Abstract
Molecular cloning and characterization of a CLAVATA1-LIKE gene in Pinus pinaster and Pinus pinea is reported. CLAVATA1 is a well-characterized gene in Arabidopsis integral to the balance between primordial differentiation and meristem proliferation. Currently, it is not known if the Arabidopsis model of in vitro caulogenesis is applicable to conifers. In this work, we study the putative role of the CLAVATA1-LIKE gene during caulogenic induction in cotyledons of P. pinea and P. pinaster after exposure to benzyladenine. Expression analysis showed that the gene was differentially expressed during the first phases of adventitious caulogenesis in cotyledons from both species, suggesting that CLAVATA1-LIKE may play a role in caulogenesis in conifers. A binary vector carrying CLAVATA1-LIKE promoter driven GFP:GUS expression was constructed. Results of genetic transformation showed GUS activity during somatic embryogenic mass proliferation and embryo maturation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adventitious shoot formation from somatic cells, also known as caulogenesis, is a postembryonic organogenic process in which shoot meristems form where shoots do not normally appear. It can occur naturally or can be induced in vitro by using the appropriate hormone balance, usually involving cytokinins, culture medium and environmental conditions. In addition to its biotechnological relevance, organogenesis in vitro provides systems for studying regulatory mechanisms of plant development (Christianson and Warnick 1988; Sugiyama 1999).
Cytokinin-induced in vitro shoot regeneration in Pinus spp. from cotyledonary explants is an efficient and reliable model for the study of caulogenesis in conifers, and it is particularly useful to study the cytokinin mode of action (Moncaleán et al. 2005; Alonso et al. 2007; Cortizo et al. 2009; Cortizo et al. 2010; Álvarez et al. 2012). The system is based on the induction of direct adventitious shoot formation (caulogenesis) in cotyledons excised from mature embryos and cultured in the presence of the cytokinin benzyladenine (BA). This caulogenic induction is determined by the dynamics of BA absorption and metabolism and does not require an intermediate callus phase, because the cotyledons are competent to form buds per se (Valdés et al. 2001; Moncaleán et al. 2005; Cortizo et al. 2009; Cuesta et al. 2009).
The knowledge obtained from in vitro culture in Arabidopsis thaliana has enabled the construction of a model that describes the molecular processes that lead to de novo meristem formation upon exposure to cytokinins (Cary et al. 2002; Meng et al. 2010), but only in regeneration systems that require an initial de-differentiation stage in an auxin-rich medium. Moreover, by combining these findings with studies involving mutants and transgenic, a model has been proposed that integrates the cytokinin signaling response and shoot development in planta (Howell et al. 2003; Shani et al. 2006). In recent years, increasing information available about the mechanism of cytokinin signal transduction and the identification of several genes involved in meristem formation in Arabidopsis, have led to a model being proposed that integrates both routes in angiosperms (Gordon et al. 2009; Sablowski 2009). Briefly, the cytokinin signal transduction pathway is mediated by a two-component phosphorelay system responsible for transmitting the initial signal to response regulators (ARRs), nuclear proteins responsible for the cytokinin effects on gene and protein function. Despite the target genes of the cytokinin primary response remaining unknown, it has been shown that cytokinins end up activating several regulatory genes involved in shoot meristem formation such as SHOOTMERISTEMLESS (STM), KNOTTED-1 (KN1), WUSCHEL (WUS) and CLAVATA-1 (CLV1) (Cary et al. 2002; Che et al. 2002; Zhang and Lemaux 2004). WUS together with CLV1 and its ligand CLV3 form a regulatory loop that controls the balance between meristem cell proliferation and differentiation (Fletcher et al. 1999; Schoof et al. 2000). It has been shown as well that WUS represses the transcription of several type-A ARRs, establishing a negative feedback loop that controls cell division in the meristem (Leibfried et al. 2005). The CLAVATA1 gene encodes a receptor kinase with a role in signal transduction associated with meristematic activity (Clark et al. 1997; Nimchuk et al. 2011). CLV3 acts as the ligand for CLV1 as part of a multimeric complex that control the balance between meristem cell proliferation and differentiation by restricting the expression of WUSCHEL to a few cells in the meristem (Fletcher et al. 1999).
Although in vitro caulogenesis in gymno- and angiosperm species share morphological and physiological features, there are key differences that may alter the underlying genetic programs. Due to the phylogenetic distance between conifers and flower plants it may be possible that some of the genes implicated in shoot organogenesis differ. Moreover, in most of the studies dealing with general topics of plant development, the particular characteristics of conifers are ignored (Carles and Fletcher 2003). This angiosperm-centric analysis is useful to obtain a general picture of adventitious shoot formation in plants, but should be validated in conifers.
As part of a study aimed at understanding the physiological and molecular mechanisms involved in adventitious shoot bud formation in pine cotyledons, we conducted a transcriptome analysis to identify early-induced genes during the first phases of adventitious caulogenesis in P. pinea cotyledons cultured in the presence of BA (Alonso et al. 2007). One of the ESTs identified in that study (GenBank EC428669) showed high similarity with leucine-rich repeat receptor protein kinase genes from various angiosperms, CLAVATA1 among them. Moreover, it was found to be overexpressed in pine cotyledons cultured for 16 h in BA-containing medium.
Due to its similarity to one of the key actors of meristem identity, in this work, we aimed to characterize and study the putative role of this CLAVATA1-LIKE gene during caulogenic induction in cotyledons of P. pinea and P. pinaster after exposure to BA. To do so, we have obtained the full coding sequence of the gene in both species, we have cloned its promoter in P. pinaster and we have studied the expression of the gene during the first stages of adventitious bud formation. Finally, in an effort to gain insight into its in planta expression we have studied the promoter activity in P. pinaster using Agrobacterium-mediated genetic transformation.
Materials and methods
CLAVATA1-LIKE gene expression in cotyledons during caulogenic induction
Plant material and culture conditions
Mature seeds from open-pollinated Pinus pinea L. trees growing in Spanish natural stands (ES-23/01 provenance) and Pinus pinaster Ait. trees (Atlantic provenance) were surface sterilized and treated according to Alonso et al. (2006); Álvarez et al. (2009a) respectively.
RNA extraction and cDNA synthesis
RNA was extracted using the NucleoSpin® RNA Plant kit (Macherey–Nagel, Germany) following the manufacturer′s instructions. The RNA concentration was measured using a picodrop microliter spectrophotometer (Picodrop Limited, United Kingdom) and the RNA integrity was tested in a 1 % agarose gel. One microgram of total RNA was reverse transcribed using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems Inc., Foster City, CA, USA) following the manufacturer’s protocol.
Cloning of a CLAVATA1-LIKE gene from P. pinea and P. pinaster
In a previous study (Alonso et al. 2007), an EST showing high similarity with a leucine rich repeat (LRR) serine/threonine protein kinase receptor was identified (GenBank EC428669). This EST was used to design specific primers for Rapid Amplification of cDNA Ends (RACE). RACE was performed using the First Choice® RLM-RACE kit (Ambion, Applied Biosystems Inc.) following manufacturer’s instructions, using the SuperTaq Plus® Taq polymerase for PCR amplification (Ambion, Applied Biosystems Inc.). Bands obtained after RACE were isolated from agarose gel and extracted with the NucleoSpin® EXTRACT II kit (Macherey–Nagel). Then, bands were cloned into the pGEMTeasy vector (Promega, Madison, WI, USA) and sequenced (at least three clones per band) in the Oviedo University DNA Analysis Facility (Spain). Once sequenced the complete cDNA, DNA was extracted using the NucleoSpin® Plant II kit (Macherey–Nagel) and the genomic sequence was obtained. The sequences obtained were compared using the Multiple Sequence Alignment CLUSTALW software (Thompson et al. 1994) and the putative protein sequences encoded were analysed using the InterProScan (http://www.ebi.ac.uk/Tools/pfa/iprscan/) and Prosite (http://prosite.expasy.org/) tools. Similarity searches in the National Center for Biotechnology Information (NCBI) public database were performed with the BLASTX program (Altschul et al. 1997). The similarity degree was represented by the BLASTX probability E-value. Sequence annotations were performed with the Geneious software (Biomatters Ltd, New Zealand). The cloned sequences were denominated CLAVATA1-LIKE (PipiCLV1L for P. pinea and PipsCLV1L for P. pinaster) due to their similarity with CLAVATA1 sequences from different angiosperms. An unrooted phylogenetic tree with the protein kinase domains of the CLV1-like proteins from selected species was generated by the UPGMA method and the Jukes-Cantor genetic distance model using the Geneious software.
Quantitative RT-PCR
To examine CLV1L gene expression, excised cotyledons were harvested at 0 h and after 12 h, 1, 2, 4 and 6 days of culture on induction medium with or without 45 μM BA for P. pinea and 10 μM BA for P. pinaster. Batches of 100 mg of random cotyledons from each treatment were frozen in liquid nitrogen and stored at −80 °C until use. RNA extraction and cDNA synthesis were performed as indicated above.
The gene expression analysis was performed by real-time PCR with an ABI PRISM 7900HT instrument (Applied Biosystems Inc.) and the Fast SYBR® Green Master Mix (Applied Biosystems Inc.). Approximately 10 ng cDNA were used per well. Amplifications were performed using the following standard protocol: 95 °C 20 s; 45 cycles of 95 °C 1 s and 60 °C 20 s. Real-time PCR specificity was assessed using negative controls (no template), RT- control (non-retrotranscribed RNA), a melting curve analysis and by gel electrophoresis of a group of selected reactions. Three biological and two technical replicates were used for analysis. Primers for PipiCLV1L and PipsCLV1L were designed with Primer3 software (Rozen and Skaletsky 2000) to amplify a 98 bp fragment of the cDNA target in both species with a 60 °C Tm and a G+C content of 40–60 %. The expression of PipiCLV1L and PipsCLV1L was normalized to the mean value of expression of two reference genes in each sample. Primers for reference genes were designed from GenBank sequences AF461687 and AY172979 (Electronic Supplementary Material 1) (Alonso et al., 2007). The relative expression ratio of the cotyledons treated with BA compared to the controls for each time tested was computed according to the comparative Ct method (Livak and Schmittgen 2001), incorporating the mean PCR efficiencies and fluorescence group threshold values as described in Ruijter et al. (2009). Results are expressed as mean expression ratios ± standard error of the three biological and two technical replicates.
Binary vector construction and genetic transformation mediated by Agrobacterium tumefaciens
Pinus pinaster CLAVATA1-LIKE promoter cloning and vector construction
Once the complete PipsCLV1 genomic sequence was obtained, gene specific primers were designed for walking upstream in the genome using the DNA Walking kit (Seegene, Inc., Korea) following the manufacturer’s instructions. Bands obtained were sequenced and aligned with the gene sequence. A PCR was performed with upstream sequence- and gene-specific primers to verify that a band of the expected length was obtained. A fragment of 1,502 bp in length upstream of the translation initiation site (pPipsCLV1L) was cloned into a Gateway® pENTR/D TOPO vector (Gateway® Recombination Cloning, Invitrogen, Life technologies Corporation, CA, USA) and introduced by att site LR recombination into pKGWFS7,0 (Gateway® destination vector) (Karimi et al. 2002). The resulting vector was called pPipsCLV1L-GFP:GUS (Electronic Supplementary Material 2).
Plant material
Embryogenic lines of P. pinaster were initiated from seeds containing immature zygotic embryos from open pollinated families growing in Asturias (Spain). The induction was based on Lelu-Walter et al. (2006), but using as induction medium WV5 salts (Coke 1996) supplemented with 1 g L−1 casein hydrolysate, 0.5 g L−1 l-glutamine, 0.06 M sucrose, 4.4 μM BA, 9 μM 2,4-dichlorophenoxyacetic acid (2,4-D) and 4 g L−1 Gelrite (Duchefa, Haarlem, The Netherlands). The pH was adjusted to 5.7 before autoclaving. Glutamine was filter-sterilised and added after autoclaving. Once initiated, cultures were subcultured every 2 weeks on 90 mm diameter, 14 mm depth Petri dishes with 20 mL of the same medium with half concentration plant growth regulators (PGR) and sealed with Parafilm® at 23 °C in darkness. Embryogenic line P5LV41 was selected based on its high growing rate and maturation capacity for subsequent assays.
Transformation procedure
The disarmed A. tumefaciens strain AGL1 (Lazo et al. 1991) was selected from previous experiments (data not shown) for genetic transformation. The binary vector pPipsCLV1L-GFP:GUS was introduced into AGL1 by the freeze–thaw method (Xu and Li 2008). Bacterial strain AGL1 pPipsCLV1L-GFP:GUS was grown at 28 ºC for 24 h in liquid YEP medium (An et al. 1988) containing 50 mg spectinomycin (Duchefa) at 250-300 rpm on an orbital shaker. The bacterial suspension was then centrifuged at 4,000 rpm, washed in 10 mM MgSO4 and resuspended in liquid WV5 medium containing 200 μM acetosyringone at a bacterial concentration of 0.6 absorbance units (OD 600 nm). The inoculation and cocultivation procedure was based on Levée et al. (1999) with some modifications. Vigorously growing P5LV41 embryogenic somatic mass (200 mg mL−1) after 7 days of the previous subculturing was disaggregated into WV5 liquid medium by brief vortex pulses and mixed with an equal volume of bacterial suspension. The resulting suspension was spread (5 mL) onto a piece of sterile filter paper (70 mm Whatman no 2). Excess liquid was drained by low-pressure vacuum pulse in a Buchner flask. Embryogenic cells and AGL1 bacteria were co-cultivated on Petri dishes with 20 mL WV5 proliferation medium without casein hydrolysate for 3 days at 28 ºC in darkness. Paper filters were then washed 4 times with 10 mL WV5 liquid medium, dried with low-pressure vacuum and placed on WV5 proliferation medium with 500 mg L−1 cefotaxime (Duchefa) to eliminate agrobacteria. After 7 days of culture, filters were transferred to selection medium consisting of WV5 proliferation medium with 500 mg L−1 cefotaxime and 15 mg L−1 kanamycin (Duchefa). Filters were replicated every 2 weeks on selection medium. Putative kanamycin-resistant growing calli were isolated, subcultured and proliferated on selection medium for at least six subcultures. Transformation efficiency was expressed as transformation events per gram of starting fresh mass.
Molecular analysis
Molecular analysis was performed on kanamycin-resistant embryogenic mass. The putative transgenic events were PCR-tested for the GFP (pKGWFS7,0) and virG (GenBank X62885) genes. Non-inoculated P5LV41 line as negative control and the AGL1 strain carrying pPipsCLV1L-GFP:GUS binary vector as positive control were used to complete the PCR analysis. Genomic DNA was extracted using the NucleoSpin® Plant II kit (Macherey–Nagel). The amplification was performed in a Biometra T-Gradient Thermoblock thermocycler with the Kapa Taq PCR kit (Kapa Biosystems Inc., Woburn, MA, USA). Reactions (10 μL) were amplified using the following PCR protocol: 95 ºC 5 min; 35 cycles of 95 ºC 30 s, 60 ºC 30 s and 72 ºC 30 s; 72 ºC 5 min. Primers used are listed in Electronic Supplementary Material 1.
The real-time PCR comparative Ct method was used for estimating copy number (Bubner and Baldwin 2004). The analysis was performed in the same conditions as above. The Pips-C61 gene (GeneBank AJ490522) was selected as endogenous control. Pips-C61 has been reported as a single copy gene in the P. pinaster genome (Reddy et al. 2003). Three biological and two technical replicates were used for analysis. Primers (Electronic Supplementary Material 1) were designed to amplify a 145 bp fragment of the GFP gene and a Pips-C61 gene fragment of 93 bp with a 60 °C Tm. The line showing the lowest ΔCt, ΔCt being the difference between Ct for transgene and Ct for endogenous control (CtGFP–CtPips-C61), was used as calibrator. PCR efficiencies and Ct were calculated using the LinRegPCR software. The copy number was calculated as E−ΔΔCt, where E = PCR efficiency, and ΔΔCt = ΔCt sample − ΔCt calibrator. The difference between Ct for transgene and Ct for endogenous control (ΔCt) is constant, independent of the amount of chromosomal DNA when PCR efficiencies of endogenous control and transgene are the same (Bubner and Baldwin 2004).
Maturation of transgenic lines
For promoting the histodifferentiation and maturation of transformed somatic embryos, 150 mg embryogenic mass were disaggregated in 2 mL sterile water. The suspension was poured onto a piece of sterile filter paper, dried by a low vacuum pulse and placed on WV5 medium supplemented with 60 g L−1 sucrose, 9 g L−1 Gelrite and a mix of amino acids consisting of 550 mg L−1 l-glutamine (Duchefa), 525 mg L−1 l-asparagine (Duchefa), 175 mg L−1 l-arginine (Duchefa), 19.75 mg L−1 l-citrulline (Sigma), 19 mg L−1 l-ornithine (Duchefa), 13.75 mg L−1 l-lysine (Duchefa), 10 mg L−1 l-alanine (Duchefa) and 8.75 mg L−1 l-proline (Duchefa) for 7 days and immediately placed on the same medium supplemented with 80 μM abscisic acid (ABA) (Duchefa). The amino acid mix and ABA were filter sterilized and added after autoclaving. Cultures were subcultured on fresh medium every 3 weeks for 3 months. Maturation was measured as the number of embryos in stage 3 per gram of fresh mass. Somatic embryo developmental stages in P. pinaster were based on those described by Ramarosandratana et al. (2001). Stage 1 is a proliferating immature embryo consisting of an embryonic region of small, densely cytoplasmic cells subtended by a suspensor. Stage 2 embryo has a prominent embryonic region that becomes opaque and develops to a stage 3 embryo on which cotyledons are visible.
Mature embryos (stage 3) of each line were isolated and placed on PGR-free WV5 medium supplemented with 30 g L−1 sucrose and 4 g L−1 Gelrite until radicle elongation and bud breaking was evident. Then, they were placed in a peat-vermiculite substrate (1:1 v/v) in the greenhouse to allow further growth.
Quantitative RT-PCR
To examine CLV1L gene expression during somatic embryo development and maturation, batches of 100 mg of somatic embryogenic mass were harvested at 0, 45 and 90 days after initiation of the culture on maturation medium. Batches were frozen in liquid nitrogen and stored at −80 °C until use. RNA extraction, cDNA synthesis and real-time PCR were performed as indicated above.
β-Glucuronidase assays during embryo development
β-Glucuronidase (GUS) activity was analysed fluorometrically and histochemically both according to Jefferson et al. (1987). The assays were carried out in 24 kanamycin-resistant independent embryogenic lines during the embryogenic mass proliferation. Fluorometric assay was performed in a TKO 100 Fluorometer (Hoefer Inc., MA, USA). Approximately 100 mg fresh mass of each independent line were used. GUS activity is expressed as picomoles of methylumbelliferone (MU) per minute and per milligram of total protein. Total protein was quantified by the Bradford method (Bradford 1976). Three independent experiments were carried out and samples were analysed in triplicate. Data are presented as the mean ± standard error. The data concerning the expression levels in relation to transgene copy number were analysed using the ANOVA test at the 5 % level.
For histochemical GUS assays approximately 50 mg fresh mass of each independent lines were used. The blue colour development of each line was inspected after 16 h incubation at 37 °C in GUS solution. Moreover, the histochemical GUS assay was also performed during the maturation process in different embryo stages (15, 45 and 90 days from the initiation of maturation process) and germinating embryos with elongated radicle.
Results
Cloning of a CLAVATA1-LIKE gene in P. pinea and P. pinaster
The coding sequence (CDS) of PipiCLV1L (GenBank HQ377525) was 3,060 nucleotides long and encoded for a predicted protein of 1,020 amino acids. The PipsCLV1L mRNA (HQ377526) was 3,741 nucleotides long, the CDS being 3,043 bp. The CLUSTALW alignment of P. pinea and P. pinaster CDS (Electronic Supplementary Material 3) showed 98 % identity.
The genomic sequence of PipsCLV1L (HQ377527) was obtained. The complete sequence was 3,860 bp including a 79 bp 5′UTR, 3,045 bp CDS with an intron of 119 and 617 bp 3′UTR. A promoter region of 1,513 bp upstream of the transcription initiation site was also obtained (Electronic Supplementary Material 4).
The predicted proteins were 1,020 and 1,015 amino acid long for P. pinea (GenBank AEP14545) and P. pinaster (GenBank AEP14546) respectively, showing 96.1 % identity in CLUSTALW alignment. In both cases the InterProScan analysis identified two hydrophobic regions in the sequence, an N-terminal signal peptide and a transmembrane region enclosed by charged residues. A leucine-rich repeat (LRR) N-terminal region was also identified flanked by paired cysteines, a feature common to many LRR proteins. Finally, a highly conserved C-terminal protein kinase domain was also identified with all 11 conserved subdomains of eukaryotic protein kinases and all invariant amino acid residues in their proper positions (Hanks and Quinn 1991). One of these subdomains encompasses a highly conserved serine/threonine kinase active site (Fig. 1).
CLUSTALW alignment of the deduced amino acid sequences for P. pinea and P. pinaster showing the predicted regions: two hydrophobic regions, a N-terminal signal peptide and a transmembrane region; a leucine-rich repeat (LRR) N-terminal region; and a highly conserved C-terminal protein kinase domain (including subdomains in roman numerals). Active site is marked with asterisk. Sequence annotations were performed with Geneious software. The protein image was created with the Prosite Image Tool (http://prosite.expasy.org/mydomains)
BLASTX analysis showed high similarity (E value = 0.0) with several proteins of the LRR receptor family in different species, the Arabidopsis CLV1 receptor kinase (GeneBank AAB58929) among them. The deduced amino acid sequence of PipiCLV1L and PipsCLV1L showed conservation of the characteristic residues found in the LRR RLK proteins in plants. The amino acid sequence alignment of protein kinase domain from predicted proteins of 11 species [A. thaliana (AAB58929), Medicago truncatula (AAW71475), Oryza sativa (BAD17641), Picea glauca (ABF73316), Populus trichocarpa (XP_002305776), Ricinus communis (XP_002517850), Solanum lycopersicum (BAK52388), Zea mays (NP_001145765), P. pinea (AEP14545), P. pinaster (AEP14546), Pinus sylvestris (CAC20842)] showed a pairwise identity of 56.2 %, showing a high conservation of protein kinase subdomains (Fig. 2). A Phylogenetic tree of the protein kinase domains showed that PipsCLV1L and PipiCLV1L are grouped together with the Picea, Populus and Ricinus proteins. Proteins from Arabidopsis, Medicago, Solanum and Zea are grouped in a sister clade, while the sequence from Pinus sylvestris appears on a separate branch (Electronic Supplementary Material 5).
Alignment of the deduced Protein Kinase domain of A. thaliana (AAB58929), M. truncatula (AAW71475), O. sativa (BAD17641), P. glauca (ABF73316), P. trichocarpa (XP_002305776), R. communis (XP_002517850), S. lycopersicum (BAK52388), Z. mays (NP_001145765), P. pinea (AEP14545), P. pinaster (AEP14546), P. sylvestris (CAC20842). Identity is represented by increasing box shading. Protein kinase subdomains are labelled in roman numerals following the nomenclature of Hanks and Quinn (1991)
CLAVATA1-LIKE expression in cotyledons during caulogenic induction and somatic embryo maturation
The dynamics of CLV1L gene expression during caulogenic induction in P. pinea and P. pinaster cotyledons are shown in Fig. 3. The expression of CLV1L was induced by BA in both species from the first moments of culture. In P. pinaster, relative expression was higher than twofold as early as 12 h after induction, showing a progressive increase up to 2.80 ± 0.07 fold after 2 days. During the last days of culture, PipsCLV1L expression decreased. In P. pinea no differential expression was observed after 12 h of culture, but after 24 h expression levels reached two-fold expression. Levels continued to rise during the next two days, reaching a peak at day 4.
Fold expression of CLAVATA1-Like in the cotyledons treated with BA compared to the controls for each time tested in P. pinaster and P. pinea during caulogenic induction as determined by Quantitative RT-PCR. Cotyledons were harvested after 12 h, 1, 2, 4 and 6 days of culture on medium with and without 45 μM BA for P. pinea and 10 μM BA for P. pinaster. Relative expression of CLV1L was measured by the comparative Ct method and normalized to the mean value of expression of two reference genes
Results also showed PipsCLV1L expression during somatic embryo maturation, with it being higher at 90 days on medium supplemented with ABA (Electronic Supplementary Material 6).
Genetic transformation with pPipsCLV1L-GFP:GUS binary vector
To assay promoter activity in P. pinaster, embryogenic cultures were transformed with the binary vector pPipsCLV1L-GFP:GUS. Transformation efficiency was of 11.2 ± 2.3 transformation events per gram of fresh mass. Twenty-four embryogenic lines of P. pinaster showing kanamycin resistance in culture were chosen for molecular analysis. Primers to amplify a GFP fragment of 355 bp included in the T-DNA and a VirG gene 200 bp fragment to discard Agrobacterium contamination were used. Results of PCR showed GFP amplification for the 24 kanamycin-resistant lines tested (Electronic Supplementary Material 7) while amplification of the VirG gene was observed only in the positive control (Agrobacterium AGL1 harbouring the pPipsCLV1L-GFP:GUS plasmid). No amplification was detected in negative control (P5LV41 non-transformed line). Copy number estimation by comparative Ct method showed 9 lines with one T-DNA insertion (37.5 %), 7 lines with two (29.2 %) and 8 lines with 3 or more copies of the T-DNA (33.3 %).
Fluorometric assay results for GUS activity showed significant differences for the different transformed lines ranging from 92.15 ± 11.01 to 16,352.55 ± 2,565.27 pmol MU per minute per mg of total protein. No significant relationships were found between GUS activity levels and transgene copy number. The highest GUS activity levels were obtained in transgenic lines with 3 or more copies of GFP:GUS fusion gene; but a high variability was observed (Fig. 4).
Fluorometric GUS assay of the 24 kanamycin-resistant lines. Fluorometric assay was performed in a TKO 100 Fluorometer (Hoefer Inc., MA, USA) according to Jefferson et al. (1987). Approximately 100 mg fresh mass of each independent line were collected 7 days after the last subculture. GUS activity is expressed as picomoles of methylumbelliferone (MU) per minute and per milligram of total protein. Total protein was quantified by the Bradford method (Bradford 1976). Different bar codes indicate transgene copy number: 1 copy (black bar), 2 copies (lined bar) and 3 or more copies (grey bar). Maturation capacity (+ or −) of each transgenic line is indicated below the line number
Mature embryos (stage 3) were obtained from 13 out of the 24 transgenic lines. No correlations were observed between capacity of maturation, number of T-DNA insertions and GUS activity (Fig. 4). The average of mature embryos per gram of fresh mass was 13.2 ± 3.8.
Mature embryos were transferred to germination medium. A 60 % showed radicle elongation and bud-break after one month and were transferred to peat-vermiculite substrate (1:1 v/v). Survival of plants was 66 %. No significant differences were observed between lines.
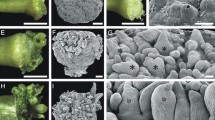
The 24 transgenic lines showed histochemical GUS staining during proliferation of the embryogenic mass. GUS activity in embryos from 15 to 45 days of maturation showed intense blue staining in the embryo heads and weak blue staining in the suspensor cells (stages 1 and 2). GUS activity in mature embryos after 90 days in ABA (stage 3) was often located in the hypocotyl and it was also observed at the root tip in germinating embryos with elongated radicle (Fig. 5).
Blue colour developed in transgenic embryogenic mass and mature embryos transformed with the pPipsCLV1L-GFP:GUS construction after incubation in GUS solution at 37 ºC for 16 h. Intense blue staining is observed in the embryo heads and weak blue staining in the suspensor cells for embryo mass proliferating and embryos maturing in stage 1 (a, b) and in stage 2 (c). Mature embryos after 90 days in ABA (stage 3) showed blue staining in cotyledons and hypocotyl (d, e). Intense blue staining is observed in the hypocotyl region when the embryo was longitudinally sectioned (e). Root tip showing intense blue staining in germinating embryo (f). Micrographs were taken with a Leica DM2000 microscope (a–c) and a Leica EZ4D stereo microscope (d–f). Bar 0.5 mm. (Color figure online)
Discussion
Despite the economical importance of conifers, studies devoted at unravelling meristem function and dynamics are scarce. This is not surprising, since conifers have long life cycles, big genomes, and are not easily amenable to in vitro culture and transformation. In this work we present the characterization of a CLAVATA1-LIKE gene in two pine species. Arabidopsis CLAVATA1 is a well-characterized gene, being a member of the LRR-XI superfamily of the RLKs in plants, essential to the balance between primordial differentiation and meristem proliferation. We studied CLV1L expression during BA-induced caulogenesis in P. pinea and P. pinaster isolated cotyledons; this treatment induces direct adventitious shoot formation in the cotyledons (Alonso et al. 2006; Álvarez et al. 2009a). Our results showed that treatment with BA increased the transcription level of CLV1L in both species from the beginning of treatment. The differential expression found in cotyledons suggests that this gene may play a role in adventitious meristem formation in pines. The CLV1L gene is expressed two-fold higher as early as one day after exposure to benzyladenine. This means that this gene is expressed before the first cell divisions that will form the new meristems (Data not shown). This is in agreement with the current view of shoot apical meristem formation, in which gene expression domains appear before the anatomical domains become visible (Groß-Hardt and Laux 2003). Thus, CLV1L may be involved, not only in meristem organisation, but also in meristem commitment and initiation in P. pinea and P. pinaster. On the other hand, in Arabidopsis, CLV1 expression is upregulated at the time of shoot commitment during incubation in cytokinin-containing shoot induction medium, once the cells have already started to divide (Cary et al. 2002). This different expression pattern could be explained by the differences in the organogenic mechanisms. In Arabidopsis de novo shoots are induced in calli from root explants, while in pines the organogenesis is direct from cotyledonary explants. Thus, the gene expression background at the moment of cytokinin exposure may be different. Nevertheless, this hypothesis must be confirmed by further studies.
Few data exist about LRR-RLK proteins in conifers. A CLV1 homologue that is expressed in the apical pole of somatic embryos has been described in P. glauca (Stasolla et al. 2003). Ávila et al. (2006) characterised a receptor-like protein kinase gene (PsRLK) in Pinus sylvestris, similar to the Arabidopsis CLAVATA1, expressed during development of seedlings and restricted to specialized phloem cells. This suggests a possible function for the receptor-like protein kinase in this particular vascular element. The phylogenetic tree (Electronic Supplementary Material 5) of the protein kinase domains of several CLV1-Like proteins shows that PipsCLV1L and PipiCLV1L are grouped together with the P. glauca sequence, in a sister clade of the one that groups the CLAVATA1 from Arabidopsis. PsRLK is grouped in a separate clade from the rest of CLV1-Like sequences from gymno- and angiosperms, indicating that it might not be a close homologue.
Somatic embryogenesis in P. pinaster has been improved in recent years (Lelu-Walter et al. 2006; Klimaszewska et al. 2007; Humánez et al. 2012) and provides a source of competent cells for genetic transformation. Although genetic transformation of conifers is possible and there are reports for the most important genera (Trontin et al. 2007), its use in studies of functional genetics is very limited, preventing the emergence of a true model system for this group of plants (Kramer 2009). Nevertheless, we have used Agrobacterium-mediated transformation of P. pinaster somatic embryos to characterize PipsCLV1L expression. In the construction used in this work (pPipsCLV1L-GFP:GUS), a 1,502 bp fragment upstream the PipsCLV1L coding sequence drives the expression of the reporter genes uidA and GFP, allowing location of the regions where CLV1L is expressed. Our results showed GUS activity in all of the transformed lines demonstrating that the construction is functional. The variability observed in the expression level of the uidA gene as estimated by GUS activity in the different transformed clones cannot be explained alone by copy number effect (Cervera et al. 2000; Álvarez et al. 2009b), therefore, phenomena such as the position effect of the insertion of transgene into the host genome (Matzke and Matzke 1998) or complex configurations of the integrated T-DNA (Stam et al. 1997) should be also considered. Our data showed a loss of maturation capacity in the different transgenic lines. This phenomenon, already reported by other authors in conifers (Tereso et al. 2006; Malabadi et al. 2008), has been related to the mutagenic effect of T-DNA insertions, which can induce the loss of gene function, in Arabidopsis (Feldmann 1991); however, this effect in conifers should be minor considering the extremely large size of the conifer genome. In addition, our data suggest that the maturation loss is not related to the number of inserted copies of T-DNA and their putative mutagenic effect. Further experiments would be necessary to confirm this point.
The analysis of GFP:GUS under the control of the PipsCLV1L promoter showed that this reporter gene is expressed in P. pinaster during somatic embryogenic mass proliferation and somatic embryo maturation, mainly in the embryo heads. These results are in concordance with those obtained from real-time PCR that showed PipsCLV1L expression during embryo maturation (Electronic Supplementary Material 6). This may indicate that PipsCLV1L plays a role during maritime pine somatic embryo development. Some LRR-RLK genes such as SERK1 are involved in somatic embryogenesis in conifers (Steiner et al. 2011). Cairney et al. (2006) also report cDNA homologues to the receptor kinase CLAVATA1 and the receptor-like protein CLAVATA2 in pine embryo-derived EST-sequences. The formation of SAM is the outcome of a successive patterning process initiated very early in embryo development (von Arnold et al. 2002). During embryo germination, we also observed GUS activity in root tips. These data suggest that the promoter sequence of PipsCLV1L controls the gene expression in different localities during plant development. Heller et al. (2012) found an EST similar to Arabidopsis CLV1 expressed in lateral root during ectomycorrhizal development in P. sylvestris. In legumes, the nodulation process is controlled by LRR-RLKs evolutionarily related to Arabidopsis CLV1 (Harris and Dickstein 2010). Several LRR-RLKs are encoded in the Arabidopsis genome, many of them showing high amino acid sequence similarity with CLAVATA1 that may play roles not only in SAM, but also throughout the plant (Stahl and Simon 2005). However, in the absence of a completed conifer genome, it is difficult to predict how many LRR-RLKs of the CLV1 type there are and what would be the true orthologue of CLV1 in gymnosperms.
In summary, the present work shows the isolation and characterization of a CLV1L gene in two pines species. Our results suggest that this gene may play an important role in adventitious shoot meristem formation and somatic embryo development in pine. In this work we also present a biotechnological tool based on homologous transformation and a basis for further studies on the genetic regulation in conifers.
References
Alonso P, Moncaleán P, Fernández B, Rodríguez A, Centeno ML, Ordás RJ (2006) An improved micropropagation protocol for stone pine (Pinus pinea L.). Ann For Sci 63:879–885
Alonso P, Cortizo M, Cantón FR, Fernández B, Rodríguez A, Centeno ML, Cánovas FM, Ordás RJ (2007) Identification of genes differentially expressed during adventitious shoot induction in Pinus pinea cotyledons by subtractive hybridization and quantitative PCR. Tree Physiol 27:1721–1730
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Álvarez JM, Majada J, Ordás RJ (2009a) An improved micropropagation protocol for maritime pine (Pinus pinaster Ait.) isolated cotyledons. Forestry 82:175–184
Álvarez R, Álvarez JM, Humara JM, Revilla Á, Ordás RJ (2009b) Genetic transformation of cork oak (Quercus suber L.) for herbicide resistance. Biotechnol Lett 31:1477–1483
Álvarez JM, Cortizo M, Ordás RJ (2012) Characterization of a type-A response regulator differentially expressed during adventitious caulogenesis in Pinus pinaster. J Plant Physiol. doi:10.1016/j.jplph.2012.07.014
An G, Ebert P, Mitra A, Ha S (1988) Binary vectors. In: Gelvin SB, Schilperoort RA (eds) Plant molecular biology manual. Kluwer, Dordrecht, pp 1–19
Ávila C, Pérez-Rodríguez J, Cánovas FM (2006) Molecular characterization of a receptor-like protein kinase gene from pine (Pinus sylvestris L.). Planta 224:12–19
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bubner B, Baldwin IT (2004) Use of real-time PCR for determining copy number and zygosity in transgenic plants. Plant Cell Rep 23:263–271
Cairney J, Zheng L, Cowels A, Hsiao J, Zismann V, Liu J, Ouyang S, Thibaud-Nissen F, Hamilton J, Childs K (2006) Expressed sequence tags from loblolly pine embryos reveal similarities with angiosperm embryogenesis. Plant Mol Biol 62:485–501
Carles CC, Fletcher JC (2003) Shoot apical meristem maintenance: the art of a dynamic balance. Trends Plant Sci 8:394–401
Cary AJ, Che P, Howell SH (2002) Developmental events and shoot apical meristem gene expression patterns during shoot development in Arabidopsis thaliana. Plant J 32:867–877
Cervera M, Pina J, Juárez J, Navarro L, Peña L (2000) A broad exploration of a transgenic population of citrus: stability of gene expression and phenotype. Theor Appl Genet 100:670–677
Che P, Gingerich DJ, Lall S, Howell SH (2002) Global and hormone-induced gene expression changes during shoot development in Arabidopsis. Plant Cell 14:2771–2785
Christianson ML, Warnick DA (1988) Organogenesis in vitro as a developmental process. HortScience 23(3):515–519
Clark SE, Williams RW, Meyerowitz EM (1997) The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell 89:575–585
Coke JE (1996) Basal nutrient medium for in vitro cultures of loblolly pines (Patent US) Westvaco Corporation, New York, NY
Cortizo M, Cuesta C, Centeno ML, Rodríguez A, Fernández B, Ordás R (2009) Benzyladenine metabolism and temporal competence of Pinus pinea cotyledons to form buds in vitro. J Plant Physiol 166:1069–1076
Cortizo M, Álvarez JM, Rodríguez A, Fernández B, Ordás RJ (2010) Cloning and characterization of a type-A response regulator differentially expressed during adventitious shoot formation in Pinus pinea L. J Plant Physiol 167:1023–1026
Cuesta C, Rodríguez A, Centeno ML, Ordás RJ, Fernández B (2009) Caulogenic induction in cotyledons of stone pine (Pinus pinea): relationship between organogenic response and benzyladenine trends in selected families. J Plant Physiol 166:1162–1171
Feldmann KA (1991) T-DNA insertion mutagenesis in Arabidopsis: mutational spectrum. Plant J 1:71–82
Fletcher JC, Brand U, Running MP, Simon R, Meyerowitz EM (1999) Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science 283:1911–1914
Gordon SP, Chickarmane VS, Ohno C, Meyerowitz EM (2009) Multiple feedback loops through cytokinin signaling control stem cell number within the Arabidopsis shoot meristem. Proc Natl Acad Sci USA 106:16529–16534
Groß-Hardt R, Laux T (2003) Stem cell regulation in the shoot meristem. J Cell Sci 116:1659–1666
Hanks SK, Quinn AM (1991) Protein kinase catalytic domain sequence database: identification of conserved features of primary structure and classification of family members. Methods Enzymol 200:38
Harris JM, Dickstein R (2010) Control of root architecture and nodulation by the LATD/NIP transporter. Plant Signal Behav 5:1365
Heller G, Lundén K, Finlay RD, Asiegbu FO, Elfstrand M (2012) Expression analysis of Clavata1-like and Nodulin21-like genes from Pinus sylvestris during ectomycorrhiza formation. Mycorrhiza 22:271–277
Howell SH, Lall S, Che P (2003) Cytokinins and shoot development. Trends Plant Sci 8:453–459
Humánez A, Blasco M, Brisa C, Segura J, Arrillaga I (2012) Somatic embryogenesis from different tissues of Spanish populations of maritime pine. Plant Cell Tissue Org Cult. doi:10.1007/s11240-012-0203-0
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Karimi M, Inz D, Depicker A (2002) GATEWAY (TM) vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7:193–195
Klimaszewska K, Trontin JF, Becwar M, Devillard C, Park YS, Lelu-Walter MA (2007) Recent progress on somatic embryogenesis of four Pinus spp. Tree For Sci Biotechnol 1:11–25
Kramer EM (2009) New model systems for the study of developmental evolution in plants. In: Jeffery WR (ed) Curr Top Dev Biol. Academic Press, Elseiver, Burlington, pp 67–105
Lazo GR, Stein PA, Ludwig RA (1991) A DNA transformation-tcompetent Arabidopsis genomic library in Agrobacterium. Nat Biotechnol 9:963–967
Leibfried A, To JPC, Busch W, Stehling S, Kehle A, Demar M, Kieber JJ, Lohmann JU (2005) WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature 438:1172–1175
Lelu-Walter M-A, Bernier-Cardou M, Klimaszewska K (2006) Simplified and improved somatic embryogenesis for clonal propagation of Pinus pinaster (Ait.). Plant Cell Rep 25:767–776
Levée V, Garin E, Klimaszewska K, Seguin A (1999) Stable genetic transformation of white pine (Pinus strobus L.) after cocultivation of embryogenic tissues with Agrobacterium tumefaciens. Mol Breed 5:429–440
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-[Delta][Delta] CT method. Methods 25:402–408
Malabadi RB, da Silva JAT, Nataraja K (2008) Agrobacterium tumefaciens-mediated genetic transformation of Pinus kesiya Royle ex Gord (Khasi Pine). Asian Australas J Plant Sci Biotech 2:7–14
Matzke AJM, Matzke MA (1998) Position effects and epigenetic silencing of plant transgenes. Curr Opin Plant Biol 1:142–148
Meng L, Zhang S, Lemaux PG (2010) Toward molecular understanding of in vitro and in planta shoot organogenesis. Crit Rev Plant Sci 29:108–122
Moncaleán P, Cortizo M, Alonso P, Fernández B, Rodríguez A, Centeno ML, Ordás RJ (2005) Organogenic responses of Pinus pinea cotyledons to hormonal treatments: BA metabolism and cytokinin content. Tree Physiol 25:1–9
Nimchuk ZL, Tarr PT, Ohno C, Qu X, Meyerowitz EM (2011) Signaling in the Arabidopsis shoot meristem stem cell niche correlates with ligand-dependent trafficking of the CLV1 receptor kinase. Curr Biol 21:345–352
Ramarosandratana A, Harvengt L, Bouvet A, Calvayrac R, Paques M (2001) Influence of the embryonal-suspensor mass (ESM) sampling on development and proliferation of maritime pine somatic embryos. Plant Sci 160:473–479
Reddy SM, Pandey AK, Melayah D, Marmeisse R, Gay G (2003) The auxin responsive gene Pp-C61 is up-regulated in Pinus pinaster roots following inoculation with ectomycorrhizal fungi. Plant Cell Environ 26:681–691
Rozen S, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132:365–386
Ruijter JM, Ramakers C, Hoogaars WMH, Karlen Y, Bakker O, van den Hoff MJB, Moorman AFM (2009) Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res 37(6):e45
Sablowski R (2009) Cytokinin and WUSCHEL tie the knot around plant stem cells. Proc Natl Acad Sci USA 106:16016–16017
Schoof H, Lenhard M, Haecker A, Mayer KFX, Jürgens G, Laux T (2000) The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100:635–644
Shani E, Yanai O, Ori N (2006) The role of hormones in shoot apical meristem function. Curr Opin Plant Biol 9:484–489
Stahl Y, Simon R (2005) Plant stem cell niches. Int J Dev Biol 49:479–489
Stam M, Mol JNM, Kooter JM (1997) Review article: the silence of genes in transgenic plants. Ann Bot 79:3–12
Stasolla C, van Zyl L, Egertsdotter U, Craig D, Liu W, Sederoff RR (2003) The effects of polyethylene glycol on gene expression of developing white spruce somatic embryos. Plant Physiol 131:49–60
Steiner N, Santa-Catarina C, Guerra MP, Cutri L, Dornelas MC, Floh EIS (2011) A gymnosperm homolog of SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE-1 (SERK1) is expressed during somatic embryogenesis. Plant Cell Tissue Organ Cult 109:41–50
Sugiyama M (1999) Organogenesis in vitro. Curr Opin Plant Biol 2:61–64
Tereso S, Miguel C, Zoglauer K, Valle-Piquera C, Oliveira MM (2006) Stable Agrobacterium-mediated transformation of embryogenic tissues from Pinus pinaster Portuguese genotypes. Plant Growth Regul 50:57–68
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Trontin JF, Walter C, Klimaszewska K, Park YS, Lelu-Walter MA (2007) Recent progress in genetic transformation of four Pinus spp. Transgenic Plant J 1:314–329
Valdés AE, Ordás RJ, Fernández B, Centeno ML (2001) Relationships between hormonal contents and the organogenic response in Pinus pinea cotyledons. Plant Physiol Biochem 39:377–384
von Arnold S, Sabala I, Bozhkov P, Dyachok J, Filonova L (2002) Developmental pathways of somatic embryogenesis. Plant Cell Tissue Org Cult 69:233–249
Xu R, Li QQ (2008) Protocol: streamline cloning of genes into binary vectors in Agrobacterium via the Gateway® TOPO vector system. Plant Methods 4:4
Zhang S, Lemaux PG (2004) Molecular analysis of in vitro shoot organogenesis. Crit Rev Plant Sci 23:325–335
Acknowledgments
We thank Dr. Kevin Dalton for proofreading the manuscript and Dr. Ruben Alvarez from University of Essex (UK) for the helpful comments. This work was supported by ‘Ministerio de Educación y Ciencia de España’ (AGL2009-12139-C02-01); ‘Plan de Ciencia Tecnología e Innovación del Principado de Asturias’ (IB08-054 and FC10-COF10-07); predoctoral grant from the ‘Ministerio de Educación y Ciencia de España’ (FPU AP2005-0140) to J.M. Álvarez and from ‘Plan de Ciencia Tecnología e Innovación del Principado de Asturias’ (BP10-098) to N. Bueno.
Author information
Authors and Affiliations
Corresponding author
Additional information
José M. Alvarez and Millán Cortizo contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Electronic Supplementary Material 1
Primer sequences. Primers used for PCR and Quantitative RT-PCR (PDF 144 kb)
Electronic Supplementary Material 2
Map of the binary vector pPipsCLV1L-GFP:GUS. A promoter fragment of 1,502 bp length was cloned into a Gateway® pENTR/D TOPO vector and introduced by att site LR recombination into the pKGWFS7,0. Sequence annotations were performed with Geneious software (PNG 51 kb)
Electronic Supplementary Material 3
P. pinaster and P. pinea CLUSTALW CDS alignment (PNG 197 kb)
Electronic Supplementary Material 4
Genomic sequence of PipsCLV1L (HQ377527) of 5,373 bp including a 1,513 bp region of the promoter, 79 bp 5’UTR, 119 bp of one intron, 3,045 bp CDS and 617 bp 3’UTR. The 1,502 bp fragment cloned in pPipsCLV1L-GFP:GUS is also indicated. Sequence annotations were performed with the Geneious software (PNG 29 kb)
Electronic Supplementary Material 5
Unrooted phylogenetic tree of the protein kinase domains of the CLV1-like proteins from various species generated using the Geneious software by the UPGMA method and the Jukes-Cantor genetic distance model. (PNG 45 kb)
Electronic Supplementary Material 6
Expression of CLAVATA1-Like in somatic embryos of P. pinaster as determined by Quantitative RT-PCR. Embryogenic masses were harvested after 0, 45 and 90 days of culture on medium supplemented with ABA. (PNG 13 kb)
Electronic Supplementary Material 7
PCR analysis from kanamycin-resistant lines using primers for a 355 bp GFP gene fragment and a 200 bp VirG gene fragment. M: 100 bp DNA ladder (Nippon Genetics). C+: AGL1 pPipsCLV1L-GFP:GUS. C-: P5LV41 non-transformed line. 1–24: kanamycin-resistant lines. (PNG 573 kb)
Rights and permissions
About this article
Cite this article
Alvarez, J.M., Cortizo, M., Bueno, N. et al. CLAVATA1-LIKE, a leucine-rich-repeat protein receptor kinase gene differentially expressed during adventitious caulogenesis in Pinus pinaster and Pinus pinea . Plant Cell Tiss Organ Cult 112, 331–342 (2013). https://doi.org/10.1007/s11240-012-0240-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-012-0240-8