et al. 2004). The WT SP and BF plants also showed a significant decrease in the RWC, upon application of Cd treatment, while transgenic plants exhibited much lower reduction of RWC. It remained constant in SP1 and SPM plants treated by both concentrations of Cd. The increase in electrolyte leakage in the BF and SP lines compared to transgenic plants suggests that the latter is better protected against oxidative damage under Cd stress.
Cd toxicity was related in part to the oxidative stress that may be due to induced accumulation of ROS such as O2 ·−, H2O2 and OH· (Andresen and Küpper 2013; Yan et al. 2015). It enhances permeability of membranes and modulates the patterns of ion leakage (Kukreja et al. 2005). MDA, a secondary toxic product of fatty acid peroxidation, was usually used as an indicator of membrane oxidative damage. H2O2 and electrolyte leakage are also considered as reliable indicators of oxidative stress (Baby and Jini 2011).
On the other hand, carotenoids which play an essential role in photosynthesis (Sekmen et al. 2012) are also known for their antioxidant capacity.
Our results showed a decrease in carotenoid level in WT plants submitted to Cd treatment, whereas SP1, BF3 and BF5 showed stable amounts of carotenoids during the Cd treatment.
Our report revealed that Cd treatment did not affect the MDA content in roots of any of the transgenic lines. A significant increase in MDA concentration was observed mainly in leaves and stems of WT plants submitted to Cd treatment. In contrast, almost no MDA accumulation was noticed in leaves of Cd-treated transgenic plants. Similarly, a significant increase in H2O2 content was observed in leaves and stems of WT plants while almost no increase was measured in transgenic plant tissues. It seems therefore that StDREB transcription factors activate antioxidant mechanisms that scavenge or prevent the formation of aggressive ROS, thereby protecting cells against oxidative damage. These data can be related to the stable carotenoid content in transgenic plants. Moreover, the evaluation of the activity of some antioxidant enzymes in Cd-treated plants further confirms this hypothesis. Indeed, the increase in activity and the expression of the SOD, CAT and GPX genes can be related to the reduced accumulation of MDA and H2O2 in leaves of all the transgenic lines tested.
The high expression levels of genes encoding antioxidant enzymes such as catalase, SOD and GPX corroborate the increased activity of these enzymes.
Similarly, the overexpression of StDREB1 in transgenic potato lines enhanced their antioxidant capacity by increasing the SOD and catalase activities under salt stress conditions (Bouaziz et al. 2015). These data described here provide further evidence of the involvement of the StDREB1 and StDREB2 transcription factors in the activation of a number of antioxidant enzymes encoding genes such as CuZnSOD, FeSOD, GPX and CAT.
Previous studies showed that overexpression of StDREB1 and StDREB2 in potato increased the transcription level of the P5CS target gene leading to an increase in free proline accumulation (Bouaziz et al. 2012, 2013). Proline serves as osmoprotectant protecting macromolecules from denaturation (Surekha et al. 2014; Imrul; Mosaddek et al. 2013). It was also examined for its antioxidant effect under stress conditions (Yan et al. 2015). Other studies reported that this osmoprotectant could be involved in metal chelation (Sharma and Dietz 2006). Higher proline accumulation has also been widely reported in response to Cd stress (Aghababaei and Raiesi 2015; Dinakar et al. 2008). Similarly, this report clearly showed that proline content was significantly increased in transgenic lines after Cd treatment; this increase was much higher than that measured in WT lines confirming the involvement of these StDREB transcription factors in the activation of proline synthesis under abiotic stress conditions.
The measurement of Cd accumulation in plant tissues showed that higher contents of Cd were detected in WT BF plants in comparison to transgenic plants. The roots of StDREB1 transgenic lines accumulated more Cd than stems and leaves. This may be associated with the fact that roots are the first tissue to be in contact with Cd (Tiryakioglu et al. 2006). However, BF WT plants showed similar Cd content in leaves and roots and the highest level was measured in stems. This report suggests that overexpression of StDREB1 and StDREB2 in transgenic potato lines, improved their tolerance to high concentrations of Cd in the medium. StDREB1 transgenic plants seem to prevent translocation of Cd to the aerial parts.
Since the NRAMP metal transporter represents an important group of transmembrane proteins involved in metal transport and homeostasis and since these proteins are considered as “general metal ion transporters” due to their ability to transport Mn2+, Zn2+, Cu2+, Fe2+, Cd2+, Ni2+ and Co2+ (Nevo and Nelson 2006), we investigated here, the expression of an NRAMP transporter in Cd-treated plants. An increase in the expression of this gene was observed suggesting that this transporter is involved in modulation of Cd toxicity (Nevo and Nelson 2006).
The higher concentration of Cd measured in roots than in stems in BF3 and BF5 plants corroborated the increase of NRAMP gene expression. These data are in agreement with those reported by Ishimaru et al. (2012) who showed that OsNRAMP5 prevents the Cd translocation of the shoots in rice plants. These findings suggest that the NRAMP transporter may control the Cd concentration in potato plants. Indeed, functional studies have shown that the AtNRAMP transporters modulate Cd and Fe toxicity in plants (Curie et al. 2000). Similarly, Sano et al. (2012) showed that overexpression of the plasma membrane transporter Nicotiana tabacum NRAMP1 gene (NtNRAMP1) in tobacco BY-2 cells increased its resistance to both Fe and Cd. These authors proposed that NtNRAMP1 moderates Fe-uptake and prevents toxicity resulting from excess Fe or Cd application. Similarly, the overexpression of OsNRAPM and EtNRAMP3 in Arabidopsis improved plant tolerance to Cd with their enhanced accumulation in roots and shoots (Tiwari et al. 2014; Thomine et al. 2000).
In conclusion, this report shows that overexpression of the StDREB1 or StDREB2 transcription factors in transgenic potato plants improves their tolerance to Cd treatment by increasing the expression of antioxidant enzymes such as SOD, GPX and CAT. It also resulted on a better control of Cd transport in aerial parts in comparison to WT, especially for StDREB1 transgenic plants.
Abbreviations
- DREB:
-
Drought-responsive element binding
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- ABA:
-
Abscissic acid
- ERF:
-
Ethylene responsive factor
- AP2:
-
Apetala2
- DRE:
-
Dehydration responsive element
- CAT:
-
Catalase
- CRT:
-
C-Repeat element
- CBF:
-
C-Repeat binding factor
- GPX:
-
Gluthation peroxidase
- MDA:
-
Malondialdehyde
- NRAMP:
-
Natural resistance-associated macrophage proteins
- TCA:
-
Trichloroacetic acid
- TBA:
-
Thiobarbituric acid
- RWC:
-
Relative water content
- ANOVA:
-
Analysis of variance
- WT:
-
Wild-type
References
Agarwal P, Agarwal P, Reddy M, Sopory S (2006) Role of DREB transcription factors in abiotic and biotic stress tolerance in plants. Plant Cell Rep 25:1263–1274
Aghababaei F, Raies F (2015) Mycorrhizal fungi and earthworms reduce antioxidant enzyme activities in maize and sunflower plants grown in Cd-polluted soils. Soil Biol Biochem 86:87–97
Aghababaei F, Raiesi F, Hosseinpur A (2014) The combined effects of earthworms and arbuscular mycorrhizal fungi on microbial biomass and enzyme activities in a calcareous soil spiked with cadmium. App Soil Ecol 75:33e42
Ahmed IM, Dai H, Zhenga W, Cao F, Zhang G, Sun D, Wu F (2013) Genotypic differences in physiological characteristics in the tolerance to drought and salinity combined stress between Tibetan wild and cultivated barley. Plant Physiol Biochem 63:49–60
Andresen E, Küpper H (2013) Cadmium toxicity in plants. In: SigelA, Sigel H, Sigel RKO (eds) Cadmium: from toxicity to essentiality. metal ions in life sciences. Springer, Dordrecht, pp 395–409
Arnon DL (1949) A copper enzyme is isolated chloroplast polyphenol oxidase in Beta vulgaries. Plant Physiol 24:1–15
Baby J, Jini D (2011) Development of salt stress-tolerant plants by gene manipulation of antioxidant enzymes. Asian J Agric Res 5:17–27
Ban Q, Liu G, Wang Y (2011) A DREB gene from Limonium bicolor mediates molecular and physiological responses to copper stress in transgenic tobacco. J Plant Physiol 168:449–458
Bates LE, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Behnam B, Kikuchi A, Celebi-Toprak F, Kasuga M, Yamaguchi-Shinozaki K, Watanabe KN (2007) Arabidopsis rd29A::DREB1A enhances freezing tolerance in transgenic potato. Plant Cell Rep 26:1275–1282
Benavides MP, Gallego SM, Tomaro ML (2005) Cadmium toxicity in plant. Braz J Plant Physiol 17:21–34
Bouaziz D, Pirrello J, Amor HB, Hammami A, Charfeddine M, Dhieb A, Bouzayen M, Gargouri-Bouzid R (2012) Ectopic expression of dehydration responsive element binding proteins (StDREB2) confers higher tolerance to salt stress in potato. Plant Physiol Biochem 60:98–108
Bouaziz D, Pirrello J, Charfeddine M, Hammami A, Jbir R, Dhieb A, Bouzayen M, Gargouri-Bouzid R (2013) Overexpression of StDREB1 transcription factor increases tolerance to salt in transgenic potato plants. Mol Biotechnol 54:803–817
Bouaziz D, Charfeddine M, Jbir R, Saidi MN, Pirrello J, Charfeddine S, Bouzayen M, Gargouri-Bouzid R (2015) Identification and functional characterization of ten AP2/ERF genes in potato. Plant Cell Tissue Organ Cult 125:155–172
Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72(248):254
Charfeddine M, Saidi MN, Charfeddine S, Hammami A, Gargouri Bouzid R (2015) Genome-wide analysis and expression profiling of the ERF transcription factor family in potato (Solanum tuberosum L.) Mol Biotechnol 57:348–358
Chen T, Yang Q, Gruber M, Kang J, Sun Y, Ding W, Zhang X (2012) Expression of an alfalfa (Medicago sativa L.) ethylene response factor gene MsERF8 in tobacco plants enhances resistance to salinity. Mol Biol Rep 39:6067–6075
Choi DW, Rodriguez EM, Close TJ, Barley CBF (2002) Gene identification, expression pattern, and map location. Plant Physiol 129:1781–1787
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162(1):156–159
Claiborne A (1985) Catalase activity. In: Greenwald RA (ed) CRC Handbook of methods in oxygen radical research. CRC Press, Boca Raton, pp 283–284
Clarkson DT, Luttge U (1989) Mineral nutrition: divalent cations, transport and compartmentalization. Prog Bot 51:93–112
Cong L, Tuan-Yao C, Zhang YX (2008) Characterization of the novel gene BjDREB1B encoding a DRE-binding transcription factor from Brassica juncea L. BiochemBiophys Res Commun 371:702–706
Curie C, Alonso, JM, Le Jean M, Ecker JR, Briat JF (2000). Involvement of NRAMP1 from Arabidopsis thaliana in iron transport. Biochem J 347:749–755
Datta K, Baisakh N, Ganguly M, Krishnan S, Yamaguchi-Shinozaki K, Datta SK (2012) Over-expression of Arabidopsis and rice stress genes inducible transcription factor confers drought and salinity tolerance to rice. Plant Biotechnol J 10:579–586
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Dinakar N, Nagajyothi PC, Suresh S, Udaykiran S, Damodharam T (2008) Phytotoxicity of cadmium on protein, proline and antioxidant enzyme activities in growing Arachis hypogaea L. seedlings. J Environ Sci 20:199–206
Dubouzet JG, Sakuma Y, Ito Y, Kasuga M, Dubouzet EG, Miura S, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) OsDREB genes in rice, Oryza sativa L. encode transcription activators that function in drought, high-salt and cold responsive gene expression. Plant J 33:751–763
Ellen G, Loon JW, Tolsma K (1990) Heavy metals in vegetables grown in the Netherlands and in domestic and imported fruits. Z Lebensm Unters Forsch 190:34–39
Floh L, Gunzler WA (1984) Glutathione peroxidase. Methods Enzymol 105:115–121
Gallego SM, Pena LB, Barcia, RA, Azpilicueta CE, Iannone MF, Rosales EP (2012) Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environ Exp Bot 83:33–46
Gaxiola RA, Fink GR, Hirschi KD (2002) Genetic manipulation of vacuolar proton pumps and transporters. Plant Physiol 129:967–973
Gonçalves JF, Nicoloso FT, da Costa P, Farias JG, Carvalho FB, da Rosa MM, Gutierres JM, Abdalla FH, Pereira JS, Dias GR, Barbosa NB, Dressler VL, Rubin MA, Morsch VM, Schetinger MR (2012) Behavior and brain enzymatic changes after long-term intoxication with cadmium salt or contaminated potatoes. Food Chem Toxicol 50:3709–3718
Gratao PL, Polle A, Lea PJ, Azevedo RA (2005) Making the life of heavy-metal stressed plants a little easier. Funct Plant Biol 32:481–494
Guerinot ML (2000) The ZIP family of metal transporters. Biochim Biophys Acta 1465:190–198
Gupta K, Agarwal PK, Reddy MK, Jha B (2010) SbDREB2A, an A-2 type DREB transcription factor from extreme halophyte Salicornia brachiata confers abiotic stress tolerance in Escherichia coli. Plant Cell Rep 29:1131–1137
Hasan SH, Talat M, Rai S (2007) Sorption of cadmium and zinc from aqueous solutions by water hyacinth (Eichchornia crassipes). Bioresour Technol 98:918–928
Hasan SA, Fariduddin Q, Ali B, Hayat Ahmad SA (2009) Cadmium: toxicity and tolerance in plants. J Environ Biol 30:165–174
Hernandez JA, Almansa MS (2002) Short-term effects of salt stress on antioxidant systems and leaf water relations of pea leaves. Physiol Plant 115:251–257
Hernandez JA, Ferrer MA, Jiminez A, Ros-Barcelo A, Sevilla F (2001) Antioxidant systems and O2/H2O2 production with NaCl-induced necrotic lesions in minor veins. Plant Physiol 127:817–831
Ishimaru Y, Takahashi R, Bashir K, Shimo H, Senoura T, Sugimoto K, Ono K, Yano M, Ishikawa S, Arao T, Nakanishi H, Nishizawa NK (2012) Characterizing the role of rice NRAMP5 in manganese, iron and cadmium transport. Sci Rep 2:286
Jaglo KR, Kleff S, Amundsen KL, Zhang X, Haake V, Zhang JZ, Deits T, Thomashow MF (2001) Components of the Arabidopsis C-repeat/dehydration responsive element binding factor cold response pathway are conserved in Brassica napus and other plant species. Plant Physiol 127:910–917
Kabata-Pendias A, Pendias H (2001) Trace elements in soils and plants, 3rd edn. CRC Press, Boca Raton
Katrin V (2014) How plants cope with heavy metals? Viehweger Bot Stud 55:35
Kukreja S, Nandwal AS, Kumar N, Sharma SK, Unvi V, Sharma PK (2005) Plant water status, H2O2, scavenging enzymes, ethylene evolution and membrane integrity of Cicer arietinum roots as affected by salinity. Biol Plant 49:305–308
Lanquar V, Lelievre F, Bolte S, Hames C, Alcon C, Neumann D et al (2005) Mobilization of vacuolar iron by AtNRAMP3 and AtNRAMP4 is essential for seed germination on low iron. EMBO J. doi:10.1038/sj.emboj.7600864
Li XP, Tian AG, Luo GZ, Gong ZZ, Zhang J, Chen SY (2005) Soybean DRE-binding transcription factors that are responsive to abiotic stresses. Theor Appl Genet 110:1355–1362
Li M, Zhang LJ, Tao L, Li W (2008) Ecophysiological responses of Jussiaea rapensto cadmium exposure. Aquat Bot 88:347–352
Licausi F, Giorgi FM, Zenoni S, Osti F, Pezzotti M, Perata P (2010) Genomic and transcriptomic analysis of the AP2/ERF superfamily in Vitis vinifera. BMC Genom 711:719
Liu CJ Guo J, Cui Y, Lü T, Zhang X, Shi G (2011) Effects of cadmium and salicylic acid on growth, spectral reflectance and photosynthesis of castorbean seedlings. Plant Soil 344:131–141
Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosyntethic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127:1781–1787
Ma Y, Zhangd L, Zhanga J, Chena J, Wu T, Zhua S, Yana Y, Zhaoa X, Zhong G (2014) Expressing a Citrus ortholog of Arabidopsis ERF1 enhanced cold-tolerance in tobacco. Sci Hortic 174:65–76
Maksymiec W (2011) Effects of jasmonate and some other signalling factors on bean and onion growth during the initial phase of cadmium action. Biol Plant 55:112–118
Morel G, Wetmore RH (1951) Fern callus tissue culture. Am J Bot 38:141–143
Mosaddek A, Huaxin D, Weite Z, Fangbin C, Guoping Z, Dongfa S, Feibo W (2013) Genotypic differences in physiological characteristics in the tolerance to drought and salinity combined stress between Tibetan wild and cultivated barley. Plant Physiol Biochem 63:49–60
Movahedi S, Tabatabaei BES, Alizade H, Ghobadi C, Yamchi A, Khaksar G (2012). Constitutive expression of Arabidopsis DREB1B in transgenic potato enhances drought and freezing tolerance. Biol Plant 56:37–42
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nakano T, Suzuki K, Fujimura T, Shinshi H (2006) Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol 140:411–432
Nevo Y, Nelson N (2006) The NRAMP family of Metalion transporters. Biochem Biophys Acta 7:609–620
Peng YL, Wang YS, Cheng H, Sun CC, Wu P, Wang LY, Fei J (2013) Characterization and expression analysis of three CBF/DREB1 transcriptional factor genes from mangrove Avicennia marina. Aquat Toxicol 141:68–76
Rae L, Lao NT, Kavanagh TA (2011) Regulation of multiple aquaporin genes in Arabidopsis by a pair of recently duplicated DREB transcription factors. Planta 234:429–444
Reis RR, da Cunha BA, Martins PK, Martins MT, Alekcevetch JC, Chalfun A Jr, Andrade AC, Ribeiro AP, Qin F, Mizoi J, Yamaguchi-Shinozaki K, Nakashima K, Carvalho Jde F, de Sousa CA, Nepomuceno AL, Kobayashi AK, Molinari HB (2014) Induced over-expression of AtDREB2A CA improves drought tolerance in sugarcane. Plant Sci 221–222:59–68
Sairam RK, Rao KV, Srivastava GC (2002) Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci 163:1037–1046
Sakuma Y, Liu Q, Dubouzet JG, Abe H, Shinozaki K, Yamaguchi-Shinozaki K (2002) DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration and cold-inducible gene expression. Biochem Biophys Res Commun 290:998–1009
Sanità di Toppi L, Gabrielli R (1999) Response to cadmium in higher plants. Environ Exp Bot 41:105–130
Sano T, Yoshihara T, Handa K, Sato M H, Nagata T, Hasezawa S (2012) Metal ion homeostasis mediated by NRAMP transporters in plant cells-focused on increased resistance to iron and cadmium ion. In: Weigert R (ed) Crosstalk and integration of membrane trafficking pathways. InTech, pp 213–228. http://www.intechopen.com/books/crosstalkand-integration-of-membrane-trafficking-pathways/metal-ion-homeostasismediated-by-nramp-transporters-in-plant-cells-focused-onincreasedresistance
Sekmen AH, Turkan I, Tanyolac ZO, Ozfidan C, Dinc A (2012) Different antioxidant defense responses to salt stress during germination and vegetative stages of endemic halophyte Gypsophila oblanceolata Bark. Environ Exp Bot 77:63–76
Senesi GS, Baldassarre G, Senesi N, Radina B (1999) Trace element inputs into soils by anthropogenic activities and implications for human health. Chemosphere 39:343–377
Sharma SS, Dietz KJ (2006) The significance of amino acids and amino acid derived molecules in plant responses and adaptation to heavy metal stress. J Exp Bot 57:711–726
Sharoni AM, Nuruzzaman M, Satoh K, Shimizu T, Kondoh H, Sasaya T, Choi IR, Omura T, Kikuchi S (2011) Gene structures, classification and expression models of the AP2/EREBP transcription factor family in rice. Plant Cell Physiol 52:344–360
Smeets K, Cuypers A, Lambrechts A, Semane B, Hoet P, Van Laere P, Vangronsveld J (2005) Induction of oxidative stress and antioxidative mechanisms in Phaseolus vulgaris after Cd application. Plant Physiol Biochem 43:437–444
Surekha Ch, Nirmala Kumari K, Aruna LV, Suneetha G, Arundhati A, Kavi Kishor PB (2014) Expression of the Vigna aconitifolia P5CSF129A gene in transgenic pigeonpea enhances proline accumulation and salt tolerance. Plant cell tiss org cult 116:27–36
Suttle J (2008) Symposium introduction: enhancing the nutritional value of potato tubers. Am J Potato Res 85:266
Szabados L, Savouré A (2009) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97
Tangahu BV, Sheikh Abdullah SR, Basri H, Idris M, Anuar A, Mukhlisin M (2011) A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int J Chem Eng. doi:10.1155/2011/939161
Thomine S, Wang R, Ward JM, Crawford NM, Schroeder JI (2000) Cadmium and iron transport by members of a plant metal transporter family in Arabidopsis with homology to Nramp genes. Proc Natl Acad Sci USA 97:4991–4996
Tiryakioglu M, Eker S, Ozkutlu F, Husted S, Cakmak I (2006) Antioxidant defense system and cadmium uptake in barley genotypes differing in cadmium tolerance. J Trace Elem Med Biol 20:181–189
Tiwari M, Sharma D, Dwivedi S, Singh M, Tripathi RD, Trivedi PK (2014) Expression in Arabidopsis and cellular localization reveal involvement of rice NRAMP, OsNRAMP1, in arsenic transport and tolerance. Plant Cell Environ 37:140–152
Tkalec M, Štefanić PP, Cvjetko P, Šikić S, Pavlica M, Balen B (2014) The effects of cadmium-zinc interactions on biochemical responses in tobacco seedlings and adult plants. PLoS One. doi:10.1371/journal.pone.0087582
Turner MA (1997) Effect of cadmium treatment on cadmium and zinc uptake by selected vegetable species. J Environ Qual 2:118–119
Vaewoerd TC, Dekker BMM, Hoekema A (1989) A small scale procedure for the rapid isolation of plant RNAs. Nucleic Acid Res 17:23–62
Viehweger K (2014) A review on How plants cope with heavy metals. Bot stud. doi:10.1186/1999-3110-55-35
Waisberg M, Joseph P, Hale B, Beyersmann D (2003) Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology 192:95–117
Weber M, Trampczynska A, Clemens S (2006) Comparative transcriptome analysis of toxic metal responses in Arabidopsis thaliana and the Cd2+ hypertolerant facultative metallophyte Arabidopsis halleri. Plant Cell Environ 29:950–963
Wei W, Chai T, Zhang Y, Han L, Xu J, Guan Z (2009) The Thlaspi caerulescens NRAMP homologueTcNRAMP3 is capable of divalent cation transport. Mol Biotechnol 41:15–21
Williams LE, Pittman JK, Hall JL (2000) Emerging mechanismsfor heavy metal transport in plants. Biochim Biophys Acta 1465:104–126
Yamasaki S, Dillenburg LC (1999) Measurements of leaf relative water content in Araucaria angustifolia. Rev Bras Fisiol Veg 11:69–75
Yan Z, Tam NFY (2013) Effect of lead stress on anti-oxidative enzymes and stress-related hormones in seedlings of Excoecaria agallocha Linn. Plant Soil 367:327–338
Yan Z, Zhang W, Chen J, Li X (2015) Methyl jasmonate alleviates cadmium toxicity in Solanum nigrum by regulating metal uptake and antioxidative capacity. Biol Plant 59:373–381
Zengin FK (2006) The effects of Co2+ and Zn2+ on the contents of protein, abscisic acid, proline and chlorophyll in bean (Phaseolus vulgaris cv. Strike) seedlings. J Environ Biol 27:441–448
Zhang H, Huang Z, Xie B, Chen Q, Tian X, Zhang X, Zhang H, Lu X, Huang D, Huang R (2004) The ethylene, jasmonate, abscisic acid and NaCl responsive tomato transcription factor JERF1 modulates expression of GCC box-containing genes and salt tolerance in tobacco. Planta 220:262–270
Acknowledgements
This work was supported by the Tunisian Ministry of High Education and Scientific Research. The authors are grateful to Anne-Lise Haenni from the Institute Jacques Monod (France) for reading and improving the English.
Authors’ contributions
MC, SC and RBM contributed to the realization of all experiments. All transgenic plants were generated by DB. RGB revised the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mariam Charfeddine and Safa Charfeddine have contributed equally in this work.
Rights and permissions
About this article
Cite this article
Charfeddine, M., Charfeddine, S., Bouaziz, D. et al. The effect of cadmium on transgenic potato (Solanum tuberosum) plants overexpressing the StDREB transcription factors. Plant Cell Tiss Organ Cult 128, 521–541 (2017). https://doi.org/10.1007/s11240-016-1130-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-016-1130-2