Abstract
In this study, several improvements and simplifications of SE protocols in Pinus pinaster (Ait.), a species of economic importance in the regions of Western Europe, are described. These improvements pertained to all stages of SE including high initiation frequencies in eight control pollinated seed families, relatively high somatic embryo maturation yield when cells were coated with particles of activated charcoal and a rapid production of plants directly in a shade house. The SE initiation frequency from isolated zygotic embryos was high (up to 100%) and plants were produced from 11 embryogenic lines representing all crosses. Based on these results, the estimated number of somatic embryos required to produce 1,000 plants varied from slightly more than the required number of plants to more than double this number depending on the line. Such an estimate is critical in developing plant production strategy when a number of embryogenic lines are considered for production of clonal plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Somatic embryogenesis (SE) of a few economically important conifer species has reached an application stage in vegetative propagation intended for clonal testing and selection of superior genotypes. The protocols to achieve maximal production of plants while reducing the cost and labour are being constantly revised and improved. SE of black, white and Norway spruce (Picea mariana, Picea glauca and Picea abies), loblolly pine (Pinus taeda) and radiata pine (P. radiata) have received the most attention thus far due to the importance of these species to the forest industry in several countries (Cyr and Klimaszewska 2002; Klimaszewska and Cyr 2002). SE of P. pinaster (maritime pine), a species grown for its wood and resin, has also received a lot of attention over the last decade (Bercetche and Pâques 1995; Lelu et al. 1999; Ramarosandratana et al. 2001a, b; Miguel et al. 2004). This research has led to improvements in SE technology for P. pinaster, particularly in the initiation and in the maturation stages where key factors have been identified. However, additional research was necessary to advance this technology towards higher SE efficiency and simpler tissue culture procedures to make the latter feasible and practical in tree breeding programs.
The selection and breeding programs for P. pinaster were initiated in France and Portugal in the early 1960s (Illy 1966; Hopkins and Butcher 1993). At present 100% of reforestration by plantation in the Aquitaine region of France is achieved with improved material from the second-generation seed orchards. In Portugal, P. pinaster is the most representative forest species (30%) and due to its high economical value it has also been introduced to Australia, New Zealand, Argentina and Chile.
This project was undertaken to improve existing protocols of P. pinaster SE and apply these enhancements to embryogenic lines derived from immature zygotic embryos of a variety of genetic backgrounds. A complete cycle of somatic plant production from seeds of eight crosses derived from the breeding program (INRA, Bordeaux, France) of maritime pine is described. The somatic seedlings obtained in the process were then acclimatized and planted in the field for clonal testing. A simplified cryopreservation protocol was also tested on a number of lines for its efficacy. Finally, critical steps, which must be considered in the operational use of SE technology for maritime pine somatic tree production, are discussed.
Material and methods
Origin of the seeds
Female strobili of Pinus pinaster (Ait.) were control pollinated in 2003 (INRA, Bordeaux, France), and the immature cones were collected in the summer of 2004. For this study, nine full sib families were produced, according to a complete factorial mating design between three mother trees from Corsican provenances (A, B and C) and three father trees from the Landes breeding population (D, E and F). The father trees were chosen among the best selections of the actual breeding population and the mother trees were chosen for their good performance in hybridization and to represent the maximum variability (three Corsican provenances). However, the cones from the cross between trees A and F were not available for this study.
Initiation of somatic embryogenesis (Experiment 1)
Prior to seed extraction, each cone was submerged in 95% (v/v) ethanol for 10 min and briefly dried in the laminar flow unit. Subsequently, scales with immature seeds were detached from the cone; seeds were picked with sterile forceps and placed on a sterile surface. It is noteworthy, that during this procedure, the immature seeds were not disinfected; yet the contamination rate of the cultures was less than 0.1% as previously observed for larch species (Lelu et al. 1994). Based on previously published results (Lelu et al. 1999), only zygotic embryos at an early stage of late embryogeny (precotyledonary stages) were excised from the surrounding megagametophytes and used as explants.
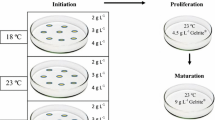
Explants were cultured in 90 mm×20 mm Petri dishes, each of which contained approximately 30 ml of a semi-solid medium (Litvay et al. 1985) modified by reducing the concentration of macro elements to 50% (except iron and EDTA) and adding 1 g l−1 casein hydrolysate (enzymatic, Sigma, CH), 0.5 g l−1 l-glutamine (Sigma), 30 g l−1 sucrose, 2,4-dichlorophenoxyacetic acid (2,4-D) at either 9 or 2.2 μM, 6-benzyladenine (BA) at either 4.4 or 2.3 (μM, and 4 g l−1 gellan gum (Phytagel™, Sigma). The medium with the higher concentrations of 2,4-D and BA was designated as mLV-S (for standard concentration of plant growth regulators (PGR)), and that with lower concentrations of the two compounds as mLV-L (for low PGR). The pH of each medium was adjusted to 5.8 after the addition of gellan gum. An appropriate aliquot of filter-sterilized stock solution of glutamine (also pH adjusted to 5.8) was added to the medium after autoclaving.
The explants were cultured in darkness at approximately 25°C and were not subcultured during the whole experiment, which lasted up to 10 weeks. Each of the 6–14 Petri dishes per combination of a cross and PGR level contained between 3 and 11 explants. There were 116 Petri dishes, altogether, 83 (72%) of which had exactly 10 explants. Several combinations of the levels of the factors were missing. The cross between mother tree A and father tree F was not used due to the lack of cones (Table 1). Among the eight remaining crosses, only four were tested at both PGR levels: the three crosses between father trees D, E and F and mother tree B, and the cross between mother tree A and father tree E. Other available crosses were tested on mLV-S only. Explants were considered to have initiated SE if the embryogenic tissue showed continuous growth and produced amounts of fresh mass (f.m.) sufficient for subculture. The response, noted for each Petri dish, was the number of explants exhibiting such behaviour.
First and subsequent subcultures of embryogenic tissue
The age of the embryogenic culture was set to zero on the day of the first subculture; subsequently, the embryonal mass was subcultured every 2 weeks onto fresh mLV-L medium of the same composition as above, except that the sucrose concentration was reduced to 20 g l−1.
Fifty to 300 mg f.m. of proliferating embryogenic tissue was collected and suspended in 4–5 ml of liquid mLV-L medium, vigorously shaken to break up the tissue pieces into a fine suspension, and poured onto a filter paper (Whatman# 2, diameter 7 cm) in a Büchner funnel. Low-pressure pulse was applied to drain the liquid, and the filter paper with attached cells was placed on the surface of fresh, semi-solid mLV-L medium and cultured in darkness at approximately 25°C for 2 weeks. This procedure yielded 3–4 g of embryogenic tissue per filter paper disc, enough to perform cryopreservation and somatic embryo maturation experiments 1 week after the second subculture on a filter paper.
Cryopreservation protocol
The cryopreservation protocol used in this study has been previously published for P. monticola (Percy et al. 2000) and since then routinely applied to other conifer species (Klimaszewska and Lelu-Walter, unpublished). Briefly, 3 g f.m. embryogenic tissue cultured on filter paper for 7 days were suspended in 12 ml liquid mLV-L supplemented with 0.4 M sorbitol for 18 h. Subsequently, 3 ml of dimethyl sulphoxide (DMSO, Sigma) was added to the suspension on ice (final DMSO concentration 7.5%). After 1.5 h, 1 ml of suspension was transferred into a cryovial and placed in Nalgene Cryo 1°C Freezing Container that was placed in a –80°C freezer for 2 h. The vials were then submerged in liquid nitrogen and stored in a vapour phase cryofreezer (−158°C). Of 12 vials frozen per embryogenic line, two were thawed after 24 or 48 h to test the viability of cells.
Factors influencing maturation of somatic embryos (Experiments 2 and 3)
Prior to the clonal production of plants from the eight crosses used in the initiation experiment (see Experiment 4 below), two maturation experiments (Experiments 2 and 3) were conducted with line MM25 obtained in 2000, which was recovered from cryopreservation, and known to produce mature somatic embryos (Lelu-Walter, unpublished results).
In these two experiments, embryogenic tissue was collected approximately 1 week after subculture and suspended (by vigorous shaking) in liquid mLV medium with 30 g l−1 sucrose and without PGRs. Five millilitres suspension was then poured onto a filter paper disc as described above and placed on a maturation medium. The maturation medium for these experiments was the mLV medium with 68 g l−1 (0.2 M) sucrose, abscisic acid (ABA, racemic, Sigma) and gellan gum (see below). The ABA stock solution was filter sterilized and added to the medium after autoclaving. The medium was dispensed into 90 mm×20 mm Petri dishes at approximately 40 ml per dish.
Cultures were placed under a 16-h photoperiod, dim light (5 μmol m−2 s−1) at approximately 24/21°C day/night temperature for 12 weeks and were not subcultured during the course of the experiments. Productivity was measured as the number of somatic embryos per Petri dish produced over the 12-week period. Modifications of this general protocol specific to each experiment are described below.
Experiment 2
The first of the two preliminary maturation experiments was designed to compare mature somatic embryo production on media with either of two ABA concentrations (80 or 120 (μM) on which either one of three initial amounts of embryogenic tissue (55, 105 or 155 mg per Petri dish) was deposited. The gellan gum concentration was 10 g l−1. The experiment was performed twice on each of two consecutive days, with five Petri dishes per ABA level, per initial amount of embryogenic tissue, per day; it thus comprised a total of 60 Petri dishes.
Experiment 3
The objective of the second maturation experiment was to assess the joint effects of the presence of activated charcoal (AC, Merck, at 10 g l−1) in the embryogenic tissue suspension, and of ABA (80 or 120 μM) and gellan gum (10 or 12 g l−1) concentrations in the maturation medium on somatic embryo production. The amount of cultured tissue was kept constant at approximately 100 mg f.m. If the liquid medium contained AC, both cells and AC particles coating the cell aggregates were collected on the filter paper. Fresh batches of the eight (= 2 AC × 2 ABA × 2 gellan gum concentrations) media were prepared for two full replicates over time: at 18 and 29 weeks of age of the embryogenic tissue cultures. There were 10 Petri dishes (9 in two instances) per medium, per culture age, for a total of 158 Petri dishes.
Maturation of somatic embryos for plant production from newly initiated lines (Experiment 4)
Based on the results of Experiments 2 and 3, an improved maturation protocol was applied to produce plants from line MM25 and 17 randomly chosen lines (from among 440 lines) from the eight crosses (two lines per cross, except for the B×E cross which provided three lines) used in the initiation experiment. From 110 to 140 mg of fresh embryonal mass collected from 5- to 10-week-old cultures (18–20 weeks for line MM25) were dissociated in 4–5 ml liquid medium containing 30 g l−1 sucrose and 10 g l−1 AC, but no PGR’s. The cells were then collected on a filter paper disc and cultured on mLV maturation medium with 68 g l−1 sucrose, 80 μM ABA, and 10 g l−1 gellan gum for 12 weeks without subculture. Photoperiod and temperature regimes were as in Experiments 2 and 3. The number of mature somatic embryos produced after a 12-week period was counted in each of the 198 Petri dishes. The number of Petri dishes varied from 3 to 10 per culture age, per line.
Conversion of somatic embryos to plants (Experiment 5)
Mature, morphologically normal, opaque somatic embryos from 12 of the 18 lines tested in Experiment 4 were picked from Petri dishes after 12 weeks of maturation and placed horizontally, all in the same orientation on the surface of mLV medium without PGRs, with 30 g l−1 sucrose and 4 g l−1 gellan gum to promote germination and conversion to plants. In this germination phase, there were 6–28 somatic embryos (usually 15–25) per (90 mm×20 mm) Petri dish each of which contained 30 ml of medium. The Petri dishes were tilted vertically at an angle of approximately 35°–40° and placed in darkness in this position at day/night temperatures of 24/21°C for 10–14 days to promote hypocotyl elongation and reduce anthocyanin accumulation. Somatic embryos were then exposed to a 16-h photoperiod (10 (μmol m−2 s−1) at 24/21°C day/night temperatures. The plantlets were subcultured once onto fresh medium of the same composition after 6–7 weeks. After 14–16 weeks on germination medium, they were transplanted to a potting mix.
Acclimatization of somatic plants
The potting mix was composed of 75% peat moss, 25% vermiculite and slow release fertilizer osmocote 750 g m−3. The trays with plantlets were placed in a shade house at INRA, Orléans, France, in mid May 2005. A plastic sheet, which was misted to maintain high humidity, covered the plantlets for the first 2–3 weeks. It was then lifted progressively. Plants were watered as needed. After 1 month, the somatic plantlets were treated as seedlings of a similar age.
Statistical analyses
All statistical models were either of the generalized linear mixed type (Brown and Prescott 1999, Chapter 3) or simple generalized linear models (McCullagh and Nelder 1989). When the response variable was a number of ‘successes’ (e.g. initiation) out of a number of ‘trials’ (e.g. explants), it was assumed to follow a binomial distribution. When it was a count (e.g. number of somatic embryos produced), it was assumed to follow a Poisson distribution. All models were fit with the GLIMMIX procedure of SAS/STAT®, Version 9, for generalized linear mixed models, which defaults to generalized linear models when the only random effect in the model is the over-dispersion parameter. This latter parameter allows for some departure from the strict binomial or Poisson assumptions. The models all involved computations on a transformed scale, the logit for binomial data, and the logarithm for Poisson counts. The logit of the probability of success per Petri dish, P, is equal to \( \log ({\frac{P}{{1 - P}}})\). The ratio of P to 1−P is called the odds of success (initiation or conversion to plant). Relevant means and their confidence limits were thus back-transformed for presentation in the tables. Unless otherwise stated, models for Poisson counts included initial fresh mass of embryogenic tissue per Petri dish as an offset variable so that the mean number of somatic embryos could be expressed on the basis of 1 g of embryogenic tissue. Unless specified otherwise, statistical tests were performed at the α=0.05 significance level.
Experiment 1
The logit of the probability that an explant initiates embryogenic tissue production was assumed to depend on the mother tree (A, B or C), the father tree (D, E or F), their interaction, the PGR concentration of the medium, and the two interactions between mother tree and PGR level and between father tree and PGR level. To limit the deleterious effects of the unbalance in the design, this initial model was then reduced hierarchically, first removing two-way interactions (PGR×mother tree, PGR×father tree and mother tree×father tree), if they did not reach significance at the 0.25 level, and the main effects of mother or father tree. Removing the main effect of PGR was not considered since the test of this effect was the main objective of this experiment. Effects retained in the final model were tested at the 0.05 level.
Experiment 2
The number of somatic embryos per Petri dish was analysed as a Poisson random variable where ABA level, initial weight of embryogenic tissue (treated as a three-level factor), their interaction, and days (considered as replicate blocks) were assumed to have fixed effects on the logarithm of the mean per Petri dish. Random effects for the sets of five Petri dishes in each day×ABA×weight combination were also included in the model. To assess the effect of initial weight on the count in this experiment, no offset variable was included in the model to correct for initial weight.
Experiment 3
The logarithm of the expected number of mature somatic embryos per Petri dish was assumed to depend on the fixed effects of AC, ABA, gellan gum, their interactions, and age (considered as blocks). Random effects for the sets of Petri dishes from each treatment×age combination were also included in the model.
Experiment 4
The expected number of mature somatic embryos per Petri dish after 12 weeks of maturation was assumed to depend on the mother tree, the father tree, their interaction, a line effect within cross, and a random effect of culture age within lines.
Experiment 5
The logit of the probability that a somatic embryo converts to a plant was assumed to depend on the mother tree, the father tree, their interaction, and the specific line within each cross. It was also assumed to depend on the random effect of replicates (culture age) within lines. The number of somatic embryos required to produce 1,000 plants was estimated for each line as \( 1,{\kern 1pt}{\kern 1pt}000/\hat P\), where\( \hat P\) is the estimated probability of conversion from somatic embryo to plant for a given line.
Results
High frequency SE initiation and proliferation of embryonal masses (Experiment 1)
The first evidence of SE initiation on both standard and low PGR media was visible on some explants after only 2 weeks of culture with the majority of explants reacting after 4–5 weeks. Most of the initiated embryonal masses proliferated after 7–9 weeks and only these were considered as initiated.
The odds of SE initiation were 3.6 times higher on mLV-S than on mLV-L medium (p≤0.0001; 95% confidence limits of this odds ratio: 2.3, 5.8). The average probability of initiation was 0.93 on mLV-S medium, and 0.80 on mLV-L medium. Hence, the standard PGR level is better than its reduced concentration. There was no evidence that the effect of PGR level on probability of initiation varied among father trees (p=0.55 for the father×PGR interaction) or mother trees (p=0.45 for the mother×PGR interaction), nor was there any indication that differences among the initiation rates of lines from the three father trees varied with mother tree (p=0.51 for the mother tree×father tree interaction). The average probability that explants initiated embryogenic tissue production varied among the three father trees from 0.87 for father tree D to 0.98 for father tree E on mLV-S medium, and from 0.65 for father tree D to 0.92 for father tree E on mLV-L medium (p≤0.0001, Table 1). No such differences could be detected between the initiation probabilities of zygotic embryos from the three mother trees (p=0.69).
Cryopreservation
The simple protocol used for cryopreservation of the 25 newly initiated embryogenic lines resulted in 100% recovery after thawing and culture on a filter paper placed on mLV-L medium (data not shown). In some lines, consistent proliferation was observed after a 2-week culture and enough material was produced for experimentation.
Factors influencing maturation of somatic embryos (Experiments 2 and 3)
There was no indication that the number of somatic embryos per gram fresh mass produced by line MM25 varied with the amount of embryogenic tissue plated on each filter paper, which ranged from 55 to 155 mg, or with the ABA concentration of the medium (p=0.16, p=0.57, respectively). Blocking by days improved the precision of the comparisons between treatment means. Overall, culture of smaller amounts of embryogenic tissue per filter paper not only resulted in similar numbers of mature somatic embryos compared to cultures with initially more tissue, but these cultures also had a reduced mass of proliferating cells that otherwise hindered the development of somatic embryos.
The average number of somatic embryos produced by line MM25 was 5.3 times higher when the embryogenic tissue suspension contained AC than when it did not (p≤0.0001; means: 149 embryos g−1 f.m. with AC, and 28 embryos g−1 f.m. without). Gellan gum concentration apparently also had an effect on somatic embryo production of this line, their average number being 73 g−1 f.m. on medium with 10 gl−1 gellan gum and 57 g−1 f.m. with 12 gl−1 gellan gum (p=0.02). However, there was some indication that the effect of gellan gum concentration differed depending on whether the embryogenic tissue was cultured with AC or without (p=0.024 for the gellan gum×AC interaction). In the presence of AC, average somatic embryo production was about 150 g−1 f.m. at both gellan gum concentrations (p=0.95 for the effect of gellan gum at this AC level); in the absence of AC, 1.6 times more somatic embryos were produced on medium with 10 g l−1 gellan gum (mean: 36 g−1 f.m.) than on medium with 12 g l−1 gellan gum (mean: 22 g−1 f.m., p=0.004, for the effect of gellan gum in the absence of AC). The concentration of ABA in the maturation medium had no apparent effect on somatic embryo production (p=0.72, for the main effect of ABA and p>0.18 for its interaction with either gellan gum concentration, AC or both). The two ages of the tissue cultures, 18 and 29 weeks, considered as block replicates, accounted for a sizeable proportion of the variation of the number of somatic embryos per gram fresh mass of embryogenic tissue (p=0.006), production being somewhat more abundant after 18 weeks of culture (77 g−1 f.m.) than after 29 weeks (55 g−1 f.m.), indicating that blocking by culture age successfully increased the precision of comparisons between the levels of the factors of interest.
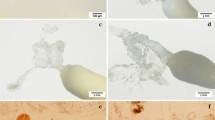
Plant production from somatic embryos of P. pinaster. a Mature somatic embryos on a germination medium at day 0 (Bar=0.5 cm). b Somatic seedlings developed after 3 weeks of culture on a germination medium, left arrow indicates root cap and right arrow indicates growing radicle (Bar=0.5 cm). c Somatic plants developed after 9 weeks on a germination medium (Bar=1 cm). d Somatic plants grown in a shade house, planted in May and photographed in October 2005 (Bar=0.5 cm)
Maturation of somatic embryos for plant production from different lines (Experiment 4)
Based on the results presented above, an improved maturation protocol was applied to produce plants in numbers sufficient for field tests. Mean somatic embryo production of lines varied considerably among mother trees (p=0.0006); the average production of lines from the three father trees also differed (p=0.02), but the nature of the differences became clear only when the interaction was examined closely (p=0.009). It suggested that the embryogenic capacity of a line depended on both parent trees and not on either one alone. Somatic embryo production was more abundant when the line resulted from a cross between mother tree B and father trees D or F, or between mother tree C and father tree D (Table 2). Although there was clear indication that lines from the cross between mother tree A and father tree E produced fewer somatic embryos, on average, the yields from the three lines of the cross between the relatively high producing mother tree B and the apparently low producing father tree E, PM10, PM18 and PM11, were 237, 125 and 54 somatic embryos per gram fresh mass, respectively, the first of these lines producing a number of embryos comparable to that of lines from high producing crosses, and ranking fourth among the 18 tested lines behind two lines from mother tree B (PM5 with father tree F and PM3 with father tree D), and one line from the cross between mother tree C and father tree D (PM12, Table 3). Variation in the yield of somatic embryos among lines within crosses accounted for a significant proportion of the overall variability of the counts on the logarithmic scale (p=0.007).
Germination and conversion to plants (Experiment 5)
Somatic embryos that were initially germinated in darkness developed long hypocotyls and rapidly growing roots (Figs. 1a and b), whereas those germinated in light had stunted hypocotyls and showed signs of stress manifested by dark red colouration of the hypocotyls caused by anthocyanin synthesis (data not shown). Shoots developed after 7 weeks on a culture medium (Fig. 1c).
The probability that somatic embryos produced plantlets varied with mother (p=0.007) and father tree (p=0.045). As for somatic embryo production, however, there was strong evidence that the effect of the father tree was not the same for all mother trees (p≤0.0001 for the mother×father interaction). The data also suggest that somatic embryos from lines of the same cross produced plants with a variable probability of success (p=0.01 for the effect of lines within crosses). Somatic embryos from the highly productive cross between mother tree B and father tree F also had the highest probability of conversion to plants (Tables 4 and 5). The next most successful cross in developing plants from somatic embryos was between the relatively low producing mother tree A and father tree E. This cross produced few somatic embryos, but those it produced had a high probability (0.77) of developing into plants.
The probability that a somatic embryo germinated and the probability that a germinated embryo produced a plant were both analysed according to a model similar to the one used in the analysis of the probability of producing plants from somatic embryos. They revealed that differences in the latter probability among lines, crosses, and mother and father trees were essentially a phenomenon of somatic embryo germination. Once a somatic embryo had germinated, the probability that it produced a plant (aerial parts) was virtually the same for all lines.
The number of somatic embryos needed to produce 1,000 plants was estimated from the line-specific probabilities of producing plants from somatic embryos as estimated from the generalized mixed model (Table 5). It varied between more than double the target number of plants (2,330 somatic embryos for line PM18), when the probability of development into plant was lowest, to 1.14 times the target number (1,140 somatic embryos for line PM5) when this probability was highest.
Acclimatization and survival of somatic plants in a shade house
The in vitro-developed plantlets were characterized by a vigorous growth of shoots and roots (Fig. 1c), and at this stage they were transplanted into potting mix and placed in a shade house under ambient conditions. After 1 month, the overall survival of plants representing 11 lines averaged 65%, but it varied from 36 to 100% depending on the line (Table 6, Fig. 1d).
Discussion
Initiation of conifer SE is known to be under strong genetic control (Park et al. 1993; Lelu et al. 1999; Klimaszewska et al. 2001; Miguel et al. 2004; Niskanen et al. 2004). In P. strobus, the estimated heritability of SE initiation was large when compared with that of other quantitative traits in forest trees, indicating that breeding and selection of responsive families would be conceivable (Klimaszewska et al. 2001). In the present study involving eight crosses of P. pinaster, the probability that explants produced embryogenic tissue was found to depend strongly on the father tree, but not on the mother tree, thus partially corroborating previous findings.
The probability that a zygotic embryo produced embryogenic tissue was estimated to be 3.6 times higher on mLV-S medium than on mLV-L medium, with mean probabilities of 0.93 on mLV-S and 0.80 on mLV-L, both very high. A few factors could contribute to this high SE initiation frequency in our study. One of them might be the fact that we controlled the developmental stage of the zygotic embryos ensuring that all the explants in the initiation experiment were developmentally uniform. Often this is not the case when whole megagametophytes with enclosed embryos are cultured on the initiation medium. Although the developmental stage of batches of immature seeds is routinely determined from preliminary samples prior to the onset of an experiment, it is difficult to ensure that all the seeds in a batch of selected cones are at the same stage. Specific father effect also seemed to contribute to this high response.
However, the procedure of excising the zygotic embryos from megagametophytes is time consuming and requires sophisticated technical skill; therefore small scale experiments were carried out in 1999 and 2000 (data not shown) also including the whole megagametophytes, which are more commonly used explants in SE of pine species. The results of SE initiations from 100 of each of the two types of explants were as follows: 43 and 50 responding megagametophytes versus 60 and 55 responding excised zygotic embryos in 1999 and 2000, respectively. These preliminary data were encouraging and indicated that an alternative type of explant might be utilized to reduce the time of dissection.
The explants remained on the same medium during the whole culture period lasting up to 10 weeks without any deleterious effect on viability of the initiated embryonal mass. During this period, the embryogenic tissue could be subcultured at any suitable time. Some explants formed ‘subculturable’ amounts of embryonal mass after only 4 to 6 weeks of culture while others did so only 5 weeks later.
One of the difficulties encountered in conifer somatic embryogenesis is the relatively long time required to amass enough material (up to 4 months, depending on the species) to begin experimentation or plant production. Increase of tissue mass through subcultures of pieces of tissue is not economical because it requires time to perform serial subcultures and not all tissue can be subcultured due to partial necrosis of the interior part of a tissue piece. Instead, we recommend carrying out the first subculture on a filter paper, as previously described in detail for Pinus strobus and P. monticola (Klimaszewska and Smith 1997; Percy et al. 2000; Klimaszewska et al. 2001). Utilization of a filter paper approximating the diameter of a given Petri dish maximizes the amount of tissue grown and minimizes the cost. Under our conditions, the tissue mass of P. pinaster increased up to eight-fold after a 2-week period (data not shown).
Another critical issue in SE of pine species is the age of culture and its influence on the ability of early somatic embryos to mature. In P. strobus and P. pinaster, embryos from a few embryogenic lines lost their maturation ability after several months of culture (Klimaszewska and Lelu-Walter, personal observations, Breton et al. 2005). This phenomenon has also been reported for other woody species such as the rubber tree (Charbit et al. 2004). It is therefore, imperative to amass several grams of tissue in a relatively short period of time (after one or two subcultures) for cryopreservation and ideally at the same time for maturation experiments, and record the plant forming ability of a given line. A rapid increase of embryogenic tissue fresh mass when cultured on a filter paper, eliminated the need for lengthy subcultures and rendered this method more economical.
Maturation of maritime pine somatic embryos was enhanced by coating the cells with particles of AC and culture on mLV medium with a high concentration of sucrose, ABA, and with either 10 or 12 g l−1 gellan gum. The nature of the interaction between the effect of AC and gellan gum concentration in the maturation medium suggested that somatic embryos were produced abundantly in the presence of AC whatever the gellan gum concentration of the medium. This result might be attributed to the hygroscopic property of AC, which created similar water availability conditions on both media, a factor critical in maturation of pine somatic embryos (Klimaszewska and Smith 1997; Klimaszewska et al. 2000). Somatic embryos that matured in the presence of AC developed faster and in greater numbers, clearly improving the productivity of the culture. Activated charcoal is known for its adsorption of residual plant growth regulators (Ebert et al. 1993; von Aderkas et al. 2002) and since the embryonal mass had been cultured on a medium with 2,4-D and BA prior to transfer onto maturation medium, the beneficial effect of coating the cells with AC particles might be attributed to this particular property. Equally, we cannot rule out the likelihood that AC adsorbed some ABA from the maturation medium. However, if this were occurring, then low concentrations of ABA would be as effective for maturation of somatic embryos. The latter has not been tested in the presented experiments, but previous work showed that 80 μM ABA was most beneficial; hence, the effect of AC seems to be complex.
The average difference in the number of mature somatic embryos among Petri dishes with initial 150, 100 or 50 mg f.m. embryonal mass was not significant, perhaps indicating the existence of a production threshold that did not depend on the initial amount of material. Alternatively, due to the abundant proliferation of non-embryogenic cells and eventually their death, many developing somatic embryos were buried in the dead cell mass and failed to mature perhaps due to changed water conditions and/or nutrient and ABA availability.
The 18 lines used for clonal plant production responded to the same maturation treatment with different numbers of mature somatic embryos. Generally, the embryogenic potential depended on the cross as well as on the specific line. The mean number of mature somatic embryos produced per gram fresh mass varied from 24 to 441 except for one line which produced an average of 2 embryos g−1 f.m. (Table 3). Similarly, the rate at which somatic embryos from 12 lines produced plants also varied among crosses and among lines of a specific cross. These empirical data are important for developing a strategy for production of a given number of plants from different lines. We therefore estimated, for each of the 12 lines tested, the number of mature somatic embryos necessary to produce 1,000 plants (Table 5). The relative yield numbers varied among the lines from 1,140 to 2,330.
Once the plants developed in Petri dishes it was beneficial to transfer them to ex vitrum conditions within a relatively short period of time to avoid deterioration. Based on the specific climatic conditions in the region of Orléans, France, the plants were transferred from Petri dishes to a potting mix and directly to a shade house in mid May 2005, and treated as seedling counterparts. Such a procedure eliminated the need for a greenhouse, simplified plant production and lowered the cost.
In conclusion, we believe that these simplified P. pinaster SE protocols should influence breeding strategies by offering an alternative tool for accelerated production of plants for clonal tests. The simplifications include:
-
1.
Seed dissection without prior disinfection.
-
2.
Culture of zygotic embryo explants of uniform developmental stage on mLV-S medium without subculturing during the whole initiation period (up to 10 weeks).
-
3.
Rapid increase of embryogenic tissue fresh mass by proliferation on filter paper discs (5–6 g f.m. material can be produced within 4 weeks from the initial 300 mg f.m.).
-
4.
Simplified cryopreservation method (no need for programmable freezer)
-
5.
Maturation of somatic embryos by coating 50–100 mg f.m. embryogenic tissue with AC particles, and culture on filter paper discs on mLV medium with 68 g l−1 sucrose, 80 μM ABA and solidified with 10 g l−1 gellan gum for 12 weeks. No subcultures are required during the entire maturation period.
-
6.
The first 10 days of germination carried out in darkness in order to elongate hypocotyls. This facilitated and improved the handling of plants at the later stage.
-
7.
Transfer of plants from Petri dishes to a potting mix during vigorous growth phase, and acclimatization under shade house conditions (with initial maintenance of high relative humidity) commencing at the appropriate date specific for the climatic region. Elimination of the need for a greenhouse.
References
Bercetche J, Pâques M (1995) Somatic embryogenesis in maritime pine (Pinus pinaster). In: Jain SM, Gupta PK, Newton RJ (eds) Somatic embryogenesis in woody plants. Gymnosperms, vol 3. Kluwer Academic Publishers, Dordrecht, Netherlands, pp 221–242
Baradat P, Pastuszka P (1992) Le pin maritime. In: Gallais A, Bannerot H (eds) Amélioration des espèces végétales cultivées. Institut National de la Recherche Agronomique, Paris, pp 695–709
Breton D, Harvengt L, Trontin J-F, Bouvet A, Favre J-M (2005) High subculture frequency, and maltose-based and hormone-free medium sustained early development of somatic embryos in maritime pine. In Vitro Cell Dev Biol Plant 41:494–504
Brown H, Prescott R (1999) Applied mixed models in medicine. Wiley, Chichester, 408 pp
Charbit E, Legavre T, Lardet L, Bourgeois E, Ferriere N, Carron MP (2004) Identification of differentially expressed cDNA sequences and histological characteristics of Hevea brasiliensis calli in relation to their embryogenic and regenerative capacities. Plant Cell Rep 22:539–548
Cyr DR, Klimaszewska K (2002) Conifer somatic embryogenesis: Applications. Dendrobiology 48:41–49
Ebert A, Taylor F, Blake J (1993) Changes of 6-benzylaminopurine and 2,4-dichlorophenoxyacetic acid concentrations in plant tissue culture media in the presence of activated charcoal. Plant Cell Tissue Organ Cult 33:157–162
Hopkins ER, Butcher TB (1993) Provenance comparisons of Pinus pinaster (Ait.) in Western Australia. CALMScience 1:55–105
Illy G (1966) Recherches sur l’amélioration génétique du pin maritime. Ann Sci Forest 23:757–948
Klimaszewska K, Cyr DR (2002) Conifer somatic embryogenesis: Development. Dendrobiology 48:31–39
Klimaszewska K, Smith DR (1997) Maturation of somatic embryos of Pinus strobus is promoted by a high concentration of gellan gum. Physiol Plant 100:949–957
Klimaszewska K, Bernier-Cardou M, Cyr DR, Sutton BCS (2000) Influence of gelling agents on culture medium gel strength, water availability, tissue water potential, and maturation response in embryogenic cultures of Pinus strobus L. In Vitro Cell Dev Biol-Plant 36:279–286
Klimaszewska K, Park Y-S, Overton C, Maceacheron I, Bonga JM (2001) Optimized somatic embryogenesis in Pinus strobus L. In Vitro Cell Dev Biol-Plant 37:392–399
Lelu MA, Bastien C, Klimaszewska K, Ward C, Charest PJ (1994) An improved method for somatic plantlet production in hybrid larch (Larix x leptoeuropaea). Part 1 Somatic embryo maturation. Plant Cell Tissue Organ Cult 36:107–115
Lelu MA, Bastien C, Drugeault A, Gouez ML, Klimaszewska K (1999) Somatic embryogenesis and plantlet development in Pinus sylvestris and Pinus pinaster on medium with and without growth regulators. Physiol Plant 105:719–728
Litvay JD, Verma DC, Johnson MA (1985) Influence of loblolly pine (Pinus taeda L.) culture medium and its components on growth and somatic embryogenesis of the wild carrot (Daucus carota L.). Plant Cell Rep 4:325–328
McCullagh P, Nelder JA (1989) Generalized linear models. Chapman & Hall, London, 511 pp
Miguel C, Gonçalves S, Tereso S, Marum L, Oliveira MM (2004) Somatic embryogenesis from 20 open-pollinated seed families of Portuguese plus trees of maritime pine. Plant Cell Tissue Org Cult 76:121–130
Niskanen A-M, Lu J, Seitz S, Keinonen K, von Weissenberg K, Pappinen A (2004) Effect of parent genotype on somatic embryogenesis in Scots pine (Pinus sylvestris). Tree Physiol 24:1259–1265
Park YS, Pond SE, Bonga JM (1993) Initiation of somatic embryogenesis in white spruce (Picea glauca): genetic control, culture treatment effects, and implications for tree breeding. Theory Appl Genet 86:427–436
Percy RE, Klimaszewska K, Cyr DR (2000) Evaluation of somatic embryogenesis for clonal propagation of western white pine. Can J For Res 30:1867–1876
Ramarosandratana A, Harvengt L, Bouvet A, Calvayrac R, Pâques M (2001a) Effects of carbohydrate source, polyethylene glycol and gellan gum concentration on embryonal-suspensor mass (ESM) proliferation and maturation of maritime pine somatic embryos. In Vitro Cell Dev Biol-Plant 37:29–34
Ramarosandratana A, Harvengt L, Bouvet A, Calvayrac R, Pâques M (2001b) Influence of the embryonal-suspensor mass (ESM) sampling on development and proliferation of maritime pine somatic embryos. Plant Sci 160:473–479
von Aderkas P, Label P, Lelu MA (2002) Charcoal affects early development and hormonal concentrations of somatic embryos of hybrid larch. Tree Physiol 22:431–434
Acknowledgements
This work was partly funded by EU (GEMINI, QLK5-CT-1999- 00942). The authors thank A. Raffin and P. Patuszka from INRA, Bordeaux for providing the cones from the breeding program. K. Klimaszewska's contribution was possible through the financial support of le Studium, Region Centre, France.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Jouanin
Rights and permissions
About this article
Cite this article
Lelu-Walter, MA., Bernier-Cardou, M. & Klimaszewska, K. Simplified and improved somatic embryogenesis for clonal propagation of Pinus pinaster (Ait.). Plant Cell Rep 25, 767–776 (2006). https://doi.org/10.1007/s00299-006-0115-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-006-0115-8