Abstract
In vitro chromosome doubling from hypocotyl segments of yellow passion fruit (Passiflora edulis Sims.) was carried out in the presence of either colchicine (0, 25, 250 and 1,250 μM) or oryzalin (0, 5, 15 and 30 μM). Murashige and Skoog (in Physiol Plant 15:473–497, 1962)(MS)-based regeneration medium containing 250 or 1,250 μM colchicine markedly affected explant development leading to browning and death of the hypocotyl segments. Oryzalin has similar effect to colchicine in inducing polyploidy. In vitro regenerated autotetraploid plants induced by 25 μM colchicine or 15 μM oryzalin were further acclimatized and cultivated in hydroponics system in greenhouse. Autotetraploids plants were more vigorous than the control diploids. The chromosome number of diploid plants was 2n = 2x = 18, whereas that found on autotetraploid plants were 2n = 4x = 36. The stomata sizes of the autotetraploids were significantly larger than those on the diploid counterparts, while the frequency of stomata was significantly reduced. Similarly, the chloroplast number of guard cells of autotetraploid plants increased significantly. Two albino plants (4%) were generated in medium with 25 μM colchicine, indicating phytotoxic effects. These plants are being grown to full maturity in order to test their potential to use in a breeding program.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Passiflora edulis Sims, the yellow passion fruit, is the species with one the highest economic importance in the Americas, Australia and Africa (Oliveira 1987). Brazil is the major worldwide producer, with an area of approximately 50,795 ha destinated to this crop production (IBGE 2009). The fruit is consumed either as fresh or processed for juice. Most the commercial passion fruit varieties available are susceptible to a large number of pests and diseases with considerable negative effects on the yield (Bruckner et al. 1995).
Passion fruit is a diploid species with 2n = 2x = 18 chromosomes (Snow and MacDougal 1983). The flowers are perfect and hermaphrodite, but self-incompatible (Rêgo et al. 1999, 2000).
Polyploidy naturally occurrence had only been previously found in a cultivated form of P. incarnata (2n = 4x = 36) (Lloyd 1963), although the diploid form is more common found. Melo et al. (2001) reported the occurrence of octoploids (2n = 8x = 72) in an unidentified species within the genus Passiflora, while all other members displayed 2n = 2x = 18.
The first polyploid plant produced by cross in Passiflora genus was described by Knight Jr (1991), a diploid hybrid F1 (Passiflora incarnata L. × P. edulis Sims), which was pollen-sterile and no fruitful. Nevertheless, fertility was restored in some individuals after doubling the chromosome number of emergent F1 seedlings treated with colchicine. However, all plants showed strong self-incompatible and several of them had low pollen viability. Crossing colchicine-treated plants, which had been converted to amphiploids, produced an allotetraploid hybrid group of four seedling progenies that had some degree of cross-compatibility. Juice of the amphiploid hybrid was lighter in color than that obtained from P. edulis fruits, but is sweeter, strongly aromatic, and may have use as the only ingredient or blended with other juice sources. Other allopolyploids plants also were obtained by somatic hybridization in Passiflora (Dornelas et al. 1995; Otoni et al. 1995).
Polyploidy, defined by the presence of more than two chromosome sets in a single individual, and it is widespread among the angiosperms. Polyploid origin can be divided into autopolyploids, which are derived from a whole-genome duplication event of the same ancestral chromosome set; and allopolyploids, which are derived from a hybridization event of alternate parental genomes followed by the genome duplication (Grosser and Gmitter 2011; Pignatta et al. 2010; Yang et al. 2011; Ochatt et al. 2011).
Polyploid fruit plants include commercially successful cultivars because of their favorable horticultural characteristics, such as large fruit size, sturdiness, high yield, disease-resistance, seedless or fewer seed production (Predieri 2001). In addition, the leaf, stem and root, which can all be useful in many crop plants, are usually bigger in polyploid plants than in diploid plants. Thus, polyploid plants may increase biomass or fruit production (Zhang et al. 2008; Leitch and Leitch 2008; Dubcovsky and Dvorak 2007).
Plant breeders have taken advantage of polyploidy in order to improve several important agronomic traits, some of which have been generated by allopolyploidisation of interspecific hybrids or by autopolyploidisation within the species (Bennett 2004).
Particularly the tetraploidy event, has several potential advantages because the organisms can result to a bigger number of genes and higher number of allelic variants. It is believed to be advantageous for plant metabolism in terms of elevated rates of synthesis or a higher variability of metabolically relevant compounds (Osborn et al. 2003; Yang et al. 2011).
Passion fruit breeding and selection goals emphasize traits related to fresh fruit production and supply for the juice industry. For fresh fruit production, the criteria include fruit size, firmness, pulp content, shape, colour, flavour and aroma. However, yield, acidity and total soluble solids are important traits for juice production. For both destinations, the development of cultivars includes the following objectives: yield (early maturity and self-fertility); fruit quality (uniform size, yield of pulp and juice, sugar: acid ratio, aroma and colour of pulp and pericarp, and turgescence), and disease resistance (Coppens D’Eeckenbrugge et al. 2001).
Colchicine is a compound that effectively arrests mitosis at the anaphase stage, and is widely used to induce polyploidy in plants. In vivo treatment of shoots, smaller axillary or sub-axillary meristems, seeds or seedlings with colchicine is traditionally employed to generate polyploid plants. However, low efficiency of polyploid plants production and a high frequency of chimeras are often associated with this method. With the development of adventitious organ regeneration techniques, in vitro induction of polyploidy has become the main method for several plant species, including Morus alba (Chakraborti et al. 1998), Punica granatum (Shao et al. 2003), Bixa orellana (Carvalho et al. 2005), Zizyphus jujube (Gu et al. 2005), Platanus acerifolia (Liu et al. 2007), Carica papaya (Sun et al. 2011), Actinidia chinensis (Wu et al. 2011), Gerbera jasmesonii (Gantait et al. 2011), among many others. It shows that in vitro induction increases efficiency and decreases occurrence of chimeras as compared to in vivo methods.
Recently, Dhooghe et al. (2011) reported several methodologies for mitotic in vitro chromosome doubling, which consists of several sub-processes. The initial process comprises the treatment of the plant material with antimitotic agents. After one or several multiplication cycles, the efficiency of polyploidization can be estimated. Besides, there are differences on antimitotic agents efficiency and the in vitro chromosome doubling protocols varies among the explant types, exposure time, concentration and application method.
However, colchicine is very harmful to human health and in some cases, shows undesirable mutagenetic activity on plants too (Van Tuyl et al. 1992; Carvalho et al. 2005). Oryzalin also inhibits mitosis activity and is one of the main chemicals used for chromosome doubling in lily (Van Tuyl et al. 1992; Allum et al. 2007), tobacco (Ramulu et al. 1991), and many other species. In lily, oryzalin effectively induced polyploids and can be considered as an alternative to colchicine (Van Tuyl et al. 1992).
The determination of ploidy levels is essential for breeding asexually propagated and polyploid crops that may have ploidy chimeras in their tissues or have several polyploid series. The different method for ploidy determination include root tip squashes, mother pollen cell squashes, pollen grain size and germinal pore counts, stomata size and density determination, and gross morphology (Doležel et al. 2007; Hodgson et al. 2010; Dhooghe et al. 2011). Recently, Yu et al. (2009) optimized conventional methodologies and developed a novel strategy for fast generation and identification of polyploids based trichome branching patterns. Nevertheless, flow cytometry is the most efficient tool to detect the ploidy level of plants (Doležel et al. 2007; Ochatt et al. 2011; Lema-Ruminska, 2011).
The present study aimed to develop an efficient means to induce autotetraploids in vitro by treating hypocotyl segments of yellow passion fruit with colchicine and oryzalin on the regeneration and development of tetraploid plantlets induction.
Materials and methods
In vitro induction of polyploidy
Seeds of yellow passion fruit (Passiflora edulis Sims.) were collected from adult plants growing in an experimental field plot at the Federal University of Viçosa, Minas Gerais State, Brazil. The coat of the seeds was removed mechanically with the help of a Mini-Vice Swivel 50 mm. Then, embryos and endosperm were dipped into 70% (v/v) ethanol for 1 min, followed by 2.5% (v/v) sodium hypochlorite and 0.1% (v/v) Tween 20 for 10 min. Afterwards, the seeds were rinsed four times with sterile distilled water. Embryos were germinated in test tubes (150 × 25 mm) containing 10 ml half strength MS medium (Murashige and Skoog 1962), supplemented with B5 vitamins (Gamborg et al. 1968), 2% (w/v) sucrose, 50 mg l−1 myo-inositol and 2.8 g l−1 Phytagel® (Sigma Chemical Co., USA).
In vitro-grown seedlings (15 days after germination) were used as source of explants. Hypocotyls segments of 10 mm long were cultured horizontally in Petri dishes (60 × 15 mm; J. Prolab, Brazil). The experimental design was completely randomized with eight treatments and five replicates, comprising five Petri dishes, each containing ten explants.
The polyploidization induction culture medium was composed by MS salts, with B5 vitamins, 100 mg l−1 myo-inositol, 3% (w/v) sucrose, 1 mg l−1 BA, 0.7% (w/v) agar (Merck, Germany), and different concentrations of microtubule depolymerising agents, colchicine at 0, 25, 250, and 1,250 μM or by oryzalin at 0, 5, 15, and 30 μM. The explants were kept on this medium and after 15 days, the explants were transferred to a fresh medium lacking antimitotic agents and remained on in this medium until the full regeneration of shoots.
The experimental design was a complete randomized, with six treatments three with colchicines and three with oryzalin plus the controls,. Each treatment was constituted by five replicates and each replicate by 10 explant/Petri dish, which was considered as an experimental unit.
The data after transformation by (x + 0.5)1/2, were subjected to the analysis of variance ANOVA and significant differences among the treatments were tested by Tukey’s test at 5% level using GENES software package (Cruz 2008).
Elongation and rooting media shoots
Regenerated shoots were excised from the original explants and then transferred to elongation and rooting medium consisted by MS salts, plus B5 vitamins, 100 mg l−1 myo-inositol, 3% (w/v) sucrose, 2.15 mg l−1 Kin, 0.88 mg l−1 IAA and 0.8% (w/v) agar (Merck, Germany). Elongated shoots (average 10 mm length) were individualized and transferred to a rooting medium with half-strength MS salts, supplemented with B5 vitamins, 3% (w/v) sucrose, 100 mg l−1 myo-inositol, 2.8 g l−1 Phytagel® and 5 μM IBA. The medium was dispensed in 10 ml aliquots per test tube (25 × 150 mm) covered with polypropylene lids.
Unless otherwise stated, the medium pH was adjusted to 5.7 ± 0.1 prior to autoclaving at 121°C, 1,05 kgf.cm2, for 15 min. Solutions of 125 μM colchicine and 10 μM oryzalin in 10% DMSO (v/v) were filtered through 0.22 μM pore size Millex-GS (Millipore™). Colchicine, oryzalin and BA were added to the medium before gelling occurred.
Cultures were maintained at 27 ± 2°C under a 16/8 h light/dark regime with 36 μmol m2 s−1 light radiation provided by two fluorescent lights (20 W, Osram, Brazil).
Stomatal analyses
To access the polyploidy level, oryzalin- or colchicine- treated plants we screened for the number of chloroplasts present at stomata guard cells. Two leaves from each established plants were sampled, from which, paradermic hand sections and abaxial epidermis imprints with the help of a nail polish. The preparation was mounted on slides for further microscopic observation. Images were captured by using a camera connected to a microscope, (Model Axio Image, Carl Zeiss, Germany), and to a computer, equipped with a digitalizing board. The determination of the number of chloroplasts per guard cell was carried out from the captured images, obtained with a 40× objective lent. For each leaf, four fields of 1 mm2 were randomly sampled to determine number of chloroplasts per guard cell.
Chromosome counting
The ploidy level was determined by chromosome counting in metaphasic cells. Under a stereo microscope (Model Stemi 2000, Carl Zeiss, Germany), meristematic regions from the root apices were removed, fixed in a methanol/acetic acid (3:1) solution, and kept in a freezer at 20°C until the analysis. The apical roots were then washed with tap water for 10 min, and then rinsed with distilled water for 5 min to remove the fixative solution. Slides were prepared by cellular dissociation, air-dried and then placed on a hot-plate at 50°C for 20 min. The slides were stained with 3% Giemsa solution in phosphate buffer pH 6.8 for 4 min, and then washed with distilled water and air-dried. An average of 200 cells were sampled and analyzed per bud flower.
Morphoagronomic characteristics
In order to compare diploid and tetraploids plants, we evaluated the following morphological, floral and fruit characteristics. Leaves were evaluated for the following traits: leaf length and width, leaf index (length/width), number of stomata (mm2), and number of stomata guard cell. For the flowers it was determined diameter (cm). The fruits were evaluated for the following traits: fruit weight (g), volume of juice per fruit (ml), soluble solids content (%), volume of juice/aril (ml), ripe fruit size (length and diameter) and number of seeds per fruit (black and white). Each evaluation was based on samples from 10 fruits.
Results and discussion
Survival and growth of colchicine-treated explants
The effect of different concentrations of colchicine and oryzalin on the survival rate of the explants was assessed 7, 15 and 30 days after of growth (Table 1). The survival rate of the explants decreased with the increasing of colchicine concentration and time of treatment. Colchicine treatment at 250 and 1,250 μM for 7 days proved to be highly toxic resulting in the death of all the explants. On the other hand, almost all the treated hypocotyls segments (94%) survived at 25 μM colchicines, regenerating diploid (2%) and autotetraploid (8%) plants (Table 1). Colchicine treated shoots along with the controls were maintained in a sustainable manner with regular subcultures at intervals of 15 days. Similar results were reported by Gantait et al. (2011) working with the induction of tetraploids using in vitro colchicine treatment on Gerbera jamesonii Bolus cv. Sciella.
In attempts to induce tetraploidy in banana resulted in negative effects on shoot regeneration and development when grown in medium with high colchicine concentrations (Van Duren et al. 1996). Likewise, Carvalho et al. (2005) induced tetraploid plants on Bixa orelana using hypocotyl segments, and also reported similar results to banana when using the same concentrations of colchicine. Phytotoxic effects of colchicine on Tripsacum dactyloids L. (Gramineae) had been previously reported by Salon and Earle (1998). According to De Jesus-Gonzalez and Weathers (2003), the efficiency of colchicine on polyploidy induction of Artemisia annua was low and at about 25% of the hairy root-treated tips died, regardless of the concentration used.
Two albino plants (4%) occurred among autotetraploid plants induced by colchicine. Albino plants were also reported in vitro induced polyploids of Nicotiana tabacum (Devreux, 1970), Datura sp. (Narayanaswamy and Chandy 1971), and Oryza sativa (Sun et al. 1979). According to these authors, the albinism results from deletions on the plastid DNA.
The treatments with oryzalin were less phytotoxic than the colchicine ones. The highest oryzalin concentration (30 μM) applied for 15 days, resulted in a survival rate of 98% viable explants, regenerating 2% autotetraploid plants. At low oryzalin concentration, shoot proliferation was statistically similar when compared to the control, reaching 100% of viable explants. Oryzalin has been reported to be less phytotoxic than colchicine and more effective for chromosomal duplication in several species (Chalak and Legave 1996; Väinölä 2000; Carvalho et al. 2005).
In relation to the antimitotic action of oryzalin, we did not observe any significant effect on the production of tetraploids (data not shown). Hebert et al. (2010) which reported the polyploid induction and regeneration of Rhododendron ‘Fragantissimum’ observed that treatment duration also did not influence ploidy level using 30 μM oryzalin. In this work, oryzalin was not more effective in inducing changes on ploidy level than colchicine.
Chromosome number determination in root-tips
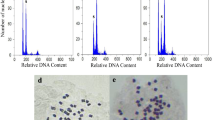
The level of polyploidy from in vitro regenerated plants was determinated by chromosome counting on root-tip of metaphasic cells. Cytological analysis revealed that out from the 14 regenerated plants, 6 were diploids (2n = 2x = 18) (Fig. 1a) and 8 autotetraploids (2n = 4x = 36) (Fig. 1b). Single spontaneous duplication was induced by in vitro culture in control plants without the help of any antimitotic agent (Table 2). And according to Snow and MacDougal (1983), the complement of chromosome number of Passiflora edulis Sims is 2n = 18. We also found a total chromosome number of 18 in diploid plantlets and 36 in the tetraploids.
Chloroplast number in guard cells
The chloroplast numbers present in guard cells was sufficient to identify the diploid and autotetraploid plants. Leaves from diploid and tetraploid plantlets had 8.4 and 16.3 chloroplasts per pair of guard cells, respectively (Fig. 1c, d), but the frequency of stomata was lower in the latter (Table 2). Diploid plants tend to have smaller stomata than their tetraploidized derivatives (Hodgson et al. 2010; Tang et al. 2011). Similar results was obtained by Otoni et al. (1995) using this technique to screen somatic hybrids on Passiflora species grown at in vitro conditions. The authors found an average number of stomatal chloroplasts of 10.7 ± 1.5 in diploid plants of P. edulis f. flavicarpa Deg. and 20.4 ± 2.8 for their somatic hybrids (allopolyploid). In the present study, tetraploids plants also had a higher number of stomatal chloroplasts (almost double) than diploids ones (Table 2). These results indicate that this methodology of chloroplast counting in guard cells was an effective and rapid way to determine polyploidy level. The polyploidy level based on chloroplast number has also been related in previous reports (Yu et al. 2009; Gu et al. 2005).
Morphoagronomic characteristics analysis
Six months after transplanting plants began to flower. Variants on the morphological characteristics of leaf, flower and fruits set were observed between 2× and 4× plants grown under the same conditions. Tetraploid plants present significant increased (P < 0.05) for the most traits evaluated, except for leaf index (leaf length/leaf width) which was 1.26 (Table 2).
The fruit size (length and diameter), amount of the juice, seeds number were evaluated in diploid and tetraploid plants of passion fruit (Table 2). Fruit weight in diploid plants was 172.0 g and for the autotetraploid 254.81 g. The number of seed per fruit in diploid was higher than tetraploids plants. This character is very important due the high positive correlation between the amount of the juice and number of seed per fruit. The amount of juice in fruit from 2× plants was of 29.1 ml while in the 4× plant was 39.93 ml (Table 2). Total soluble solids content for the tetraploids was significantly higher than the content found in fruits from diploid plants. In the present study, tetraploids fruits also had a higher amount juice per seed (almost double) than diploid plants. The juice was pleasant in flavor and strongly aromatic than juice of P. edulis f flavicarpa in this respect. Thus, there is a reason to expect that juice of selections from the tetraploid can have many uses for the same juice like for the purple or yellow passion fruit.
Different results were obtained by Knight Jr. (1991) working with 10 clones allotetraploid hybrid group of Passiflora incarnata L. × P. edulis f. flavicarpa Deg., which were compared for specific attributes. The number of seeds per fruit determined ranged from one to 129. And, the amount of juice per fruit ranged from 0.5 to 31.5 ml, except for total soluble solids content for the allotetraploid plants, which ranged from 13.2 to 17.2%.
Tetraploid plants grew faster, they were more vigorous, and had flowers with longer duration and size when compare with control diploid plants (Fig. 2). According to Samford (1983) and Predieri (2001), the effects of polyploidy on plants, include significant enlargement of fruits and flowers, longer lasting flowers, overcome barriers for hybridization, no seed or fewer seed, enhanced pest resistance and stress tolerance.
Large fruit size, sturdiness, high productivity, disease-resistance, no seed or fewer seed are attributes of polyploidy in many plants (Predieri 2001), as well as increase biomass or product yields (Zhang et al. 2008). Effects of gigas expression were described for several characteristics of agronomics interest in Capsicum annuum (L) by Kulkarni and Borse (2010). As observed by Bennett (2004), polyploidy should be utilized by plant breeders because of the advantage from polyploidy to improve agronomic traits of economically important plants. Some of these plants have been generated by allopolyploidisation of interspecies hybrids or by autopolyploidisation within species, as occurred in this work.
In this work, it was determined that stomata length and chloroplast number in guard cells is a suitable parameter to identify putative yellow passion fruit autotetraploids.
In conclusion, this study presents the first report of autotetraploid plants of yellow passion fruit derived from hypocotyls segments in vitro cultivated. The protocol described here was effective with 16% of the plants being autotetraploids, suggesting that their chromosomes were doubled.
This study is a novel and successful attempt to use polyploidy to demonstrate targeted trait improvement throughout the selection for improved juice fruit production and better soluble solids content in yellow passion fruit, as well as, to increase the size of this beautiful flower, which is the most important characteristics as an ornamental plant.
The developed genetic material in this present work is being used for further selections throughout the segregating generations. Hybridization of these novel tetraploids with other breeding material, with commercially important traits, is also being carried out.
References
Allum JF, Bringloe DH, Roberts AV (2007) Chromosome doubling in a Rosa rugosa Thunb. hybrid by exposure of in vitro nodes to oryzalin: the effects of node length, oryzalin concentration and exposure time. Plant Cell Rep 26:1977–1984. doi:10.1007/s00299-007-0411-y
Bennett MD (2004) Perspectives on polyploidy in plants-ancient and neo. Biol J Lin Soc 82:411–423. doi:10.1111/j.1095-8312.2004.00328.x
Bruckner CH, Casali VWD, Moraes CF, Regazzi AJ, Silva EAM (1995) Self-incompatibility in passion fruit (Passiflora edulis Sims). Acta Hortic 370:45–57
Carvalho JFR, Carvalho CR, Otoni WC (2005) In vitro induction of polyploidy in annatto (Bixa orellana). Plant Cell Tiss Organ Cult 80:69–75. doi:10.1007/s11240-004-8833-5
Chakraborti SP, Vijayan K, Roy BN, Quadri SMH (1998) In vitro induction of tetraploidy in mulberry (Morus alba L.). Plant Cell Rep 17:799–803. doi:10.1007/s002990050486
Chalak L, Legave JM (1996) Oryzalin combined with adventitious regeneration for an efficient chromosomoe doubling of trihaploid kiwifruit. Plant Cell Rep 16:97–100. doi:10.1007/BF01275459
Coppens d’Eeckenbrugge G, Segura SD, de Jaramillo EH, Góngora GA (2001) Passion fruits. In: Charrier A, Jacquot M, Hamon S, Nicolas D (eds) Tropical plant breeding. Science Publishers and CIRAD, Enfield, pp 381–401
Cruz CD (2008). Programa genes: diversidade genética. Editora UFV. Viçosa (MG). 278p
De Jesus-Gonzalez L, Weathers PJ (2003) Tetraploid Artemisia annua hairy roots produce more artemisinin than diploids. Plant Cell Rep 21:809–813. doi:10.1007/s00299-003-0587-8
Devreux M (1970) New possibilities for in vitro cultivation of plant cells. Tech Eur Comm 9:105–110
Dhooghe E, Van Laere K, Eeckhaut T, Leus L, Van Huylenbroeck J (2011) Mitotic chromosome doubling of plant tissues in vitro. Plant Cell Tiss Organ Cult 104:359–373. doi:10.1007/s11240-010-9786-5
Doležel J, Greilhuber J, Suda J (2007) Flow cytometry with plants: an overview. In: Doležel J, Greilhuber J, Suda J (eds) Flow cytometry with plant cells. Wiley, Weinheim, pp 41–65
Dornelas MC, Tavares FCA, Oliveira JC, Vieira MLC (1995) Plant regeneration from protoplast fusion in Passiflora spp. Plant Cell Rep 15:106–110. doi:10.1007/BF01690264
Dubcovsky J, Dvorak J (2007) Genome plasticity a key factor in the success of polyploid wheat under domestication. Science 316:1862–1865. doi:10.1126/science.1143986
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean roots cells. Exp Cell Res 50:151–158
Gantait S, Mandal N, Bhattacharyya S, Das PK (2011). Induction and identification of tetraploids using in vitro colchicines treatment of Gerbera jamesonii Bolus cv. Sciella Plant Cell Tiss Organ Cult. doi:10.1007/s11240-011-9947-1
Grosser JW, Gmitter FG Jr (2011) Protoplast fusion for production of tetraploids and triploids: applications for scion and rootstock breeding in citrus. Plant Cell Tiss Organ Cult 104:343–357. doi:10.1007/s11240-010-9823-4
Gu XF, Yang AF, Meng H, Zhang JR (2005) In vitro induction of tetraploid plants from diploid Zizyphus jujuba Mill cv. Zhanhua. Plant Cell Rep 24:671–676. doi:10.1007/s00299-005-0017-1
Hebert CJ, Touchell DH, Ranney TG, LeBude AV (2010) In vitro shoot regeneration and polyploid induction of rhododendron ‘Fragrantissimum improved’. Hortscience 45:801–804
Hodgson JG, Sharafi M, Jalili A, Díaz S, Montserrat-Martí G, Palmer C, Cerabolini B, Pierce S, Hamzehee B, Asri Y, Jamzad Z, Wilson P, Raven JA, Band SR, Basconcelo S, Bogard A, Carter G, Charles M, Castro-Díez P, Cornelissen JHC, Funes G, Jones G, Khoshnevis M, Pérez-Harguindeguy N, Pérez-Rontome′ MC, Shirvany FA, Vendramini F, Yazdani S, Abbas-Azimi R, Boustani S, Dehghan M, Guerrero-Campo J, Hynd A, Kowsary E, Kazemi-Saeed F, Siavash B, Villar-Salvador P, Craigie R, Naqinezhad A, Romo-Díez A, de Torres Espuny L, Simmons E (2010) Stomatal versus genome size in angiosperms: the somatic tail wagging the genomic dog? Ann Bot 105:573–584. doi:10.1093/aob/mcq011
IBGE (2009) Levantamento sistemático da produção agrícola. Rio de Janeiro
Knight RJ Jr (1991) Development of tetraploid hybrid passion fruit clones with potential for the North Temperate zone. HortScience 26:1541–1543
Kulkarni M, Borse T (2010) Induced polyploidy with gigas expression for root traits in Capsicum annuum (L.). Plant Breed 129:461–464. doi:10.1111/j.1439-0523.2009.01696.x
Leitch AR, Leitch IJ (2008) Genomic plasticity and the diversity of polyploid plants. Science 320:481–483. doi:10.1126/science.1153585
Lema-Ruminska J (2011) Flow cytometric analysis of somatic embryos, shoots, and calli of the cactus Copiapoa tenuissima Ritt. forma monstruosa. Plant Cell Tiss Organ Cult. doi:10.1007/s11240-011-9941-7
Liu G, Li Z, Bao M (2007) In vitro induction of tetraploids in Phlox subulata L. Euphytica 157:145–154. doi:10.1007/s10681-007-9457-8
Lloyd RM (1963) Tetraploid Passiflora incarnata in North Carolina. Rhodora 65:79–80. doi:10.1007/BF02866495
Melo NF, Cervi AC, Guerra M (2001) Karyology and cytotaxonomy of the genus Passiflora L. (Passifloraceae). Plant System Evol 226:69–84. doi:10.1007/s006060170074
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Narayanaswamy S, Chandy LP (1971) In vitro induction of haploid, diploid and triploid androgenic embryos and plantlets in Datura metel L. Ann Bot 25:535–542
Ochatt SJ, Patat-Ochatt EM, Moessner A (2011) Ploidy level determination within the context of in vitro breeding. Plant Cell Tiss Organ Cult 104:329–341
Oliveira JC (1987) Melhoramento genetic. In: Ruggiero C (ed) Maracujá, vol 1. Editora Legis Summa, Ribeirão Preto, pp 218–246
Osborn TC, Pires JC, Birchler JA, Auger DL, Chen ZJ, Lee HS, Comai L, Madlung A, Doerge RW, Colot V, Martienssen RA (2003) Understanding mechanisms of novel gene expression in polyploids. Trends Genet 19:141–147. doi:10.1016/S0168-9525(03)00015-5
Otoni WC, Blackhall NW, d’Utra Vaz FB, Casali VW, Power JB, Davey MR (1995) Somatic hybridization of the Passiflora species, P. edulis f. flavicarpa Degener and P. incarnata L. J Exp Bot 46:777–785. doi:10.1093/jxb/46.7.777
Pignatta D, Dilkes BP, Yoo SY et al (2010) Differential sensitivity of the Arabidopsis thaliana transcriptome and enhancers to the effects of genome doubling. New Phytol 186:194–206. doi:10.1111/j.1469-8137.2010.03198.x
Predieri S (2001) Mutation induction and tissue culture in improving fruits. Plant Cell Tiss Org Cult 64:185–210. doi:10.1023/A:1010623203554
Ramulu KS, Verhoeven HA, Dijkhuis P (1991) Mitotic blocking micronucleation and chromosome doubling by a oryzalin, amiprophosmethyl and colchicines in potato. Protoplasma 160:65–71. doi:10.1007/BF01539957
Rêgo MM, Bruckner CH, Finger FL, Siqueira DL, Fernandes A (1999) Self-incompatibility in passion fruit: evidence of two loci genetic control. Theor Appl Genet 98:564–568. doi:10.1007/s001220051105
Rêgo MM, Rêgo ER, Bruckner CH, Silva EAM, Finger FL, Pereira KJC (2000) Pollen tube behavior in yellow passion fruit following compatible and incompatible crosses. Theor Appl Genet 101:685–689. doi:10.1007/s001220051531
Salon PT, Earle ED (1998) Chromosome doubling and mode of reproduction of induced tetraploids of eastern gamagrass (Tripsacum dactyloids L.). Plant Cell Rep 17:881–885. doi:10.1007/s002990050502
Samford JC (1983) Ploidy manipulations. In: Moore JN, Janick J (eds) Methods in fruit breeding. Purdue Univ. Press, West Lafayette, pp 100–123
Shao J, Cheng C, Deng X (2003) In vitro induction of tetraploid in pomegranate (Punica granatum). Plant Cell Tiss Organ Cult 75:241–246. doi:10.1023/A:1025871810813
Snow N, MacDougal JM (1983) New chromosome reports in Passiflora (Passifloraceae). System Bot 18:185–210
Sun CS, Wu SC, Wang CC, Chu CC (1979) The deficiency of soluble proteins and plastid ribossomal RNA in the albino pollen plantlets of rice. Theor Appl Genet 55:193–197. doi:10.1007/BF00268112
Sun DQ, Lu XH, Liang GL, Guo OG, Mo YW, Xie JH (2011) Production of triploid plants of papaya by endosperm culture. Plant Cell Tiss Organ Cult 104:23–29. doi:10.1007/s11240-010-9795-4
Tang ZQ, Chen DL, Song ZJ, He YC, Cai DT (2011) In vitro induction and identification of tetraploid plants of Paulownia tomentosa. Plant Cell Tiss Organ Cult 102:213–220. doi:10.1007/s11240-010-9724-6
Väinölä A (2000) Polyploidization and early screening of Rhododendron hybrids. Euphytica 112:239–244. doi:10.1023/A:1003994800440
Van Duren M, Morpurgo R, Doležel J, Afza R (1996) Induction and verification of autotetraploids in diploid banana (Musa acuminata) by in vitro techniques. Euphytica 88:25–34. doi:10.1007/BF00029262
Van Tuyl JM, Meijer B, Van Dien MP (1992) The use of oryzalin as an alternative for colchicines in vitro chromosome doubling of Lilium and Nerina. Acta Hortic 325:625–630
Wu JH, Ferguson AR, Murray BG (2011) Manipulation of ploidy for kiwifruit breeding: in vitro chromosome doubling in diploid Actinidia chinensis Planch. Plant Cell Tiss Organ Cult. doi:10.1007/s11240-011-9949-z
Yang X, Ye CY, Cheng ZM, Tschaplinski TJ, Wullschleger SD, Yin W, Xia X, Tuskan XA (2011) Genomic aspects of research involving polyploid plants. Plant Cell Tiss Organ Cult 104:387–397. doi:10.1007/s11240-010-9826-1
Yu Z, Haage Z, Streit VE, Gierl A, Ruiz RAT (2009) A large number of tetraploid Arabidopsis thaliana lines, generated by a rapid strategy, reveal high stability of neo-tetraploids during consecutive generations. Theor Appl Genet 118:1107–1119. doi:10.1007/s00122-009-0966-9
Zhang Z, Daí H, Xiao M, Liu X (2008) In vitro induction of tetraploids in Phlox subulata L. Euphytica 159:59–65. doi:10.1007/s10681-007-9457-8
Acknowledgments
The authors thank CNPq and CAPES for financial support this investigation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rêgo, M.M., Rêgo, E.R., Bruckner, C.H. et al. In vitro induction of autotetraploids from diploid yellow passion fruit mediated by colchicine and oryzalin. Plant Cell Tiss Organ Cult 107, 451–459 (2011). https://doi.org/10.1007/s11240-011-9995-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-011-9995-6