Abstract
bZIP transcription factors are involved in diverse cellular processes including stress response pathways in plants. In the present study, we identified a bZIP gene, MusabZIP53, from banana EST database and subsequently characterized it by overexpression in transgenic banana plants. Expression profiling in native banana plants proved that MusabZIP53 was strongly up-regulated by cold and drought stress and by ABA treatment in both leaf and root tissues. Transgenic banana plants constitutively overexpressing MusabZIP53 displayed growth retardation from early stages of transformation/regeneration protocol and mature greenhouse hardened transgenic plants displayed a distinct dwarf phenotype. Genes belonging to several families known to be involved in abiotic stress perception and mitigation were found to be differentially regulated in these transgenic plants. These included genes coding for dehydration response element binding proteins, late embryogenesis abundant proteins, anti-oxidant enzymes, aquaporins, polyphenol oxidases, Aux/IAA proteins and proteins involved in amino acid metabolism. Presence of a conserved untranslated ORF in the 5′ UTR region of MusabZIP53 gene and detection of enhanced levels of sucrose in the leaves of transgenic MusabZIP53 overexpressing banana plants indicated an important role for this bZIP gene in sucrose homeostasis in banana plants. Further, strong up-regulation of four polyphenol oxidase coding transcripts in MusabZIP53 overexpressing plants coupled with induction of these transcripts in banana leaves by cold and ABA treatments pointed towards possible involvement of MusabZIP53 in the control of total polyphenol oxidase activity in banana plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Being sessile organisms, plants are continuously exposed to different abiotic stresses like as high salt, cold and drought which in effect seriously impact the optimal plant growth and developmental processes. In order to respond to these challenges to their well being, plants have developed multiple mechanisms which work at physiological, cellular and molecular levels and enable the plant to tide over these adverse environmental conditions (Shinozaki and Yamaguchi-Shinozaki 2000). Plant hormone abscisic acid (ABA) plays an especially vital role in the signaling processes associated with these abiotic stresses in plants. Generally when a plant encounters adverse environment conditions, ABA levels increase and this leads to a chain of signal transduction events wherein multiple pathways are modulated to finally reorient the internal milieu of a plant cell such that it now has enhanced capacity to overcome the stress period (Leung and Giraudat 1998). Most genes which are observed to respond to drought, high salt and cold stress treatments are also up-regulated by exogenous ABA application (Rabbani et al. 2003). On the other hand, there are other genes which have been observed to get induced under abiotic stress conditions but are not found responsive to exogenous ABA treatments (Chinnusamy et al. 2004). The above said two groups of genes are therefore recognized as being part of either ABA-dependent or ABA-independent stress response pathways in plants. Concurrently, promoter analyses of common abiotic stress-inducible genes in different plant systems have led to the identification of two major cis-acting elements: the ABA-responsive element [(C/T)ACGTGGC] which is recognized by ABA-dependent mechanisms (Choi et al. 2000) and the DRE/CRT element [A/GCCGACNT] which is recognized by ABA independent framework (Nakashima and Yamaguchi-Shinozaki 2006). Modulation of gene expression through ABA-responsive elements is predominantly regulated by basic leucine zipper protein (bZIP) transcription factors (Chinnusamy et al. 2004). bZIPs constitute a large family of transcription factor proteins having a highly conserved bZIP domain composed of two invariate structural features: a basic domain (rich in basic amino acids) required for sequence specific DNA binding, and the leucine zipper domain which contains at least 3–4 repeats of leucine at every 7th position (heptad leucine repeat) that mediates dimerisation through hydrophobic bonding (Landschulz et al. 1988). Most bZIP proteins can form homodimers and are able to bind DNA having ACGT core sequence. Outside of this bZIP domain, bZIPs also contain other regions which may work as transcriptional activators and these include proline-rich, glutamine-rich, and acidic domains (Schindler et al. 1992). bZIP proteins are spread throughout the plant kingdom where they are involved in a multitude of physiological processes including but not limited to seed maturation and germination (Alonso et al. 2009), photomorphogenesis and light signaling (Mallappa et al. 2006), flower development and fertility (Zou et al. 2008), senescence (Yang et al. 2001) and abiotic stress responses (Hossain et al. 2010a, b).
In Arabidopsis and rice respectively, 61 and 84 members of bZIP gene family have been identified since the completion of their genome sequences. In contrast, 123 bZIP like genes have been identified in banana genome (D’Hont et al. 2012). Despite its status as one of the most important food and fruit crops of the world, research in banana stress response networks has lagged way behind comparable species like rice. In the last decade however, several concerted efforts have been made to understand the stress response regulons of banana. One such study reported stress-inducible expressed sequence tags (ESTs) from the stress tolerant Brazilian banana variety named ‘Cachaco’ (http://www.ncbi.nlm.nih.gov). Realizing the important role played by bZIP proteins in abiotic stress pathways in well studied plants like rice and Arabidopsis, we identified a putative bZIP gene from the ‘Cachaco’ EST library (Genbank accession no. DN239778) based on the assumption that comparative analysis of stressed and non-stressed tissue derived EST data sets can provide reasonable prediction about differential expression of moderately abundant ESTs (Wei et al. 2006).
Close homologs of this putative bZIP transcription factor gene have been documented to be stress-inducible in related plant species and in several cases overexpression of these bZIP genes have led to improved abiotic stress tolerance in transgenic plants (Wang et al. 2010). Further, specific bZIP genes have also been documented to be involved in sugar-regulated transcriptional control of multiple metabolic enzymes in plant cells (Hanson et al. 2008). This sucrose mediated control is exerted through an untranslated ORF (uORF) present in the 5′ UTR sequence of the specific bZIP genes (Thalor et al. 2012). Apart from definite involvement in abiotic stress response pathways and sugar signaling in plants, specific bZIP genes have been documented to play important roles in plant senescence (Yang et al. 2001). Since plant senescence is generally related with browning of the ageing plant tissue, polyphenol oxidases (PPOs) have been proposed to play important roles in these senescence processes (Kar and Mishra 1976). PPOs are especially important in context of banana fruit where any level of chilling injury imparts a PPO mediated browning and undesirable odour (Gooding et al. 2001). Induction of bZIP coding genes in response to cold stress together with their involvement in plant senescence points towards a possible correlation between bZIP proteins and chilling induced PPO activity which needs thorough investigation.
In the present study, we have carried out detailed characterization of the identified bZIP gene (Genbank accession no. DN239778) by profiling its expression in response to different abiotic stress conditions by northern blotting followed by its overexpression using a strong constitutive promoter in transgenic banana plants. Differential expression of several genes was observed in the transgenic banana plants. Further during the course of these overexpression studies we have also concluded that this bZIP gene takes part in sucrose homeostasis and exerts a coordinated control over multiple PPO coding genes in banana thereby establishing that this bZIP gene (MusabZIP53) is an important link between abiotic stress, ABA signaling and PPO activity.
Materials and methods
Primers
The primer sequences used in this study are listed in Table S1.
Amplification and sequence analysis of MusabZIP53 gene
Total RNA was extracted from young leaves of banana cv. Karibale Monthan by using Concert Plant RNA Reagent (Invitrogen, USA). This RNA was cleaned up and subjected to on column DNase treatment using RNeasy Plant Mini Kit (Qiagen, Germany). Approx. 5 μg of this RNA was utilized in synthesis of first strand cDNA using Oligo (dT)12–18 primer (Invitrogen, USA) and AccuScript Reverse Transcriptase (Stratagene, USA). The full-length coding sequence of MusabZIP53 gene was amplified from this cDNA using Pfu Ultra AD DNA Polymerase (Stratagene, USA) using the following thermal cycling conditions 94 °C for 5 min followed by 30 cycles each comprising of 94 °C for 1 min, 56 °C for 1 min and 72 °C for 1 min with a final extension at 72 °C for 15 min. Putative translated protein sequence for MusabZIP53 cDNA was obtained using ExPASy translate tool (http://au.expasy.org/tools/dna.html). This predicted MusabZIP53 protein sequence was aligned with its closest homologs (determined by Blastp program of NCBI) by using ClustalW2 program (http://www.ebi.ac.uk/Tools/clustalw2/index.html) and Box Shade server (http://www.ch.embnet.org/software/BOX_form.html). The conserved bZIP domain of MusabZIP53 protein was identified using ExPASy prosite server (http://au.expasy.org/prosite/). Evolutionary relationships for MusabZIP53 protein were determined using MEGA 5 software. Genomic context of MusabZIP53 gene was analyzed using the banana genome database. Further, the 5′ and 3′ UTRs of MusabZIP53 were predicted based on the banana EST and transcriptome datasets available in NCBI database.
Expression profiling of MusabZIP53 in banana tissues under various stress treatments by northern blotting
In vitro plantlets of banana cv. Karibale Monthan were hardened in the controlled greenhouse for 2–3 months. Uniform sized banana plants with 4–5 healthy leaves were exposed to cold stress at 8 ± 2 °C in a plant growth chamber set at 16 h light/8 h dark regime. For imparting drought stress, plants were uprooted and washed to remove the attached soil and then left to dehydrate on blotting sheets in the greenhouse. For ABA treatment, plants were treated with 100 μM ABA solution. Plants exposed only to sterile water were taken as experimental controls. Leaf and root samples derived from treated plants were immediately frozen in liquid nitrogen and stored at −80 °C freezer. Three independently treated samples for each treatment were mixed uniformly before the RNA extraction. Total RNA was extracted from the treated tissues using Concert Plant RNA Reagent (Invitrogen, USA) and RNeasy Plant Mini Kit (Qiagen, Germany) as described above and subsequently resolved using a 1.2 % (w/v) FA-MOPS agarose gel. These RNA samples were allowed to slowly migrate in 1× MOPS buffer at an applied field strength of 4–5 V/cm for 2 h. Subsequently, the RNA containing gel was washed twice with 20× SSC and then the RNA was allowed to be transferred onto positively charged nylon membrane by capillary transfer in 20× SSC buffer. RNA was subsequently cross linked to the membrane by baking at 120 °C for 30 min. DIG-labeled DNA probes targeted against MusabZIP53 cDNA sequence were used to probe the nylon membrane. Prehybridization was done at 60 °C for 2 h followed by hybridization with DIG-labeled DNA probes at 55 °C overnight. After the hybridization, the nylon membrane was washed twice each with 2× SSC and 0.1 % (w/v) SDS at room temperature and 0.1× SSC and 0.1 % (w/v) SDS at 60 °C. Chemiluminescent detection of hybridization signals was performed according to the manufacturer instructions (DIG High Prime DNA Labeling and Detection Starter Kit II, Roche Applied Science make) in a chemiluminescent enabled gel documentation system.
Construction of plant expression vector for overexpression of MusabZIP53
Plant expression cassette for overexpressing MusabZIP53 in transgenic banana plants was assembled in the MCS of pCAMBIA-1301 plant expression vector. Initially, the nos 3′ UTR amplified using pBI121 plant binary vector as template was inserted in SacI and EcoRI restriction sites in MCS of pCAMBIA-1301. Later, the Zea mays polyubiquitin promoter amplified using Zea mays genomic DNA and the MusabZIP53 cDNA amplified from the banana cv. Karibale Monthan leaf derived cDNA were inserted into the modified pCAMBIA-1301 expression vector by performing a three-way ligation reaction in which restriction sites specific for HindIII, PstI and KpnI restriction enzymes were utilized. The newly constructed binary vector was denoted as pMusabZIP53-1301. This expression vector, having the expression cassette [pZmUbi-MusabZIP53-nos] was later sequenced using appropriate primers to confirm the sequence of the cloned MusabZIP53 coding region. pMusabZIP53-1301 binary vector was then electroporated into Agrobacterium tumefaciens strain EHA 105 and subsequently used for transformation of banana embryogenic cells.
Agrobacterium-mediated genetic transformation of banana embryogenic suspension culture cells, generation of transgenic banana plants and their phenotypic assessment
Overnight grown culture of Agrobacterium tumefaciens strain EHA105 harboring pMusabZIP53-1301 binary vector was resuspended at an OD600 nm of 0.1 in the morning and grown further till an OD600 nm of 0.6–0.8 was obtained. This culture was centrifuged at 6,500g for 10 min and then the Agrobacteria were resuspended in M2 medium (Cote et al. 1996) added with 100 μM ACS. This bacterial suspension was utilized for transforming banana cv. Rasthali embryogenic suspension culture cells as described previously (Ganapathi et al. 2001). Five to seven days post-subculture cells (0.5 ml PCV) were sieved through a 85-μm sieve before being cocultivated with Agrobacterium for 30 min with intermittent shaking. These cells were then aspirated onto glass filter discs with the help of Buchner apparatus and then transferred on to semi-solid M2 medium added with 100 μM ACS. The plates containing the cells on glass filter discs were incubated in dark for 3 days at 25 ± 1 °C. After 3 days, the cells along with the filter discs were transferred to fresh semi-solid M2 medium supplemented with cefotaxime (400 mg l−1). Three days hence the cells were removed from the filters and cultured on banana embryo induction medium supplemented with cefotaxime (400 mg l−1) and hygromycin (5 mg l−1). Embryo development could be observed in 3–4 weeks and then the developing embryos were subcultured on the same medium for three subculture cycles of 3 weeks each duration along with hygromycin selection. Fully grown embryos were transferred to MS medium added with BAP (0.5 mg l−1) for efficient germination. The germinated embryos were subsequently subcultured onto banana multiplication medium (Ganapathi et al. 2008) to obtain multiple copies of the same transformed line. Individual shoot were isolated from the multiple shoot cultures and then transferred to MS medium supplemented with NAA (1 mg l−1) for efficient root development. Multiple shoot cultures and the rooted plantlets obtained after transformation with pMusabZIP53-1301 were compared with equivalent controls to analyze the effects of overexpression of MusabZIP53 on the growth and development of transgenic banana tissues. Photographic evidence of representative samples was accordingly recorded at all of these stages. The complete putatively transformed plantlets obtained were later hardened in the greenhouse using soilrite mix and then used for all the analysis.
Molecular analysis of transgenic banana plants
Genomic DNA isolated from leaves of different transgenic lines (using GenElute plant genomic DNA miniprep kit, Sigma, USA) was used as template in PCR reactions together with primers targeted against hygromycin phosphotransferase gene present within the two T-DNA borders of pMusabZIP53-1301 binary vector. Genomic DNA sourced from untransformed control banana plant was used as controls in these PCR reactions. Results obtained from genomic DNA PCR of the putatively transformed banana lines were further confirmed by Southern blot analysis. Approx. 20 μg of genomic DNA isolated from the leaves of four putatively transformed lines and untransformed control plants was restricted with KpnI restriction enzyme before being resolved in a 0.8 % (w/v) TAE agarose gel. This DNA was then transferred onto positively charged nylon membrane by capillary transfer using 20× SSC buffer. Subsequent to the cross linking of this DNA on to the nylon membrane by baking at 120 °C for 30 min, DIG-labeled probes targeted against hygromycin phosphotransferase gene were used to probe the membrane. Stringency washes and chemiluminescent detection of hybridization signals was performed according to the manufacturer instructions (DIG High Prime DNA Labeling and Detection Starter Kit II, Roche Applied Science make) in a chemiluminescent enabled gel documentation system.
Overexpression of MusabZIP53 transcript in transgenic banana plants was confirmed by northern blotting. Total RNA isolated from transgenic leaves as well as from leaves of untransformed control plants were used for this northern blot which was done as described above for expression profiling of MusabZIP53 gene in different abiotic stress conditions. Three independently grown leaf samples for each line were mixed uniformly before the RNA extraction.
Assessment of stress tolerance characteristics of MusabZIP53 overexpressing lines
Leaf disc stress tolerance assays were performed to ascertain response of MusabZIP53 overexpressing lines towards cold, drought and salt stress conditions. Leaf discs cut out of leaves derived from untransformed control plants and the four transgenic lines were exposed to cold (8 °C) for 14 days, 250 mM mannitol for 1 week and 250 mM NaCl for 1 week in 1/10 MS media in a 16 h light/8 h dark regime. The leaf discs were subsequently photographed to demonstrate and record the differential damage in the two groups of samples. Further, the level of stress damage was quantitatively estimated by determining the malondialdehyde levels in the treated leaf discs (as described in Shekhawat et al. 2011b). All the leaf discs experiments were performed in groups of five leaf discs and each treatment was repeated at least thrice.
Analysis of differential expressed genes in MusabZIP53 overexpressing banana plants
To determine the genes which are directly or indirectly regulated by MusabZIP53 gene in banana plants, we identified genes coding for dehydration response element binding (DREB) proteins, late embryogenesis abundant (LEA) proteins, anti-oxidant enzymes, aquaporins, PPOs, and Aux/IAA proteins from banana EST and transcriptome databases maintained at NCBI and also from the recently released banana genome database hosted at CIRAD, France. Several other putative MusabZIP53 targets were identified based on previous studies wherein close homologs of MusabZIP53 (AtbZIP11, AtbZIP53, TBZ17) were overexpressed in Arabidopsis and tobacco (Hanson et al. 2008; Alonso et al. 2009; Thalor et al. 2012). In total, 189 genes were identified based on the above criteria and subsequently specific primers for these genes were designed for use in real-time quantitative RT-PCR reactions wherein leaf cDNAs derived from one of the MusabZIP53 overexpressing transgenic lines and untransformed control plant were used as template. Three independently grown leaf samples for transgenic and untransformed plants were mixed uniformly before the RNA extraction. Total RNA extraction and cDNA preparation for these studies were performed as described above. REST-MCS software was used to calculate relative expression values for these genes (Pfaffl et al. 2002) and primers specific to banana EF1α gene (Chen et al. 2011) were used for normalization of expression values.
Analysis of sucrose content and sucrose mediated regulation of MusabZIP53 protein
Since the 5′ UTR of MusabZIP53 shows significant homology with an untranslated ORF documented to be involved in sucrose signaling in other plants, sucrose levels were estimated in leaves of MusabZIP53 overexpressing plants using a modified anthrone method (Finley and Fellers 1973). Herein, 500 mg of leaf tissue was first crushed under liquid nitrogen, followed by addition of 10 ml 80 % (v/v) ethanol to the crushed leaves. This mixture was collected in test tube and heated in a boiling water bath for 15 min. Subsequently, the mixture was made up to 50 ml with 80 % (v/v) ethanol. One milliliter of this solution was then added with 9 ml of freshly prepared Fehling’s solution and heated in a boiling water bath for 30 min. Subsequently, 1 ml of this solution was added with 10 ml of freshly prepared anthrone reagent and incubated at 40 °C for 30 min. Finally the absorbance was read at 610 nm. A standard curve of sucrose was prepared using the same protocol to calculate the exact amount of sucrose in the transgenic and control leaves. Sucrose estimation was repeated thrice with independent samples for each line and untransformed control plant.
To validate the role of the conserved uORF in sucrose signaling, we gave sucrose starvation treatment to banana embryogenic cells for 24 h followed by isolation of total RNA and cDNA preparation as described before. Expression levels of two genes (coding for a aspargine synthetase and a PPO enzyme) which were shown to be highly induced in MusabZIP53 overexpressing transgenic banana plants were checked before and after the sucrose starvation treatment to ascertain the effect of sucrose starvation on the translation level of MusabZIP53 protein in banana cells.
Analysis of PPO gene transcription in abiotic stress and protein activity
Since multiple PPO coding genes were shown to be up-regulated in MusabZIP53 overexpressing transgenic plants, possible correlation between cold stress, ABA treatment, MusabZIP53 up-regulation and PPO gene induction was investigated by studying the expression pattern of the four PPO coding genes (up-regulated in MusabZIP53 overexpressing transgenic banana plants) in response to ABA treatment (100 μM for 24 h) and cold stress (8 °C for 24 h). Total RNA isolated from the leaves of these treated plants was used to make cDNA as described above and this was then used in real-time quantitative RT-PCR reactions using primers specific for the four PPO coding genes mentioned above. Calculation of relative expression values and banana EF1α gene based normalization of expression values was performed as described above. Further, PPO activity in the leaves of MusabZIP53 overexpressing transgenic banana plants together with untransformed controls was determined using catechol as a substrate as described before (Sreedharan et al. 2012). Also, PPO activity in ABA treated and cold stressed leaves of control banana plant was determined similarly. PPO activity estimation was repeated thrice with independent samples for each line and untransformed control plant and also for cold and ABA treatment.
Results
Amplification and sequence analysis of MusabZIP53 gene
A novel bZIP transcription factor gene was identified from banana EST library (Genbank accession no. DN239778) based on its differential abundance in contrasting sets of abiotic stress tissue derived EST sequences. Full length coding sequence of this gene was amplified from banana cv. Karibale Monthan leaf derived cDNA and then sequenced. Since AtbZIP53 is the closest homolog of this gene among all Arabidopsis bZIP genes, it was named as MusabZIP53 to reflect this homology. Full length coding sequence of MusabZIP53 consists of 435 nucleotides. A 493 bp long 5′ UTR and a 225 nucleotide long 3′ UTR of MusabZIP53 gene were also identified after analysis of several MusabZIP53 homologous sequences sourced from EST and transcriptome libraries of banana maintained at NCBI (Fig. S1). MusabZIP53 cDNA encodes a protein consisting of 144 amino acids, having a molecular weight of 16.46 kDa and a theoretical pI of 8.11. The bZIP domain of MusabZIP53 protein, similar to its close homologs, possesses a basic domain which is rich in basic amino acids and a leucine zipper domain having heptad leucine repeat. Multiple sequence alignment of MusabZIP53 protein sequence with its closest homologs indicated that apart from the respective bZIP domains, these homologs share very little homology with each other indicating specific function for each of these proteins in the respective species (Fig. 1a, b). MusabZIP53 gene and all the close homologs have long 5′ UTRs. Investigations in close homologs like AtbZIP53 have revealed the presence of conserved uORFs in these long 5′ UTRs (Thalor et al. 2012). In fact, a highly conserved uORF was identified in the 5′ UTR of MusabZIP53 gene also. The significance of this uORF in context of regulation of MusabZIP53 activity is dealt with later. Further, no intron was identified in either the coding or untranslated regions of MusabZIP53 gene when its mRNA sequence was compared with its genomic complement available in the banana genome database (locus identifier GSMUA_Achr11T05140_001).
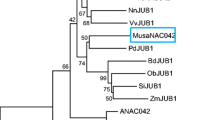
Alignment analysis and evolutionary relationships of MusabZIP53 (a) Alignment of MusabZIP53 protein sequence with sequences from Populus trichocarpa (EEE80784), Petroselinum crispum (CAC00657), Ricinus communis (EEF47269), Tamarix hispida (ACN71235), Capsicum annuum (ABB17073), Arabidopsis (NP_191801) and Oryza (ABA98933). The BZIP domain is boxed and shows relatively high homology as compared to the flanking regions. b Phylogenetic relationship of MusabZIP53 with other closely related bZIP sequences from different plant species namely Populus trichocarpa (EEE80784), Petroselinum crispum (CAC00657), Ricinus communis (EEF47269), Tamarix hispida (ACN71235), Capsicum annuum (ABB17073), Nicotiana tabacum (AAK92214), Arabidopsis thaliana (NP_191801), Theobroma cacao (EOX95259), Capsella rubella (EOA25509), Solanum lycopersicum (NP_001234339), Cicer arietinum (XP_004494422), Vitis vinifera (XP_002276485), Cucumis sativus (XP_004152226), Craterostigma plantagineum (AAZ72654), Glycine max (NP_001237222), Malus domestica (ADL36616), Antirrhinum majus (CAA74022), Vigna unguiculata (BAM93582), Prunus persica (EMJ03088) and Oryza (ABA98933). This bootstrapped tree with 1,000 replicates was constructed using ClustalW2 and MEGA 5 tools
Expression profiling of MusabZIP53 in banana tissues under various stress treatments by northern blotting
Since MusabZIP53 was initially identified from the banana EST library based on its differential abundance in abiotic stress tissue derived contrasting EST datasets, northern blotting was performed to confirm its abiotic stress inducibility. MusabZIP53 was found to be strongly induced in cold and drought stressed banana leaves as well as roots (Fig. 2a, b). This in turn validated the assumption that differential abundance in abiotic stress tissue derived contrasting EST datasets indicates stress responsive behavior of a particular gene. Further, the northern blots also indicated that the steady state levels of MusabZIP53 gene in control untreated conditions were quite low as compared to its abiotic stress induced levels. The levels of MusabZIP53 transcripts did not induce significantly in salt stressed banana leaves and roots (data not shown). Further, to confirm the involvement of ABA in the cold and drought induced expression of MusabZIP53, we checked the expression levels of MusabZIP53 in ABA treated leaf and roots of banana. ABA treatment strongly induced the levels of MusabZIP53 transcripts in banana indicating that the cold and drought inducibility of MusabZIP53 is probably ABA dependent (Fig. 2c).
Expression profiling of MusaBZIP53 under different abiotic stress stimuli. Northern blotting was performed using total RNA isolated from root and leaf of cold and drought stressed banana plants. MusaBZIP53 was found to be induced in both leaf (a) and root (b) tissues of stressed plants. Similarly, MusabZIP53 transcript was found to be induced in both leaf and root of banana plants treated with 100 μM ABA (c). rRNA bands have been shown to depict equal loading of total RNA in each lane
Generation of transgenic banana plants and their phenotypic assessment
Banana cv. Rasthali embryogenic suspension culture cells were genetically transformed using Agrobacterium tumefaciens harboring a plant binary vector designed to overexpress MusabZIP53 (pMusabZIP53-1301) constitutively in transgenic banana plants (Fig. 3a–e). Three to four weeks from transformation, several whitish embryos developed on banana embryo induction medium supplemented with hygromycin (5 mg l−1). These embryos were later subcultured on fresh medium of the same composition to obtain secondary embryos. The embryos so generated were cultured on embryo germination medium to facilitate the emergence of first shoots from them. These were later subcultured on to banana multiplication medium having BAP for multiple shoot induction. Since each emerging shoot represented a unique transformation event, multiple shoot induction was necessary to generate multiple copies of the same transformation event. These shoots were then separated and put for rooting on MS medium added with NAA. Multiple copies of each transformation event were hardened in a contained greenhouse using soilrite mix.
Generation of transgenic banana cv. Rasthali plants overexpressing MusaBZIP53. a T-DNA region of binary vector pMusaBZIP53-1301 designed to constitutively overexpress MusaBZIP53 in transgenic banana plants. b Transformed embryos on embryo induction medium. c Transgenic multiple shoots on multiple shoot induction medium. d Transgenic rooted plantlets on rooting medium along with untransformed control plantlets. e Transgenic hardened plants in greenhouse (2-months old). f Two years old transgenic plants together with a 6 months old untransformed control plant. g, h Close-up of the dwarfed transgenic plants
Severe stunting was observed in putatively transformed transgenic tissues at all stages of transformation/regeneration protocol. The embryos were slow to emerge from the transgenic tissues and they were significantly lower in number as compared to the equivalent controls. Further, multiple shoot induction and rooting of these putatively transgenic tissues was also slow. Subsequently, the hardened plants were observed to be severely dwarf as compared to controls (Fig. 3f–h). In the absence of vertical growth of banana pseudostem in these plants, there was increase in the girth of these plants overtime. The root growth was also severely stunted. The leaves of these transgenic plants showed a very typical morphology in that they were significantly thicker than the control leaves of the same length and a transverse section demonstrated that the number of aerenchyma were almost double in the transgenic leaves of the same size as the control leaf (Fig. S2). Further, due to the overall stunting, the leaves appear to form a stiff rosette on top of the plant.
Molecular analysis of transgenic banana plants
Since the growth of transformed tissues was slow and stunted from early stages, only four putative transgenic banana lines could be successfully regenerated and established in the contained greenhouse. Genomic DNA PCR analysis of the four MusabZIP53 overexpressing lines displayed a single 788-bp fragment derived from hygromycin phosphotransferase coding sequence present on the T-DNA of the transformation vector whereas it was absent in genomic DNA derived from untransformed control plants (Fig. 4a). To further confirm the transgenic nature of these transgenic banana plants, Southern blotting of restricted genomic DNA derived from these transgenic lines was undertaken using a DIG-labeled probe generated using hygromycin phosphotransferase gene coding region. Restriction enzyme KpnI was used to restrict these genomic DNAs as it is expected to cut the T-DNA of the overexpression vector only once and therefore the number of bands visible after chemiluminescence imaging can directly be recorded as the copy number of the T-DNAs integrated in the banana genome in these transgenic lines. All the four transgenic lines analyzed had a single copy of T-DNA integrated into the banana genome. The different sizes of the bands observed in these four lines established that these transgenic lines were the derived from independent transformation events (Fig. 4b). Further, to ascertain whether the T-DNAs have indeed integrated into transcriptionally active euchromatin regions of banana genome and MusabZIP53 is actually being overexpressed in the transgenic lines, northern blotting was performed using DIG-labeled DNA probes targeted against MusabZIP53 coding sequence. In the total RNA derived from untransformed control leaf, a single band corresponding to the basal level of native MusabZIP53 gene expression was noticed. In contrast, in all the four transgenic lines, in addition to this MusabZIP53 native transcript, a smaller transcript (derived from the integrated T-DNAs) was visible at a much higher intensity confirming the expression of MusabZIP53 from the constitutive expression cassette carried on the T-DNA. Since the native MusabZIP53 gene has an unusually long 5′ UTR, the native MusabZIP53 band is of significantly higher nucleotide size than the one derived from the T-DNA wherein the mature 5′ UTR derived from Zea mays polyubiquitin promoter is smaller in size (Fig. 4c).
Molecular analysis of MusabZIP53 overexpressing transgenic banana plants. a Genomic DNA-PCR analysis of untransformed control and transgenic plants. A 788-bp amplification band derived from hygromycin phosphotransferase gene carried on the T-DNA was amplified from genomic DNA derived from pMusabZIP53-1301 transformed banana lines (B1, B2, B3 and B4) whereas it was absent in untransformed banana plants (C). b Genomic Southern blot analysis of MusabZIP53 overexpressing banana plants (B1, B2, B3 and B4). Genomic DNA derived from young leaves was digested with KpnI before being immoblised and filter hybridized with DIG-labeled probes generated using hygromycin phosphotransferase gene sequence as template. Approx. positions of the DNA marker bands are indicated. c Northern blot analysis of MusabZIP53 overexpressing transgenic lines using probe against MusabZIP53 protein coding sequence. Note the presence of two bands in each of the transgenic lines (B1, B2, B3 and B4), one corresponding to the native copy of MusabZIP53 gene and the other shorter and denser one derived from the T-DNA. The total RNA derived from the untransformed control plant (C) shows only one native MusabZIP53 band
Assessment of stress tolerance characteristics of MusabZIP53 overexpressing lines
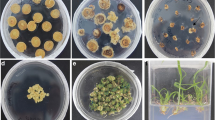
Since the transgenic plants were stunted and very slow growing as compared to the controls, whole plant assays for abiotic stress tolerance were not considered in the case of MusabZIP53 overexpressing plants. Instead, uniform sized leaf discs cut out from transgenic and control leaves were cut out using a leaf borer and used for abiotic stress tolerance test. For all the three assays performed (cold, drought and salt stress) leaf discs derived from transgenic plants performed better as indicated by visual assessment of the damage done to the leaf discs (Fig. 5a–c). The visual assessment was further confirmed by estimating the levels of malondialdehyde in the leaf discs post stress treatment. The MDA levels were found to be highest in the control leaf discs indicating maximum damage in control untransformed leaves (Fig. 5d).
Leaf disc assay for abiotic stress tolerance of MusabZIP53 overexpressing banana lines. Leaf disc derived from MusaBZIP53 overexpressing transgenic banana plants (B1, B2, B3 and B4) and untransformed controls (Control) were exposed to cold stress (a), drought stress (b) and salt stress (c) before being photographed. d MDA equivalents in leaf discs of MusabZIP53 overexpressing lines and untransformed control plants subjected to cold, drought and salt stress. Data represents mean ± SD
Analysis of differentially expressed genes in MusabZIP53 overexpressing banana plants
In order to gain clues for understanding the role of MusabZIP53 in the stunting effects and also the abiotic stress tolerance noticed in the transgenic banana plants, we attempted to identify genes whose expression is significantly modulated by MusabZIP53 overexpression in these plants. Since bZIP transcription factors are known to modulate expression of multiple gene families, we investigated differential expression of genes known to be involved in abiotic stress responses in other well studied model plants like Arabidopsis and rice. Accordingly, genes coding for DREB proteins, LEA proteins, PPOs, different anti-oxidant enzymes and those coding for aquaporins and Aux/IAA proteins were identified from banana EST and transcriptome databases maintained at NCBI and also from the recently released banana genome database. Apart from that, based on previous studies on related bZIP proteins, genes coding for several proteins involved in amino acid metabolism were identified from different banana sequence databases and investigated. While performing these studies, the size of transformed and untransformed leaves was considered to be the paramount parameter and hence transformed and untransformed leaves of approximately same size were chosen for studying differential expression of genes (Fig. S2).
Among the 92 DREB protein coding genes tested, we could detect differential expression in 10 genes. Similarly, among the 30 LEA protein coding genes, 8 PPO coding genes, 7 anti-oxidant protein coding genes, 13 aquaporin coding genes and 23 Aux/IAA protein coding genes, respectively 2 LEA protein coding genes, 4 PPO coding genes, 3 anti-oxidant protein coding genes, 1 aquaporin coding genes and 3 Aux/IAA protein coding genes were differentially regulated in MusabZIP53 overexpressing plants. Further, among the 16 genes coding for proteins involved in amino acid metabolism, 2 genes coding for asparagine synthetases were noticed to be highly up-regulated in MusabZIP53 overexpressing plants. In total, 25 genes were found to be differentially regulated out of a total of 189 tested. Also most of these genes were up-regulated whereas only five were found to be down-regulated (Fig. 6).
Differential expression of genes known to be involved in abiotic stress reponses in MusabZIP53 overexpressing transgenic banana lines. In total, 25 genes out of a total of 189 tested were found to be differentially expressed. Banana genome database locus identifiers have been used to depict each differentially regulated gene. From left to right, the first 10 genes are DREB protein coding genes followed by 4 PPO coding genes, 2 LEA protein coding genes, 3 anti-oxidant protein coding genes, 3 Aux/IAA protein coding genes, 2 asparagine synthetase coding genes and one aquaporin coding gene. The gene expression values have been normalized against Musa EF1α cDNA expression levels and the x-axis represents the expression level of the respective gene in untransformed control plant. Values are mean ± SE
Analysis of sucrose content and SIRT based regulation of MusabZIP53 protein
Plants overexpressing close homologs of MusabZIP53 have been reported to have elevated sucrose levels in their leaves. Further, these genes have also been reported to possess a highly conserved uORF in their long 5′ UTRs (Fig. 7a). These studies have postulated that these bZIP genes possessing this uORF are involved in sucrose sensing such that their translation is slowed down if the amount of cellular sucrose increases and vice versa. In our studies also, we observed the levels of sucrose were higher by approx. 40 % in the transgenic leaves as compared to the equivalent controls (Fig. 7b). Further to prove that sucrose indeed affects the translation of MusabZIP53 gene, we used banana embryogenic suspension culture cells growing in modified MS medium with 4.5 % (w/v) sucrose. When these cells were starved of sucrose for 24 h, we noticed that the transcript levels of two genes which are up-regulated in untreated MusabZIP53 overexpressing leaves were induced to significant levels indicating that sucrose starvation indeed leads to elevated translation levels of MusabZIP53 in banana cells leading to induction of the two genes as described above (Fig. 7c).
SIRT in MusabZIP53 gene and enhanced sucrose content in MusabZIP53 overexpressing lines. a Schematic representation of the structure of MusabZIP53 gene with its conserved untranslated ORF present in the 5′ UTR and its homologous sequences from other related plant species. b Enhanced sucrose contents in the transgenic banana lines (B1, B2, B3 and B4) as compared to the untransformed controls (c). Elevated transcript levels of two putative targets of MusabZIP53 protein in sucrose starvation conditions. GSMUA_Achr1T21220_001 codes for asparagines synthetase enzyme and GSMUA_AchrUn_randomT25220_001 codes for a PPO. The gene expression values have been normalized against Musa EF1α cDNA expression levels and the x-axis represents the expression level of the respective gene in control conditions (4.5 % w/v sucrose concentration). Values are mean ± SE
Analysis of PPO gene transcription in abiotic stress and protein activity
Out of a total of eight PPO coding genomic loci detected in banana genome database, we could determine sequences corresponding to only four of these in the extensive EST and transcriptome databases of banana maintained at NCBI. Further, we could not detect the other four PPO sequences by RT-PCR using our banana tissues probably indicating that these four are not expressed to detectable levels. Further, all the four PPO sequences detected in EST and transcriptome databases were found to be up-regulated in MusabZIP53 overexpressing plants (Fig. 6). Since all the four expressed PPO coding genes were up-regulated in the transgenic MusabZIP53 overexpressing plants, we wanted to investigate whether the MusabZIP53 gene exerts a coordinated control of the total PPO activity in banana and whether these PPO genes are in any way related to abiotic stress response pathways in banana plant. Hence, PPO coding gene transcript levels were determined in cold and ABA stressed banana leaves. All the four PPO coding transcripts demonstrated to be up-regulated in MusabZIP53 overexpressing plants were found to be induced in ABA treated banana leaves also whereas in case of cold stressed leaves, two of these were induced to high levels whereas the other two were induced to moderate levels (Fig. 8a). To validate the correlation between elevated transcript levels and increased protein activity, total PPO activity was determined in the leaves of four transgenic plants and in the untransformed controls and also in cold and ABA treated untransformed banana plants. Approx. 20–25 % higher PPO activity was noticed in all the four transgenic lines indicating higher transcript levels do lead to increased protein activity, although increased transcript levels could not be linearly correlated with increase in protein activity (Fig. 8b). Further, higher PPO activity was noticed in ABA and cold stressed banana leaves as compared to the equivalent untreated controls and this increase in PPO activity was commensurate with the higher PPO activity in untreated MusabZIP53 overexpressing plants (Fig. 8b). These results are significant since alteration of PPO activity in response to abiotic stress (most commonly cold temperatures) affects the visual and sensory quality of banana fruits.
Analysis of PPO gene transcriptional levels in abiotic stress and protein activity. a All the four PPO coding genes found to be expressed in banana tissues (GSMUA_AchrUn_randomT25220_001, GSMUA_Achr7T03450_001, GSMUA_Achr8T34370_001, GSMUA_Achr10T20540_001) are induced in response to ABA or cold treatment. The gene expression values have been normalized against Musa EF1α cDNA expression levels and the x-axis represents the expression level of the respective PPO coding gene in control conditions. Values are mean ± SE. b Total PPO activity is higher in all the transgenic lines generated as compared to equivalent controls. Further, total PPO activity increase in ABA and cold treated leaves is in line with increased transcription of PPO coding genes. Values are mean ± SD
Discussion
bZIP transcription factors have been implicated in several important cellular processes and hence investigations into the roles played by various members of bZIP gene family in plants is important. Many of these bZIP transcription factors have been in the past associated with abiotic stress responses wherein their overexpression has resulted in improved abiotic stress tolerance in the transgenic plants. ThbZIP1 gene from Tamarix hispida enhanced the salt stress tolerance in transgenic tobacco plants (Wang et al. 2010). Transgenic Arabidopsis plants overexpressing three soybean bZIP genes displayed better tolerance to salt and freezing stress as compared to respective controls (Liao et al. 2008). Transgenic tobacco plants overexpressing a wheat bZIP gene Wlip19 showed significantly improved freezing stress tolerance (Kobayashi et al. 2008). Arabidopsis bZIP gene ABF3 improved abiotic stress tolerance in rice without stunting the growth of transgenic rice plants (Oh et al. 2005). OsABF1 and OsABF2 bZIP transcription factor genes from rice have been shown to be positive regulators of abiotic stress tolerance (Hossain et al. 2010a, b). As is evident, in most cases these investigations have involved genes derived from important model plants like Arabidopsis or staples like rice while the equally significant plants like banana, with huge food security role and trade potential, have been largely left untouched. However, probably as a result of realization of the importance of banana in global food paradigm, several studies have been recently undertaken to investigate banana abiotic stress signaling and its mitigation. These studies have mostly focused on characterization of specific genes predicted to be involved in abiotic stress responses. These include characterization of MusaDHN-1 (Shekhawat et al. 2011b), MusaSAP1 (Sreedharan et al. 2012) MusaWRKY71 (Shekhawat et al. 2011a, Shekhawat and Ganapathi 2013), MpRCI (Feng et al. 2009) and MpAsr (Liu et al. 2010; Dai et al. 2011). A bZIP gene, MabZIP3, from banana was recently reported to be responsive to methyl jasmonate, abscisic acid, chilling stress and pathogen Colletotrichum musae (He et al. 2013). The present study deals with the characterization of another important abiotic stress inducible bZIP gene from banana. As our results demonstrate, MusabZIP53 plays an important part in different abiotic stress response pathways in banana which ultimately lead to a coherent response upon the onset of abiotic stress.
MusabZIP53 was initially identified based on its differential abundance in the two contrasting EST datasets derived from abiotic stress tolerant banana cultivar ‘Cachaco’. Since it was found to be more abundant in the EST library derived from stressed ‘Cachaco’ tissue, we predicted it to be involved in abiotic stress tolerance in banana plant. Similar predictions based on such libraries have been validated by us in the past by performing detailed studies on multiple genes wherein overexpression of full length coding sequences of these genes resulted in transgenic banana plants displaying improved tolerance towards multiple abiotic stress stimuli (Shekhawat et al. 2011a, b). Once MusabZIP53 was confirmed to be significantly induced under different abiotic stress stimuli by northern blotting, we postulated that a positive correlation must exist between MusabZIP53 up-regulation and abiotic stress tolerance in banana plants. Towards this end, MusabZIP53 was constitutively overexpressed in transgenic banana plants using highly efficient Zea mays polyubiquitin promoter. Although the resulting transgenic plants were severely stunted and dwarf with a very different phenotype than the untransformed controls, abiotic stress assays performed using leaf discs confirmed that these plants tolerate abiotic stress conditions much better that the equivalent controls. Genes belonging to multiple gene families reported to be involved in abiotic stress perception and tolerance were observed to be differentially regulated in MusabZIP53 overexpressing banana plants. These included genes coding for DREB proteins, LEA proteins, aquaporins, anti-oxidant enzymes, PPOs and others. Further, the fact that a single bZIP gene was able to regulate genes coding for several different protein classes indicated that this bZIP gene probably acts higher up in the abiotic stress regulatory networks to simultaneously affect several cellular processes in the aftermath of stress perception in banana plants.
Although several studies have previously reported growth stunting as a side effect of overexpression of potent transcription factor genes in plants, MusabZIP53 overexpressing plants were very unique in the sense that even after 3.5 years of growth in rich organic manure supplemented soil mix with ample growing room, they did not raise even 30 cm in height. To couple that, the stunting effect was even more pronounced in the leaves which were rather very stiff and arranged in a uniform rosette around the pseudostem. Although, we were not able to pinpoint the definite reasons behind this severe stunting, simultaneous expression of multiple genes which otherwise express only under strong abiotic stress conditions may be responsible for this type of stunting phenotype. The differential regulation of several DREB transcription factor genes in these transgenic plants was a clear indicator of this fact. Since each transcription factor gene is expected to modulate the expression of several effector genes at the cellular level, the cellular homeostasis in these plants is strongly altered. The transcriptional load arising out of expression of several genes and proteins, which normally are required only for smaller periods of time immediately in the aftermath of stress perception and whose expression is mostly maintained at lower insignificant levels in unstressed conditions, probably induces a strong inhibitory effect on plant growth and development. Even the MusabZIP53 gene itself is maintained at very low cellular levels in the banana plants under control conditions and gets induced to high levels only under stressed conditions. Since the duration of abiotic stress in natural conditions is quite short, the effect of induction of these stress specific effector genes does not significantly affect the growth trajectory of the plants under stress. In contrast, in MusabZIP53 overexpressing plants, since the transcript levels of MusabZIP53 are always high owing to its constitutive overexpression, the target genes and proteins which are otherwise only momentarily induced are also up-regulated constitutively. This probably explains the extreme dwarf phenotype that is observed in the transgenic MusabZIP53 overexpressing plants. Even so, Arabidopsis homolog of one of the DREB genes found to be up-regulated in MusabZIP53 overexpressing plants (referred to as TINY), is known to cause similar stunting of transgenic plants when it is overexpressed (Sun et al. 2008). Therefore in our study where several DREB genes are simultaneously differentially expressed, such stunting effect is but expected.
Another unique feature of these transgenic plants was that they had almost 40 % higher sucrose content as compared to equivalent controls. Similar results were reported in studies wherein close homologs of MusabZIP53 (Thalor et al. 2012) were overexpressed in transgenic plants. These two genes share a conserved uORF in the 5′ UTR with MusabZIP53 and have been implicated in cellular responses to low energy conditions such as darkness, low sugar and stress conditions. According to Thalor et al. (2012) in tobacco, in low cellular energy conditions, TBZ17 transactivates asparagine synthetase gene and induces metabolic reprogramming, thereby turning on the sucrose synthesis pathway. Further, if the end product sucrose reaches an upper limit, the excess sucrose acts via the conserved uORF to suppress the translation of TBZ17 in a feedback manner. This phenomenon was referred to as sucrose induced repression of translation. On the other hand, in plants overexpressing TBZ17 without the linked uORF, even if sucrose concentration reaches an upper threshold, there is no translation repression and the TBZ17 translation continues increasing the total sucrose content of the cells (Thalor et al. 2012). The same feedback loop appears to be conserved in banana also with MusabZIP53 sharing the critical conserved uORF with TBZ17 and AtbZIP53. Similar to TBZ17 mediated up-regulation of asparagine synthetase coding gene in tobacco, we also found that two banana asparagine synthetase coding genes are highly up-regulated in the transgenic MusabZIP53 overexpressing plants. And finally, like the TBZ17 and AtbZIP53 overexpressing tobacco and Arabidopsis plants, the banana plants overexpressing MusabZIP53 were also stunted and the leaves were thicker than the controls. Lastly, since a very strong constitutive promoter (derived from Zea mays polyubiquitin) was used in our banana studies, the level of stunting was very severe in our case.
During the course of our studies aimed at investigating the differentially expressed genes in MusabZIP53 overexpressing plants, we noticed that all the four PPO coding genes (out of a total of eight such genomic loci) which are expressed in banana plants, are induced in MusabZIP53 overexpressing plants. Since there have been no reports of induction of PPO coding genes in plants overexpressing close homologs of MusabZIP53, we found the coordinated up-regulation of all the four expressed PPO coding genes in our transgenic plants very interesting. Role of PPOs in oxidation of plant flavonoids has been studied in detail in the past (Pourcel et al. 2007) and a positive correlation is known to exist between stress tolerance and induction of PPOs. Since PPOs are especially important in context of banana owing to the negative sensory and visual characteristics they impart to the banana fruit (Gooding et al. 2001). We wanted to investigate the correlation between abiotic stress induction of MusabZIP53 and the up-regulation of these PPO coding genes in the transgenic plants overexpressing MusabZIP53. As expected, all the four PPO coding genes that are induced in MusabZIP53 overexpressing plants were also found to be induced in cold as well as ABA treated control untransformed banana plants. Further, the total PPO activity was also higher in the stressed tissues as compared to the controls. Taken together, these results indicate that probably MusabZIP53 plays an intermediary role between the perception of abiotic stress and up-regulation of PPO coding transcripts. In one of earlier studies, we had reported a similar up-regulation of one of these PPO coding genes in transgenic plants overexpressing a zinc finger containing stress associated protein gene, MusaSAP1 (Sreedharan et al. 2012). This transcript was also demonstrated to be inducible in response to methyl jasmonate treatment and wounding stress as was the case with MusaSAP1 gene itself. Results of the present study are unique in the sense that all four expressed PPO gene transcripts are induced to significant levels in the transgenic banana plants indicating major role for MusabZIP53 gene in regulation of PPO activity in banana plants.
In conclusion, the study presented here proved that MusabZIP53 gene plays important roles in abiotic stress related signaling in banana. Further, it also takes part in sucrose homeostasis in banana plant through a linked uORF present in its 5′ UTR. Also, our findings pertaining to the coordinated induction of all four expressed PPO coding genes by MusabZIP53 gene (in effect controlling the total cellular PPO activity) assign a hitherto unknown role for bZIP genes in plants.
Abbreviations
- ABA:
-
Abscisic acid
- ACS:
-
Acetosyringone
- BAP:
-
6-Benzylaminopurine
- PCV:
-
Packed cell volume
- PPO:
-
Polyphenol oxidase
- NAA:
-
Napthalene acetic acid
- SIRT:
-
Sucrose induced repression of translation
References
Alonso R, Onate-Sanchez L, Weltmeier F, Ehlert A, Diaz I, Dietrich K, Vicente-Carbajosa J, Droge-Laser W (2009) A pivotal role of the basic leucine zipper transcription factor bZIP53 in the regulation of Arabidopsis seed maturation gene expression based on heterodimerization and protein complex formation. Plant Cell 21:1747–1761
Chen L, Zhong HY, Kuang JF, Li JG, Lu WJ, Chen JY (2011) Validation of reference genes for RT-qPCR studies of gene expression in banana fruit under different experimental conditions. Planta 234:377–390
Chinnusamy V, Schumaker K, Zhu JK (2004) Molecular genetic perspectives on cross-talk and specificity in abiotic stress signalling in plants. J Exp Bot 55:225–236
Choi HI, Hong JH, Ha JO, Kang JY, Kim SY (2000) ABFs, a family of ABA-responsive element binding factors. J Biol Chem 275:1723–1730
Cote FX, Domergue R, Monmarson S, Schwendiman J, Teisson C, Escalant JV (1996) Embryogenic cell suspensions from the male flower of Musa AAA cv. Grand Nain. Physiol Plant 97:285–290
D’Hont A, Denoeud F, Aury JM, Baurens FC, Carreel F, Garsmeur O, Noel B, Bocs S, Droc G, Rouard M, Da Silva C, Jabbari K, Cardi C, Poulain J, Souquet M, Labadie K, Jourda C, Lengelle J, Rodier-Goud M, Alberti A, Bernard M, Correa M, Ayyampalayam S, McKain MR, Leebens-Mack J, Burgess D, Freeling M, Mbeguie AMD, Chabannes M, Wicker T, Panaud O, Barbosa J, Hribova E, Heslop-Harrison P, Habas R, Rivallan R, Francois P, Poiron C, Kilian A, Burthia D, Jenny C, Bakry F, Brown S, Guignon V, Kema G, Dita M, Waalwijk C, Joseph S, Dievart A, Jaillon O, Leclercq J, Argout X, Lyons E, Almeida A, Jeridi M, Dolezel J, Roux N, Risterucci AM, Weissenbach J, Ruiz M, Glaszmann JC, Quetier F, Yahiaoui N, Wincker P (2012) The banana (Musa acuminata) genome and the evolution of monocotyledonous plants. Nature 488:213–217
Dai JR, Liu B, Feng DR, Liu HY, He YM, Qi KB, Wang HB, Wang JF (2011) MpAsr encodes an intrinsically unstructured protein and enhances osmotic tolerance in transgenic Arabidopsis. Plant Cell Rep 30:1219–1230
Feng DR, Liu B, Li WY, He YM, Qi KB, Wang HB, Wang JF (2009) Over-expression of a cold-induced plasma membrane protein gene (MpRCI) from plantain enhances low temperature resistance in transgenic tobacco. Environ Exp Bot 65:395–402
Finley JW, Fellers DA (1973) Sucrose determination by a modified anthrone method. Application with sweetened wheat–soy blend and corn–soy–milk. Cereal Chem 50:210–214
Ganapathi TR, Higgs NS, Balint-Kurti PJ, Arntzen CJ, May GD, Van Eck JM (2001) Agrobacterium-mediated transformation of embryogenic cell suspensions of the banana cultivar Rasthali (AAB). Plant Cell Rep 20:157–162
Ganapathi TR, Sidhaa M, Suprasannaa P, Ujjappa KM, Bapat VA, D’Souza SF (2008) Field performance and RAPD analysis of gamma-irradiated variants of banana cultivar ‘giant cavendish’ (AAA). Int J Fruit Sci 8:147–159
Gooding PS, Bird C, Robinson SP (2001) Molecular cloning and characterization of banana fruit polyphenol oxidase. Planta 213:748–757
Hanson J, Hanssen M, Wiese A, Hendriks M, Smeekens S (2008) The sucrose regulated transcription factor bZIP11 affects amino acid metabolism by regulating the expression of asparagine synthetase 1 and proline dehydrogenase 2. Plant J 53:935–949
He S, Shan W, Kuang J-f, Xie H, Xiao Y-y, Lu W-j, Chen J-y (2013) Molecular characterization of a stress-response bZIP transcription factor in banana. Plant Cell Tissue Organ Cult 113:173–187
Hossain MA, Lee Y, Cho JI, Ahn CH, Lee SK, Jeon JS, Kang H, Lee CH, An G, Park PB (2010a) The bZIP transcription factor OsABF1 is an ABA responsive element binding factor that enhances abiotic stress signaling in rice. Plant Mol Biol 72:557–566
Hossain MA, Cho JI, Han M, Ahn CH, Jeon JS, An G, Park PB (2010b) The ABRE-binding bZIP transcription factor OsABF2 is a positive regulator of abiotic stress and ABA signalling in rice. J Plant Physiol 167:1512–1520
Kar M, Mishra D (1976) Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol 57:315–319
Kobayashi F, Maeta E, Terashima A, Kawaura K, Ogihara Y, Takumi S (2008) Development of abiotic stress tolerance via bZIP-type transcription factor LIP19 in common wheat. J Exp Bot 59:891–905
Landschulz WH, Johnson PF, McKnight SL (1988) The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science 240:1759–1764
Leung J, Giraudat J (1998) Abscisic acid signal transduction. Annu Rev Plant Physiol Plant Mol Biol 49:199–222
Liao Y, Zou HF, Wei W, Hao YJ, Tian AG, Huang J, Liu YF, Zhang JS, Chen SY (2008) Soybean GmbZIP44, GmbZIP62 and GmbZIP78 genes function as negative regulator of ABA signaling and confer salt and freezing tolerance in transgenic Arabidopsis. Planta 228:225–240
Liu HY, Dai JR, Feng DR, Liu B, Wang HB, Wang JF (2010) Characterization of a novel plantain Asr gene, MpAsr, that is regulated in response to infection of Fusarium oxysporum f. sp. cubense and abiotic stresses. J Integr Plant Biol 52:315–323
Mallappa C, Yadav V, Negi P, Chattopadhyay S (2006) A basic leucine zipper transcription factor, G-box-binding factor 1, regulates blue light-mediated photomorphogenic growth in Arabidopsis. J Biol Chem 281:22190–22199
Nakashima K, Yamaguchi-Shinozaki K (2006) Regulons involved in osmotic stress-responsive and cold stress-responsive gene expression in plants. Physiol Plant 126:62–71
Oh SJ, Song SI, Kim YS, Jang HJ, Kim SY, Kim M, Kim YK, Nahm BH, Kim JK (2005) Arabidopsis CBF3/DREB1A and ABF3 in transgenic rice increased tolerance to abiotic stress without stunting growth. Plant Physiol 138:341–351
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30:e36
Pourcel L, Routaboul JM, Cheynier V (2007) Flavonoid oxidation in plants: from biochemical properties to physiological functions. Trends Plant Sci 12:29–36
Rabbani MA, Maruyama K, Abe H, Khan MA, Katsura K, Ito Y, Yoshiwara K, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) Monitoring expression profiles of rice genes under cold, drought, and high-salinity stresses and abscisic acid application using cDNA microarray and RNA gel-blot analyses. Plant Physiol 133:1755–1767
Schindler U, Menkens AE, Beckmann H, Ecker JR, Cashmore AR (1992) Heterodimerization between light-regulated and ubiquitously expressed Arabidopsis GBF bZIP proteins. EMBO J 11:1261–1273
Shekhawat UKS, Ganapathi TR (2013) MusaWRKY71 overexpression in banana plants leads to altered abiotic and biotic stress responses. PLoS One. doi:10.1371/journal.pone.0075506
Shekhawat UKS, Ganapathi TR, Srinivas L (2011a) Cloning and characterization of a novel stress-responsive WRKY transcription factor gene (MusaWRKY71) from Musa spp. cv. Karibale Monthan (ABB group) using transformed banana cells. Mol Biol Rep 38:4023–4035
Shekhawat UKS, Ganapathi TR, Srinivas L (2011b) MusaDHN-1, a novel multiple stress-inducible SK3-type dehydrin gene, contributes affirmatively to drought- and salt-stress tolerance in banana. Planta 234:915–932
Shinozaki K, Yamaguchi-Shinozaki K (2000) Molecular responses to dehydration and low temperature: differences and cross-talk between two stress signaling pathways. Curr Opin Plant Biol 3:217–223
Sreedharan S, Shekhawat UKS, Ganapathi TR (2012) MusaSAP1, a A20/AN1 zinc finger gene from banana functions as a positive regulator in different stress responses. Plant Mol Biol 80:503–517
Sun S, Yu JP, Chen F, Zhao TJ, Fang XH, Li YQ, Sui SF (2008) TINY, a dehydration-responsive element (DRE)-binding protein-like transcription factor connecting the DRE- and ethylene-responsive element-mediated signaling pathways in Arabidopsis. J Biol Chem 283:6261–6271
Thalor SK, Berberich T, Lee SS, Yang SH, Zhu X, Imai R, Takahashi Y, Kusano T (2012) Deregulation of sucrose-controlled translation of a bZIP-type transcription factor results in sucrose accumulation in leaves. PLoS One 7:e33111
Wang Y, Gao C, Liang Y, Wang C, Yang C, Liu G (2010) A novel bZIP gene from Tamarix hispida mediates physiological responses to salt stress in tobacco plants. J Plant Physiol 167:222–230
Wei H, Dhanaraj AL, Arora R, Rowland LJ, Fu Y, Sun L (2006) Identification of cold acclimation-responsive Rhododendron genes for lipid metabolism, membrane transport and lignin biosynthesis: importance of moderately abundant ESTs in genomic studies. Plant Cell Environ 29:558–570
Yang S, Berberich T, Sano H, Kusano T (2001) Specific association of transcripts of tbzF and tbz17, tobacco genes encoding basic region leucine zipper-type transcriptional activators, with guard cells of senescing leaves and flowers. Plant Physiol 127:23–32
Zou M, Guan Y, Ren H, Zhang F, Chen F (2008) A bZIP transcription factor, OsABI5, is involved in rice fertility and stress tolerance. Plant Mol Biol 66:675–683
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shekhawat, U.K.S., Ganapathi, T.R. Transgenic banana plants overexpressing MusabZIP53 display severe growth retardation with enhanced sucrose and polyphenol oxidase activity. Plant Cell Tiss Organ Cult 116, 387–402 (2014). https://doi.org/10.1007/s11240-013-0414-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-013-0414-z