Abstract
Protoplast fusion technology has been utilized in many crops to generate allotetraploid somatic hybrids, and sometimes autotetraploids as a byproduct of the process. A brief history of this technology development is provided, along with a simple protocol developed for citrus, which can be easily adapted to other plants. Protoplast fusion has become a significant tool in ploidy manipulation that can be applied in various cultivar improvement schemes. In rare cases, a new somatic hybrid may have direct utility as an improved cultivar; however, the most important application of somatic hybridization is the building of novel germplasm as a source of elite breeding parents for various types of conventional crosses for both scion and rootstock improvement. Somatic hybridization is generating superior allotetraploid breeding parents for use in interploid crosses to generate seedless triploids. Seedlessness is a primary breeding objective for new fresh fruit citrus varieties, and several thousand triploid hybrids have been produced using somatic hybrids as the tetraploid parent. Protoplast fusion is also being utilized to produce somatic hybrids that combine complementary diploid rootstocks, which have shown good potential for tree size control. Tree size control has gained importance as a means of reducing harvesting costs, maximizing the efficiency of modern cold protection methodology, and facilitating the adaptation of new fruit production systems. Successful somatic hybridization in citrus rootstock improvement has enabled rootstock breeding at the tetraploid level via sexual hybridization, which can yield maximum genetic diversity in zygotic progeny upon which to impose selection for the many traits required in improved rootstock cultivars, including disease and insect resistance, broad adaptation, tree size control, and the ability to consistently produce high yields of quality fruit. Recent progress and successful examples of these applications are discussed. Finally, a discussion of the genetic potential of somatic hybrids as breeding parents, including meiotic behavior and inheritance is provided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant somatic hybridization via protoplast fusion has become an important tool for ploidy manipulation in plant improvement schemes, allowing researchers to combine somatic cells from different cultivars, species, or genera, resulting in novel allotetraploid and autotetraploid genetic combinations. This technique can facilitate conventional breeding, gene transfer, and cultivar development by bypassing some problems associated with conventional sexual hybridization including sexual incompatibility, nucellar embryogenesis, and male or female sterility (Grosser and Gmitter 1990). Applications of somatic hybridization in crop improvement are constantly evolving, and original experiments generally targeted gene transfer from wild accessions to cultivated selections that were either difficult or impossible to accomplish by conventional methods (Grosser et al. 1996). The most common target using somatic hybridization is the generation of symmetric allotetraploid hybrids that contain the complete nuclear genomes of both parents. The greatest level of success for fruit breeding has occurred in Citrus, primarily due to the highly successful model of fusing embryogenic suspension-derived protoplasts with leaf-derived protoplasts, resulting in the regeneration of somatic hybrid plants from nearly 500 different parental combinations (previously reviewed by Grosser et al. 2000; Grosser and Gmitter 2005). Somatic hybrid recovery following protoplast fusion is often facilitated by hybrid vigor (Guo and Grosser 2005). New somatic hybrid combinations rarely produce fruit with adequate quality as necessary to be released as a new cultivar, but it is possible that a few new somatic hybrids may be released as improved cultivars (Guo et al. 2004a). It has become clear that the most important application of somatic hybridization in plant breeding programs is the building of novel germplasm as a source of elite breeding parents for various types of conventional crosses. In Citrus, somatic hybridization is being used to generate key allotetraploid breeding parents for use in interploid crosses to generate seedless triploids (Grosser and Gmitter 2005). In our program, such allotetraploid somatic hybrids have already been used as pollen parents in interploid crosses, followed by embryo rescue when necessary, to generate several thousand triploid hybrids for mandarin, grapefruit/pummelo, and acid lime/lemon improvement. A few monoembryonic allotetraploid somatic hybrids have also been used as females in interploid crosses to generate hundreds of grapefruit-pummelo type hybrids. With this latter approach, triploid hybrids can be recovered directly from seed without the need for embryo rescue; however, it has been more difficult to generate monoembryonic allotetraploid somatic hybrids because the embryogenic callus lines used as effective fusion partners have been derived from polyembryonic donors that produce nucellar-derived embryos. It is extremely difficult to obtain embryogenic callus from monoembryonic cultivars in citrus.
Somatic hybridization has also made significant contributions to citrus rootstock improvement. The initial strategy was to produce allotetraploid hybrids that combined complementary diploid rootstock selections. Many such hybrids have been produced, propagated by rooted cuttings, and evaluated in commercial rootstock trials (Grosser et al. 1995; Grosser and Chandler 2003). Nearly all the somatic hybrids tested produced citrus trees smaller than their diploid parents, indicating their potential for tree size control and use in emerging citrus production systems featuring high tree densities (Grosser et al. 1995, 2003; Grosser and Gmitter 2005; Ananthakrishnan et al. 2006). Subsequently, many of these same hybrids have been evaluated for nucellar seed production and nursery performance. A few of these ‘tree size controlling rootstocks’ are expected to be released for commercial use. The large population of allotetraploid somatic hybrid rootstocks, including a few good monoembryonic hybrids, has allowed for the creation of a rootstock breeding program at the tetraploid level. This approach can generate tremendous genetic diversity in zygotic progeny and is a powerful tool to package all necessary rootstock attributes into robust yet tree-size controlling new rootstocks (Grosser et al. 2003, 2007). Performance feedback from field trials of somatic hybrid rootstocks has guided the selection of parents used in such tetraploid crosses. This review will provide a basic protocol for somatic hybridization via protoplast fusion, and specific applications of ploidy manipulation that are now possible to facilitate citrus scion and rootstock breeding, because of the maturation of the technology.
Brief history
As mentioned, plant somatic hybridization via protoplast fusion has become an important tool for the generation of novel genotypes and ploidy manipulation in plant improvement schemes. The development of this technology became possible following the first successful isolation of plant protoplasts by Cocking (1960). This was followed by the first successful report on somatic hybridization in tobacco by Carlson et al. (1972). Since this time, hundreds of reports have been published during the past four decades which extend the procedures to additional plant genera and that evaluate the potential of somatic hybrids in many crops including citrus, rice, rapeseed, tomato, and potato. Excellent general reviews of the subject have appeared over the years including those by Gleba and Sytnik (1984), Bravo and Evans (1985), Waara and Glimelius (1995), and Johnson and Veilleux (2001); and for specific commodities including potato (Orczyk et al. 2003) and citrus (Grosser and Gmitter 1990, 2005; Grosser et al. 2000). Significant accomplishments of somatic hybridization research include the production of wide intergeneric hybrids that combine sexually incompatible or difficult to hybridize species to increase genetic diversity for crop improvement (Dudits et al. 1980; Grosser et al. 1996; Skarzhinskaya et al. 1996; Escalante et al. 1998; Bastia et al. 2001; Wang et al. 2003; Xu et al. 2003). Also of interest is the successful accession of genes that confer disease resistance from wild species (Fock et al. 2000; Collonnier et al. 2003). However, many of the reported wide somatic hybrids have exhibited genetic instability (Szcerbakowa and Bottowicz 2003).
Unfortunately, the potential of the technology predicted during the 1970s and 1980s has never quite materialized, primarily because the somatic hybrid products of protoplast fusion have not generally had direct value as new cultivars, and because of the advances in plant molecular biology that have shifted the direction of plant improvement research more toward genetic transformation approaches, focused on specific genes. Also, efficient protoplast-to-plant regeneration systems have yet to be worked out for many important crop species. Many of the allotetraploid somatic hybrids reported in the literature were produced by laboratory researchers that were not associated directly with applied plant breeding programs, and thus the somatic hybrid plants ended up as merely products of academic exercises. This is not the case with citrus, as somatic hybridization technology has reached full maturity and become a significant part of integrated citrus improvement programs, with major contributions to both scion and rootstock improvement (Gmitter et al. 2007). It has become quite clear that the real value of allotetraploid somatic hybrids, at least in citrus, is their value as superior breeding parents in interploid crosses for scion improvement, and breeding at the tetraploid level for rootstock improvement. The successful applications of the technology outlined for citrus in this review should stimulate efforts to realize such potential for applications of somatic hybridization and ploidy manipulation in other commodities, especially other fruit crops.
Protocol
Somatic hybrids in Citrus are most commonly produced using the model of fusing protoplasts isolated from embryogenic callus or suspension cultures of one parent with leaf-derived protoplasts of the second parent. At least one parent in any fusion combination must be embryogenic to provide the capacity for plant regeneration from the protoplast fusion products. The following protocol (updated from Grosser and Gmitter 1990) has been used successfully to produce somatic hybrid plants from nearly 300 parental combinations at the University of Florida/IFAS, Citrus Research and Education Center, Lake Alfred, FL, USA. It was developed with a goal of minimizing genetic combination specificity. The protocol can be easily fine-tuned and adapted to other plant genera and species (Grosser 1994), as was the case with avocado (Witjaksono et al. 1998) and grape (Xu et al. 2007). Successful protoplast culture media for a specific plant species can be developed by combining the previously successful tissue culture basal medium for the given species with appropriate osmoticum and the 8P multi-vitamin and sugar alcohol additives of Kao and Michayluk (1975). Subsequent plant regeneration schemes should be dependent on growth regulator combinations already developed for any given commodity.
Embryogenic callus/suspension induction and maintenance
Embryogenic callus in citrus can be initiated from nucellar tissue of undeveloped ovules that can be removed from either immature or mature fruit of polyembryonic cultivars. Fruit is surface sterilized prior to ovule removal under aseptic conditions in a laminar flow hood. Removed undeveloped ovules are cultured on DOG medium (EME + 5 mg/l kinetin, see Table 1) for callus induction. Cultured ovules should be subcultured every 3–4 weeks until white or yellow callus emerges from the ovules. In some cases, developing callus will be accompanied by proliferating somatic embryos, and friable callus should be separated from the somatic embryos for continued callus proliferation. Callus induction is generally inefficient, and a large quantity of ovules (several hundred) should be cultured for each cultivar of interest. Proliferating friable callus should be subcultured every 4 weeks. After a significant quantity of callus is obtained, callus lines can be habituated by subculturing on growth regulator free EME or H+H medium (Table 1). At this point, each callus line should be checked by analysis of robust genetic markers, such as SSRs, to assure nucellar rather than zygotic origin; we have occasionally found that embryogenic callus can originate from zygotic embryos in undeveloped aborted ovules (Chen et al. 2008). It generally takes a year to have adequate quantities of embryogenic callus for suspension initiation. Suspension cultures are initiated by transfer of approximately 2 g of callus tissue to a 125-ml Erlenmeyer flask (preferably with a Teflon-coated rim) containing 20 ml H+H liquid medium. Suspension cultures are then shaken continuously on a rotary shaker at 125–150 rpm. After 2 weeks, another 20 ml of H+H liquid medium is added to each flask to bring the total to 40 ml. Rapidly growing suspension cultures can then be maintained by subculture every 2 weeks, by splitting the contents of one flask into two and restoring the volume to 40 ml with fresh H+H medium.
Protoplast isolation
From embryogenic callus or suspension culture, cells used for protoplast isolation should be in the log phase of growth, with best results using suspension cells from 4 to 12 days into the 2 week subculture cycle. Transfer 1–2 g friable callus tissue into a 60 × 15 mm petri dish (for suspension, transfer approximately 2 ml suspension with a wide-mouth pipette and drain off the liquid using a Pasteur pipette). Resuspend the cells in 2.5 ml 0.7 M BH3 medium (Table 2; BH3 + 34 g/l sucrose) and then add 1.5 ml of filter sterilized Enzyme Solution containing 0.7 M mannitol, 12.0 mM CaCl2, 6.0 mM MES buffer, 1.4 mM NaH2PO4, 2% Onozuka RS cellulase, 2% Macerase, at pH 5.6. Seal dishes with Nescofilm and incubate overnight on a shaker slowly rotating at 20 rpm, either in low light or darkness.
For the leaf parent donor, fully expanded but not completely hardened off leaves should be taken from plants grown either in a growth chamber or heavily shaded greenhouse. Alternatively, in vitro grown leaves are desirable because they do not require decontamination prior to protoplast isolation. Leaf material can be decontaminated by immersion in 1 N HCl for a few seconds followed by a 12–15 min immersion in 10–15% commercial bleach (6% sodium hypochlorite) containing 3 drops of Liquinox soap or another suitable surfactant, followed by a 5-min rinse and two 10-min rinses in double-distilled H2O. Damaged vascular tissue and the leaf midveins should be removed with a sharp scalpel. Remaining leaf material is feathered or cut into thin strips with a sharp scalpel and incubated in 3 ml of Enzyme Solution (see above) combined with 8 ml 0.7 M BH3 medium in a 125 ml side-armed Erlenmeyer flask (with the side-arm covered with Miracloth to prevent contamination). Leaf material in the enzyme cocktail is evacuated for 15 min at 50 kPa to facilitate enzyme infiltration. Preparations are sealed and incubated as above. It should be noted that newly initiated and younger callus/suspension cultures consist of cells with higher starch content. This can reduce protoplast yields and viability due to higher levels of protoplast breakage. This problem diminishes over time with continuous subcultures.
Protoplast purification
Resulting protoplasts should be purified on a sucrose/mannitol gradient, as follows. Following incubation, preparations are passed through a 45 μm stainless steel or nylon mesh screen to remove undigested cell clumps and debris from broken cells. Protoplast-containing filtrates are then centrifuged for 4–10 min at 100g in 15 ml calibrated screw-top centrifuge tubes. The supernatant is removed with a Pasteur pipette, and the pellet containing the protoplasts is gently resuspended in 5 ml of a 25% sucrose solution containing CPW nutrients (27.2 mg/l KH2PO4, 100 mg/l KNO3, 150 mg/l CaCl2, 250 mg/l MgSO4, 2.5 mg/l Fe2(SO4)3.6H2O, 0.16 mg/l KI, 0.00025 CuSO4, pH 5.8) (Frearson et al. 1973). This is followed by slowly pipetting 2 ml of a 13% mannitol solution (containing CPW salts) directly on top of the sucrose layer (avoid mixing). Centrifuge for 6 min at 100 g. Viable protoplasts form a band at the interface between the sucrose and the mannitol. Carefully remove the protoplasts from this interface and resuspend them in the appropriate volume of BH3 medium in preparation for somatic fusion.
Protoplast fusion: PEG method
The following protocol for the chemical PEG (polyethylene glycol) method of protoplast fusion is simple, efficient, inexpensive and non-toxic to plant protoplasts. Electrofusion also works quite well (Guo and Deng 1998). Mix approximately equal volumes of purified protoplasts from each parental source in BH3 medium (Table 2) and centrifuge for 4 min at 100 g. Resuspend the pellet of mixed protoplasts in a volume of BH3 medium equal to 4 × to 20 × the volume of the original pellet (10 × is recommended for initial experiments, with subsequent adjustments based on obtained plating efficiencies). Pipette two drops of the resuspended mixture into 60 × 15 plastic Petri dishes. Immediately add 2 drops of fresh PEG solution (40% polyethylene glycol 8000, 0.3 M glucose, and 66 mM CaCl2 at pH = 6) to each fusion Petri dish and incubate 8 min. If using stored PEG solution, it may be necessary to readjust the pH. Add 2 drops of A + B solution (9:1 v:v mixed immediately prior to use, A = 0.4 M glucose, 66 mM CaCl2, and 10% dimethylsulfoxide at pH = 6; and B = 0.3 M glycine at pH = 10.5 using KOH pellets) to each fusion Petri dish. Following another incubation of 12 min, add 12–15 drops of BH3 medium to the periphery of the fusing protoplasts. After a 5 min incubation, carefully remove the PEG plus [A + B] solution with a Pasteur pipette (without removing protoplasts) and replace it with 15 drops BH3 medium. After incubating another 10 min, remove the BH3 medium with a Pasteur pipette and replace it with 12–15 drops fresh BH3 medium. Repeat this washing step two more times, always carefully avoiding the loss of protoplasts. After the final wash, protoplasts can be cultured directly in the fusion Petri dish either in a shallow pool (8–12 drops medium) or thin-layer culture (1.5 ml medium) in either BH3 medium, EMEP medium (Table 2), or a 1:1 v:v mixture of BH3 and EMEP (Grosser and Gmitter 1990). Seal plates with Nescofilm, and culture in either darkness or low-light, preferably in sealed plastic boxes.
Protoplast culture and plant regeneration
Following the incubation of the protoplast cultures for 4–6 weeks, they are supplemented with medium containing reduced osmoticum, which is accomplished by adding 10–12 drops of a 1:2 (v:v) mixture of BH3 medium and 0.38 M EME medium (Table 1, EME + 76.7 g/l sucrose). After incubation for another 2 weeks, cultures can be transferred to solid medium in 100 × 20 petri dishes with further osmoticum reduction as follows: add 2 ml of a 1:2 (v:v) mixture of BH3 medium and liquid EME medium (Table 1) to each fusion dish and pour entire contents onto solid medium plates containing standard agar-solidified EME-maltose medium (containing 50 g/l maltose instead of sucrose to facilitate embryo induction). The liquid medium containing the protoplast-derived colonies should be spread evenly over the entire plate, forming a shallow layer. Too much liquid medium will drown the growing colonies. Vigorously growing cultures may require dilution in order to achieve somatic embryo induction. Recovered somatic embryos are enlarged on 1,500 embryo enlargement medium and germinated on B + medium (Table 1), as described previously in Grosser and Gmitter (1990). Often, recovered embryos are abnormal and fail to germinate. These can be dissected into large sections and cultured on DBA3 shoot induction medium (Table 1). Resulting shoots can be rooted on RMAN medium (Table 1). When embryos produce roots but no shoots, they can be decapitated to remove any abnormal tissue, and cultured on either DBA3 or RMAN for adventitious shoot induction and whole plant recovery.
Somatic hybrid validation
Prior to transfer to soil, recovered in vitro plantlets can be screened by flow cytometry to determine the ploidy level, using a tabletop Partec flow cytometer (Model D-48161, Münster, Germany, or any other suitable model), as previously described (Khan and Grosser 2004). When using suspension cultures obtained from newly initiated callus lines of high totipotency, numerous diploid plants often regenerate from unfused protoplasts. In such cases, a larger population of regenerated plants is necessary to recover tetraploids. Older cultures (usually more than 3 years) tend to lose their totipotency, and there is generally an increase in the percentage of tetraploids recovered from somatic fusion experiments. Tetraploids identified by flow cytometry are then micro-grafted to small greenhouse grown rootstock seedlings (usually Carrizo citrange) to expedite whole plant recovery. Use of a trifoliate rootstock such as Carrizo allows easy identification and removal of adventitious shoots regenerating directly from the rootstock. Rooted plants can be transferred to any suitable commercial potting mixture and maintained under cover at high humidity for 2–3 weeks for acclimatization. Recovered tetraploids should undergo molecular analysis to determine if they are autotetraploids or allotetraploid somatic hybrids, with both having potential value to subsequent conventional breeding schemes. Previously, somatic hybrids have been validated by isozymes, RAPD or CAPS (Grosser and Gmitter 2005); more recently unambiguous and reliable EST-SSR markers with fragments separated on a sequencer, has been the method of choice (Chen et al. 2008). EST-SSRs have a further advantage of ploidy level validation in many instances, as up to four different alleles may be identified at a single locus in a somatic hybrid plant. Cytoplasmic genome analysis in somatic hybrids has been done using RFLPs (Kobayashi et al. 1991; Moreira et al. 2000), and more recently by CAPS analysis (Guo et al. 2004b).
Applications
Ploidy manipulation in scion improvement
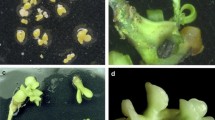
Although the fruit of some somatic hybrids approaches cultivar quality and a few may be released as new cultivars (Fig. 1), (Guo et al. 2004a), their most important value to breeding programs is as unique tetraploid breeding parents. Seedlessness has become a primary breeding objective of all citrus fresh fruit improvement programs, as seedless fruits are much preferred in the marketplace. Somatic hybridization via protoplast fusion technology provides the opportunity to combine complementary elite diploid scions into allotetraploid somatic hybrids. Flowering somatic hybrids are being used as breeding parents in interploid crosses with selected complementary diploid parents to generate triploid progeny. Autotetraploids, often a by-product of somatic hybridization experiments and also produced by other in vitro techniques, are also used as parents in interploid crosses. However, more variation in triploid progeny is generally observed when using allotetraploid parents. In citrus, two types of embryos can be produced in seed, either of nucellar (genotypically equivalent to the mother tree) or zygotic origin. Oranges, grapefruit, lemons, and many mandarins produce seed that generate multiple embryos predominantly of nucellar origin (referred to as polyembryonic), making them difficult to use as female parents in conventional crosses. Citrus accessions that produce monoembryonic seed generally contain zygotic embryos, and of course these are the preferred seed parents for breeding. The number and diversity of high quality, monoembryonic diploid parents is limited, though some that have been used by various programs around the world include ‘Clementine’ selections, ‘Fallglo’, ‘Fortune’, and ‘Temple’ for mandarin breeding. In the UF-CREC citrus breeding program, we are also utilizing a number of good new monoembryonic parents from our diploid citrus breeding program, many shown previously to possess good general combining ability, producing families with relatively high percentages of hybrids that yield fruit with acceptable quality. For grapefruit/pummelo improvement, selected high quality pummelos are being used as seed parents.
In general, interploid crosses of diploid seed parents with tetraploid pollen parents are problematic because fully developed seeds containing viable embryos are not usually recovered. In most cases when fully developed seeds are occasionally recovered, these generally grow into seedlings that are tetraploid, presumably the product of an unreduced gamete from the seed parent being fertilized by the pollen parent. The problem of abnormal seed development from 2× X 4× crosses has been attributed to an unfavorable embryo:endosperm ratio, resulting in endosperm failure and subsequent embryo abortion. In vitro embryo rescue is required to circumvent this for efficient triploid embryo recovery (Viloria et al. 2004, 2005). If a tetraploid seed parent is used, this problem does not exist, as is the case with the unreduced gametes coming from diploid seed parents. Unfortunately, the number of high-quality monoembryonic tetraploids is currently quite limited. Moreover, production of monoembryonic tetraploid somatic hybrids is difficult, because the fusion technology requires the use of an embryogenic callus or suspension culture arising from a nucellar parent to provide the necessary totipotency, and in general most somatic hybrids derived from such a donor parent reveal the dominance of nucellar embryony. To date it has not been possible to generate embryogenic callus cultures of monoembryonic citrus types. However, very recently we have combined nucellar parents with monoembryonic parents, with the latter being the leaf-derived protoplast donor. A few of these hybrids have proven to be monoembryonic and are being utilized in interploid crosses as females (for example ‘Succari’ sweet orange + ‘Hirado Buntan Pink’ zygotic pummelo). We are still waiting for several other such hybrids to pass through juvenility to flowering. Our program has generated more than 12,000 triploid citrus hybrids from interploid crosses, with a few thousand of these being fathered by somatic hybrids (Gmitter and Grosser 1993, 2005; Viloria and Grosser 2004; Grosser et al. 2000, 2006). Somatic hybrid parents successfully used in these crosses are listed in Table 3. Our first triploid hybrids are now overcoming juvenility, and seedless triploid fruits have been obtained in all three categories to provide proof of concept (Fig. 2). For mandarin improvement, additional breeding objectives beyond seedlessness include easy-peeling, good external/internal color, good flavor, a range of maturity dates, and good shipping ability/shelf-life. Our program is continuing efforts to generate improved breeding parents, and we recently reported 9 new somatic hybrids and 5 autotetraploids produced by protoplast fusion, with focus on the zipper-skin trait (Grosser et al. 2010).
Examples of seedless fruits from triploid hybrids produced by interploid crosses using somatic hybrid pollen parents. Left ‘Sugar Belle’ × ‘Nova’ + ‘Succari’ somatic hybrid; Middle ‘Todo del Ano’ lemon × ‘Mexican’ lime + ‘Valencia’ somatic hybrid; Right ‘Todo del Ano’ lemon × ‘Hamlin’ + Femminello’ somatic hybrid
For acid lemon/lime improvement, primary breeding objectives include improved cold-hardiness and disease resistance (citrus canker, citrus tristeza virus, and witches broom), and potential new industrial oils. We have previously shown that kumquat-derived resistance to citrus canker can be transferred to triploid lime-like hybrids when using canker resistant ‘Lakeland limequat’ as a parent (Viloria et al. 2004). For grapefruit/pummelo improvement, primary breeding objectives include improved resistance to citrus canker, fruit quality (flavor and color), and extended maturity seasons. Also of interest is the potential to reduce or eliminate furanocoumarins in new grapefruit-like cultivars. Furanocoumarins are the chemicals found in all commercial grapefruit cultivars that interact negatively with prescription drugs, preventing many elderly consumers from enjoying grapefruit. We have found that several of our pummelo breeding parents and triploid progeny have little or no furanocoumarins (Chen et al., submitted).
Horticultural characteristics of triploid trees are also important, and these are also naturally affected by parentage. We are observing significant differences in important traits such as the level of thorniness and the length of juvenility. For example, a small population of ‘Clementine’ derived triploids from unreduced gametes are slower to bear fruit and much more thorny than triploid hybrids from a cross of ‘Sugar Belle’ (‘Clementine’ × ‘Minneola’) with the ‘Nova’ mandarin + ‘Succari’ sweet orange somatic hybrid (Fig. 3). Thus, it is advantageous to conduct a broad range of interploid crosses using elite parents to identify the best parental combinations to achieve specific breeding objectives.
Budwood from first-generation triploid hybrids showing differences in thorniness. The three on the left are triploid hybrids obtained from ‘Clementine’ via unreduced gametes; the three on the right are hybrids of ‘Sugar Belle’ × the somatic hybrid ‘Nova’ mandarin + ‘Succari’ sweet orange. The latter 3 triploids also fruited earlier than the ‘Clementine’ derived hybrids
Somatic hybrids in rootstock improvement
Diseases, including citrus blight (a disease of unknown etiology) and quick-decline caused by citrus tristeza virus (CTV), kill millions of citrus trees annually worldwide. More recently, the Diaprepes root weevil/Phytophthora complex has become an important problem in Florida (Grosser et al. 2003, 2007). Rootstocks have a significant impact on tree productivity and survivability, and improved rootstocks can greatly improve the profitability of citriculture. The primary objective of our rootstock breeding program is packaging the necessary biotic and abiotic resistances together with wide soil adaptation, productivity, and the ability to control tree size. In Florida, smaller trees are desirable to reduce harvesting costs and to maximize the efficiency of cold-protection, and may now be needed for new production systems that minimize the impact of citrus canker and HLB (Huanglongbing, or citrus greening). Allotetraploid somatic hybrids produced by protoplast fusion are now playing a key role in this effort.
The original strategy we employed using somatic hybridization for rootstock improvement was to combine complementary diploid rootstocks via protoplast fusion to generate tetraploid somatic hybrid rootstocks. More than 100 such somatic hybrid rootstock combinations have been produced, propagated by rooted cuttings, and many have been entered into commercial field trials (Grosser and Chandler 2003). Tetraploid citrus rootstocks have continuously shown the ability to reduce tree size, including somatic hybrid rootstocks (Tables 4, 5). Yield and tree size data from field trials indicates that somatic hybrid rootstocks can produce adequate yields of high-quality sweet orange fruit on small-medium trees. If planted at optimum high-density row and tree spacing, somatic hybrids such as sour orange + Rangpur lime or sour orange + Palestine sweet lime, can yield over 22 tons (20,000 kg) fruit per acre on trees approximately 3–4 m in height. In our initial field experiments to evaluate somatic hybrid rootstocks, we identified several good dwarfing rootstocks as compared to the industry standard Flying Dragon trifoliate orange. These somatic hybrids exhibited better nursery performance than Flying Dragon; however, later showed some deficiencies in other important characteristics. For example, all the somatic hybrids made with the Cleopatra mandarin embryogenic callus line produced inadequate numbers of nucellar seeds for standard nursery propagation (Table 4). Since Cleopatra mandarin is quite seedy, this could be due to some mutation in the embryogenic callus line used to make the somatic hybrids. Another somatic hybrid of Hamlin + Flying Dragon performed well initially (Table 4), but had poor survival at some field locations, precluding its further potential as a commercial rootstock. As mentioned, the sour orange + Rangpur somatic hybrid performed very well in trials where it was grown as rooted cuttings (Table 4), and it produces fruit that are seedy. Unfortunately, it has been shown that the seedlings are of zygotic origin and therefore cannot be used for standard nursery propagation, which relies on nucellar embryony to yield uniform rootstock seedlings. The evaluation of additional somatic hybrid rootstocks has resulted in the identification of several promising selections for tree size control that do not exhibit the deficiencies mentioned above, and some of these are described in Table 5. These somatic hybrid rootstocks have yield efficiencies higher than the standard industry dwarfing rootstock Flying Dragon trifoliate orange, and produce adequate nucellar seed for standard nursery propagation. Due to their parentage, they are expected to have better soil adaptation than Flying Dragon, which does not perform well on high pH, calcareous soils. Following additional testing, we expect to release some of these somatic hybrid rootstocks for commercial use.
Breeding rootstocks at the tetraploid level
Conventional breeding at the tetraploid level using selected somatic hybrid rootstocks as parents provides an opportunity to recombine alleles from three or four proven diploid rootstocks, upon which to impose subsequent selection. This powerful approach can maximize the genetic diversity in zygotic progeny. We have coined the term ‘tetrazyg’ to identify allotetraploid sexual hybrids produced in this manner. Combining this with new approaches being employed to evaluate new material has the potential to greatly shorten the time required to release an improved rootstock. This is a good example of combining biotechnology with traditional breeding methods.
Selection of seed parents
As mentioned above, the sour orange + Rangpur somatic hybrid produces very high yield efficiency per canopy volume when used as a rootstock. However, this hybrid produces seed containing embryos of zygotic origin, and is therefore not amenable to standard nursery propagation. On this basis, it was selected as a first seed parent for breeding at the tetraploid level. The choice of the second seed parent was based on serendipity. The somatic hybrid, ‘Nova’ mandarin + ‘Hirado Buntan’ pummelo (zygotic), originally produced for scion improvement, was planted in some extra space adjacent to somatic hybrid rootstock trial in a challenging high pH, calcareous soil. One year after planting, a severe infestation of Diaprepes root weevils occurred, and nearly all the trees in the rootstock trial were destroyed, with no rootstocks (including six commercial control rootstocks) showing adequate tolerance of the pest. However, the Nova + HBP somatic hybrid continued to perform well, showing soil adaptation, and tolerance to the Diaprepes/Phytophthora complex. Fruit from seed trees of this somatic hybrid were seedy, though the resulting seedlings were zygotic. This robust somatic hybrid was therefore selected as a second primary female parent for the new tetraploid breeding program.
Selection of pollen parents and greenhouse screening
Somatic hybrids that performed well in initial field trials (i.e., sour orange + Carrizo, Cleopatra + trifoliate orange, sour orange + Palestine sweet lime) were selected as pollen parents. Seed from crosses of the primary female parents mentioned above and these pollen parents was germinated directly in a high pH, calcareous soil/Phytophthora screen to eliminate weak seedlings, as described by Grosser et al. (2003, 2007). For some crosses, a simultaneous preliminary salinity screen was imposed by watering the tetrazyg seedlings exclusively with 3,500 ppm NaCl for 3 mo; a few tetrazyg hybrids were able to tolerate this. Superior “tetrazyg” hybrids were grafted with sweet orange infected with a quick decline isolate of tristeza virus to quickly determine their resistance. Selected “tetrazyg” hybrids were propagated by topworking and/or rooted cuttings to provide clonal material for further evaluation. This approach has great potential to shorten the time required to develop a new rootstock. The program was initiated at the CREC in 1999, and so far more than 600 genetically diverse “tetrazyg” hybrids have been selected for further evaluation.
Preliminary evidence from greenhouse and field evaluations indicates a generally strong performance by most of the ‘tetrazyg’ selections (Fig. 4). Many of the tetrazygs are clearly outperforming the commercial rootstock rough lemon planted in reset positions where the original trees were killed by citrus blight at a location near St. Cloud, FL. Many seed trees of the first “tetrazygs” produced are now fruiting. Most produce adequate seed numbers (including the hybrids made with the low seeded Cleopatra + trifoliate orange pollen parent), and the hybrids are segregating for the nucellar/zygotic seedling trait as expected, based on SSR analysis of small populations of seedling trees (Table 6). Seed from nucellar selections have been provided to commercial citrus nurseries for subsequent field trials, and the nursery performance has been excellent. These results support the great potential for this approach to rootstock improvement; however, several years of continuing field studies will be necessary to determine the productivity and survivability of these new rootstocks over time. We are currently attempting to identify “tetrazyg” rootstocks that can grow trees very quickly, but then shift to heavy fruit production during year 3 and thereafter, with trees never growing beyond a medium size. If successful, such rootstocks would contribute substantially to the success of new production systems being developed for Florida, with a goal of bringing groves into production more quickly and shortening grove rotation times as necessary to maintain profitability in the presence of HLB.
Left Uniform ‘tetrazyg’ nucellar seedling rootstocks in a commercial nursery; Right 5-year old ‘Valencia’ tree on tetrazyg Orange #14 between two Valencia trees on the commercial rootstock rough lemon of the same age planted as citrus blight resets in the Alligator Grove c/o Mr. Orie Lee, east of St. Cloud, Florida (note: the rough lemon trees appear to be affected by disease)
Genetic and genomic studies, and consequent breeding potential, of citrus somatic hybrids
As described in detail above, the use of somatic scion hybrids as pollen (and potentially as seed) parents enhances the opportunities to produce new seedless hybrids for selection and cultivar development, from triploid offspring produced by interploid hybridization. As such, it should be of great value to have information on interactions of the two combined genomes and their effects on gene expression and phenotype, and of somatic hybrid meiotic behavior and its implications on inheritance (e.g. disomic vs. tetrasomic vs. intermediate), to devise efficient strategies for triploid breeding based on complete understanding of the genetic control and transmission of critical traits from somatic hybrid parents to triploid offspring. Likewise, such information is relevant to any long range breeding strategies employed using sexual hybridization for breeding at the tetraploid level in citrus.
Recently, there have been reports published that have revealed interesting information on the base phenotypic behavior of a selected citrus somatic hybrid, compared with the donor parents and using RT–PCR to study gene expression differences in key pathways. Bassene et al. (2009a) reported on the components of fruit quality (sugars and acids in the juice, and aromatic compounds found in peel oil) in a somatic hybrid of Willowleaf mandarin (Citrus deliciosa Ten.) + Eureka lemon [Citrus limon (L.) Burm.] and compared these to the diploid parents. They observed that some characters, such as acids, were most like those of the lemon parent, though fructose was closer to mandarin, while sucrose and glucose levels were intermediate, suggesting partial dominance of lemon for these traits. Peel oil aromatics of the somatic hybrid fruit were mostly similar to the mandarin, though the presence of one mandarin specific compound, N-methylanthranilate, was dramatically decreased. In general, it can be concluded that, at least in this case where the two parents possess significantly different fruit phenotypes, the genes controlling the biosynthetic pathways of the compounds studied are not inherited in an additive fashion, and may be subject to dosage effects, likely over-dominance, co-dominance, and other complex interactions in expression. Further, fruit carotenoid levels were compared in the same allotetraploid somatic hybrid and its donor parents, as well as levels of gene expression through the carotenoid biosynthetic pathway (Bassene et al. 2009b). Total carotenoids were 60 times greater in the Willowleaf parent compared with the Eureka lemon, with very low levels of only b-carotene and b-cryptoxanthin in the lemon; in contrast, these two plus five others were found at high levels in Willowleaf. The somatic hybrid produced the same carotenoid compounds as found in Willowleaf fruit, but at tenfold lower levels; the abscisic acid content (a downstream product of the carotenoid pathway) by contrast was significantly higher in the hybrid than either parent. As might be expected, gene expression for most genes in the pathway, revealed by RT–PCR, was significantly higher for Willowleaf than Eureka lemon. However, several upstream genes, particularly CitDxs, were down regulated in the somatic hybrid, indicating a level of partial dominance of lemon in that part of the pathway. Additionally, CitZep and Cit Nceds, critical enzymatic steps leading to ABA synthesis from carotenoid substrates, were expressed at levels in the hybrid that exceeded both donor parents. The combination of lower levels of upstream gene expression with the greater downstream consumption of carotenoids, leading to increased ABA levels and supported by increased gene expression, could explain the comparative phenotypic observations of the hybrid with the donors.
There have been few studies of meiotic behavior of citrus somatic hybrids. Fatta Del Bosco et al. (1999) compared meiosis of diploid Valencia sweet orange (C. sinensis L. Osbeck) and Femminello lemon (C. limon), with their somatic hybrid. They concluded that intergenomic pairing was taking place, based on the increased frequency of multivalents observed at diakinesis. Also, the frequency of univalents (as many as 10 univalents per cell in the hybrid) was found to be intermediate in the hybrid relative to the parents; as a consequence of pairing behavior, chromosomally unbalanced gametes were produced along with a high frequency of polyads. Despite the variable chromosomal content, pollen germination and viability were equal or even greater in the somatic hybrid compared with the diploid donors, so such pollen could be used to create triploid hybrids. The unbalanced number of chromosomes in pollen gave rise to backcross offspring that were concluded to be possibly aneuploid, triploid, and tetraploid based on measurements of DNA content by flow cytometry. Although the authors concluded that the sweet orange and lemon chromosomes were pairing and therefore presumably undergoing recombination, no information was provided from either molecular or phenotypic characterizations to demonstrate this as fact.
More recently, Chen et al. (2008) studied meiosis and microsporogensis in two additional citrus somatic hybrids, Hamlin sweet orange + Rough Lemon (Citrus jambhiri Lush) (HR) and Key lime (Citrus aurantifolia (Christm.) Swing.) + Valencia sweet orange (KV), and their diploid donor parents. Similar to the results reported by Fatta Del Bosco et al. (1999), these somatic hybrids also had increased frequencies of multivalent pairing and univalents, much lower frequencies of tetrad production, but sufficient levels of pollen viability and germination to enable their utilization in hybridizations. These authors, however, also noted unpublished data from meiotic analysis of a somatic hybrid of C. reticulata cv. Cleopatra and Poncirus trifoliata cv. Argentine, the latter being a sexually compatible genus related to Citrus; in this case, pairing was characterized by a high frequency of bivalents, perhaps a consequence of the more distant relatedness of the chromosome of each donor parent, and potentially resulting in amphidiploid-like behavior if used as a breeding parent. The authors predicted somatic hybrid breeding behavior to be intermediate between auto- and allo-tetraploids. Stift et al. (2008) in fact have demonstrated through study of segregation at SSR loci in natural autotetraploids of yellow cress (Rorippa amphibia and R. sylvestris) that they behaved as amphidiploids, that is, they display disomic inheritance. However, when segregation patterns from an F1 hybrid (the equivalent of a citrus somatic, allotetraploid hybrid) were studied, it was found that inheritance indeed fit an intermediate model, between disomic and tetrasomic inheritance. The modes of inheritance were inconsistent among individuals for a common locus, and likewise inconsistent within individuals at different loci. Clearly, such unpredictability will make linkage mapping and genetic studies of inheritance from citrus somatic hybrids difficult to study. The relatedness of the donors and their chromosomes is a factor that will influence the degree of recombination that might occur, and this in turn will affect the genotypes and phenotypes of the triploid hybrid offspring.
There is some information on the phenotypic characteristics of citrus triploids derived from the use of autotetraploid pollen parents in interploid hybridizations. Reforgiato Recupero et al. (2005) noted that in such crosses, the characteristics of the male parent generally dominated in the fruit phenotypes (size, color, quality, and appearance) of the triploid offspring. Further they noted substantially less variation among the triploid offspring compared with equivalent 2× X 2× crosses, using the corresponding diploid pollen parent. They attributed this phenomenon to the duplex nature of the autoploid genome, from which the heterozygous condition (AAaa) segregates 1AA:4Aa:1aa in pollen. Based on the above information relating to meiosis and genome interactions of citrus somatic hybrids, it is not surprising that offspring from crosses using citrus somatic hybrid parents exhibit greater phenotypic variation in the triploid offspring than that from autotetraploid citrus (Gmitter and Grosser, unpublished data).
Although studies of meiotic behavior and inheritance of specific important plant traits from somatic hybrid breeding parents will be challenging, the effort to conduct such studies may be a good investment going forward with polyploid breeding of citrus, both to create triploids and at the tetraploid level as well. A better understanding of the particular breeding behavior of individual parents potentially can lead to optimization of parental combinations, for somatic hybrid creation as well as in interploid or tetraploid sexual hybridizations. Studying the modes of inheritance at single loci, and even more so QTLs, will require sophisticated new statistical models to analyze and to predict breeding behavior of somatic hybrids of citrus.
Concluding remarks
Somatic hybridization via protoplast fusion has become a relevant biotechnology that facilitates ploidy manipulation in efforts to develop improved plant cultivars. Initial successes have been primarily to access disease resistance genes from related wild species that are difficult or impossible to hybridize by conventional breeding methods. Although not apparent at the onset, the most important application of the technique may be the production of novel breeding parents for use in unique conventional breeding strategies. In the CREC citrus breeding program, somatic hybridization is having a profound impact on fresh citrus cultivar development through production of superior tetraploid breeding parents via symmetric fusion of elite diploid cultivars, followed by interploid crosses to generate seedless triploids for mandarin, grapefruit/pummelo and acid lemon/lime improvement. Due to the heterozygous nature of allotetraploids obtained via somatic fusion, greater genetic and phenotypic diversity can be found from interploid conventional crosses using the somatic hybrid allotetraploid as compared to an autotetraploid parent. The evaluation of numerous interploid crossing combinations can lead to the identification of superior allotetraploid parents that should contribute to rapid progress in the development of improved seedless fresh fruit citrus cultivars.
For rootstock improvement, feedback from the field evaluation of somatic hybrid rootstocks has identified superior parents for use in a unique tetraploid rootstock-breeding program that maximizes genetic diversity in tetraploid zygotic progeny. Evolving screening methodologies are facilitating efficient identification of superior hybrids for ongoing greenhouse and field evaluations. Currently available EST-SSR genotyping techniques are being utilized to determine unambiguously seedling type (nucellar or zygotic origin), and whether candidate rootstocks are amenable to traditional nursery propagation method. Continued research should result in the release of improved, widely adapted “tetrazyg” rootstocks that exhibit all required disease tolerance/resistance and tree size control. In this regard, the approaches outlined here could be adapted to other commodities with similar breeding objectives, especially avocado, grape, and some tropical fruit species, as well as other commodities that utilize rootstocks.
References
Ananthakrishnan G, Calovic M, Serrano P, Grosser JW (2006) Production of additional allotetraploid somatic hybrids combining mandarins and sweet orange with pre-selected pummelos as potential candidates to replace sour orange rootstock. In Vitro Cell Dev Biol Plant 42:367–371
Bassene JB, Berti L, Costantino G, Carcouet E, Kamiri M, Tomi F, Dambier D, Ollitrault P, Froelicher Y (2009a) Inheritance of characters involved in fruit quality in a citrus interspecific allotetraploid somatic hybrid. J Agric Food Chem 57:5065–5070
Bassene JB, Froelicher Y, Dhuique-Mayer C, Mouhaya W, Ferrer RM, Ancillo G, Morillon R, Navarro L, Ollitrault P (2009b) Non-additive phenotypic and transcriptomic inheritance in a citrus allotetraploid somatic hybrid between C. reticulata and C. limon: the case of pulp carotenoid biosynthesis pathway. Plant Cell Rep 28:1689–1697
Bastia T, Scotti N, Cardi T (2001) Organelle DNA analysis of Solanum and Brassica somatic hybrids by PCR with universal primers. Theor Appl Genet 102:1265–1272
Bravo JE, Evans DA (1985) Protoplast fusion for crop improvement. Plant Breed Rev 3:193–218
Carlson PS, Smith H, Dearing RD (1972) Parasexual interspecific plant hybridization. Proc Natl Acad Sci USA 69:2292–2294
Chen CX, Grosser JW, Ćalović M, Serrano P, Pasquali G, Gmitter J, Gmitter FG Jr (2008) Verification of mandarin + pummelo somatic hybrids by EST-SSR marker analysis. J Am Soc Hortic Sci 133:1–7
Cocking EC (1960) Enzymatic degradation of cell wall for protoplast formation. Nature 187:927–929
Collonnier C, Fock I, Daunay MC, Servaes A, Vedel F, Siljak-Yakovlev S, Souvannavong V, Sihachakr D (2003) Somatic hybrids between Solanum melongena and S. sisymbrifolium as a useful source of resistance against bacterial and fungal wilts. Plant Sci 164:849–861
Dudits D, Fejér O, Hadlaczky G, Koncz C, Lázár G, Horváth G (1980) Intergeneric gene transfer mediated by protoplast fusion. Mol Gen Genet 179:283–288 (TC:83)
Escalante A, Imanishi S, Hossain M, Ohmido N, Fukui K (1998) RFLP analysis and genomic in situ hybridization (GISH) in somatic hybrids and their progeny between Lycopersicon esculentum and Solanum lycopersicoides. Theor Appl Genet 96:719–726
Fatta Del Bosco S, Tusa N, Conicella C (1999) Microsporogenesis in a Citrus interspecific tetraploid somatic hybrid and its fusion parents. Heredity 83:373–377
Fock I, Collonnier C, Purwito A, Luisetti J, Souvannavong V, Vedel F, Servaes A, Ambroise A, Kodja H, Ducreux G, Sihachakr D (2000) Resistance to bacterial wilt in somatic hybrids between Solanum tuberosum and Solanum phureja. Plant Sci 160:165–176
Frearson EM, Power JB, Cocking EC (1973) The isolation, culture and regeneration of Petunia leaf protoplasts. Dev Biol 33:1130–1137
Gleba YY, Sytnik KM (1984) Protoplast fusion: genetic engineering in higher plants. Springer, Berlin
Gmitter FG Jr, Grosser JW (1993) Ploidy level manipulation for scion cultivar development. Proceedings international mandarin festival, October 29–31, 1993, Azuma-cho, Kagoshima-ken, Japan
Gmitter FG Jr, Grosser JW, Castle WS, Moore GA (2007) A comprehensive citrus genetic improvement program. In: Kahn IH (ed) Citrus genetics, breeding and biotechnology, vol 2. CAB International, Wallingford, pp 9–18
Grosser JW (1994) Observations and suggestions for improving somatic hybridization by plant protoplast isolation, fusion, and culture. Hortscience 29:1241–1243
Grosser JW, Chandler JL (2003) New Citrus rootstocks via protoplast fusion. Acta Hort 622:491–497
Grosser JW, Gmitter FG Jr (2009) In vitro breeding provides new and unique opportunities for conventional breeding. Acta Hortic 829:65–72
Grosser JW, Gmitter FG Jr (1990) Protoplast fusion and citrus improvement, Chapter 10. In Plant Breeding Reviews 8. Timber Press Inc, Portland, pp 339–374
Grosser JW, Gmitter FG Jr (2005) 2004 SIVB Congress symposium proceedings thinking outside the cell—applications of somatic hybridization and cybridization in crop improvement, with citrus as a model. In Vitro Cell Dev Plant 41:220–225
Grosser JW, Gmitter FG Jr, Castle WS, Chandler JL (1995) Production and evaluation of citrus somatic hybrid rootstocks: progress report. Proc Fla State Hortic Soc 108:140–143
Grosser JW, Mourao FAA, Gmitter FG Jr, Louzada ES, Jiang J, Baergen K, Quiros A, Cabasson C, Schell JL, Chandler JL (1996) Allotetraploid hybrids between Citrus and seven related genera produced by somatic hybridization. Theor Appl Genet 92:577–582
Grosser JW, Ollitrault P, Olivares-Fuster O (2000) Invited review—somatic hybridization in citrus: an effective tool to facilitate variety improvement. In Vitro Cell Dev Biol Plant 36:434–449
Grosser JW, Graham JH, McCoy CW, Hoyte A, Rubio HM, Bright DB, Chandler JL (2003) Development of “tetrazyg” rootstocks tolerant of the diaprepes/phytophthora complex under greenhouse conditions. Proc Fla State Hortic Soc 116:262–267
Grosser JW, Ananthakrishnan G, Ćalović M, Serrano P, Chandler JL, Gmitter FG Jr, Guo WW (2006) Applications of somatic hybridization and cybridization in scion and rootstock improvement with focus on citrus. Proc IS Biotechnol Temp, Fruit Crops and Tree Species. Acta Hortic 738:73–82
Grosser JW, Graham JH, Hoyte A, Rubio HM, Bright DB, Gmitter J, Chen CX, Gmitter FG Jr (2007) Continued development of rootstocks tolerant of the phytophthora-diaprepes complex via greenhouse screening. Proc Fla State Hortic Soc 120:103–109
Grosser JW, An HJ, Calovic M, Lee D, Chen C, Vasconcellos M, Gmitter FG Jr (2010) Production of new allotetraploid and autotetraploid citrus breeding parents: focus on zipperskin mandarins. HortScience 45:1–4
Guo WW, Deng XX (1998) Somatic hybrid plantlets regeneration between Citrus and its wild relative, Murraya paniculata via protoplast electrofusion. Plant Cell Rep 18:287–300
Guo WW, Grosser JW (2005) Somatic hybrid vigor in citrus: direct evidence from protoplast fusion of an embryogenic callus line with a transgenic mesophyll parent expressing the GFP gene. Plant Sci 168:1541–1545
Guo WW, Cai XD, Grosser JW (2004a) Somatic cell cybrids and hybrids in plant improvement. In: Daniell H, Chase CD (eds) Molecular biology and biotechnology of plant organelles, vol 24. Kluwer, Dordrecht, pp 635–659
Guo WW, Prasad D, Serrano P, Gmitter FG Jr, Grosser JW (2004b) Citrus somatic hybridization with potential for direct tetraploid scion cultivar development. J Hortic Sci Biotech 79:400–405
Johnson AAT, Veilleux RE (2001) Somatic hybridization and application in plant breeding. Plant Breed Rev 20:167–225
Kao KN, Michayluk MR (1975) Nutritional requirements for growth of Vicia hajastana cells and protoplasts at a very low population density in liquid media. Planta 126:105–110
Khan IA, Grosser JW (2004) Regeneration and characterization of somatic hybrid plants of Citrus sinensis (sweet orange) and Citrus micrantha, a progenitor species of lime. Euphytica 137:271–278
Kobayashi S, Ohgawara T, Fujiwara K, Oiyama I (1991) Analysis of cytoplasmic genomes in somatic hybrids between navel orange (Citrus sinensis Osb.) and “Murcott tangor”. Theor Appl Genet 82:6–10
Moreira CD, Chase CD, Gmitter FG, Grosser JW (2000) Inheritance of organelle genomes in citrus somatic cybrids. Mol Breed 6:401–405
Murashige T, Tucker DPH (1969) Growth factor requirements for citrus tissue culture. In: Proceedings of first international citrus symposium, vol 3, pp 1155–1161
Orczyk W, Przetakiewicz J, Nadolska-Orczyk A (2003) Somatic hybrids of Solanum tuberosum: application to genetics and breeding. Plant Cell Tissue Org Cult 73:245–256
Reforgiato Recupero G, Russo G, Recupero S (2005) New promising Citrus triploid hybrids selected from crosses between monoembryonic diploid female and tetraploid male parents. HortScience 40:516–520
Skarzhinskaya M, Landgren M, Glimelius K (1996) Production of intertribal somatic hybrids between Brassica napus L. and Lesquerella fendleri (Gray) Wats. Theor Appl Genet 93:1242–1250
Stift M, Berenos C, Kuperus P, van Tienderen PH (2008) Segregation models for disomic, tetrasomic and intermediate inheritance in tetraploids: a general procedure applied to Rorippa (yellow cress) microsatellite data. Genetics 179:2113–2123
Szcerbakowa A, Bottowicz D, Wilgat B (2003) Interspecific somatic hybrfids of Solanum bulbocastanum (+) and S. tuberosum H-8105. Physiol Plant 25:365–373
Viloria Z, Grosser JW (2004) Acid citrus fruit improvement via interploid hybridization using allotetraploid somatic hybrid and autotetraploid breeding parents. J Am Soc Hortic Sci 130:392–402
Viloria Z, Droulliard DL, Graham JH, Grosser JW (2004) A stomatal inoculation method for screening triploid hybrids of ‘Lakeland’ limequat for resistance to Asiatic Citrus Canker (Xanthomonas axonopodis pv.citri). Plant Dis 88:1056–1060
Viloria Z, Grosser JW, Bracho B (2005) Immature embryo culture and seedling development of acid citrus fruit dereived from interploid hybridization. Plant Cell Tissue Organ Cult 82:159–167
Waara S, Glimelius K (1995) The potential of somatic hybridization in crop breeding. Euphytica 85:217–233
Wang YP, Sonntag K, Rudloff E (2003) Development of rapeseed with high erucic acid content by asymmetric somatic hybridization between Brassica napus and Crambe abyssinica. Theor Appl Genet 106:1147–1155
Witjaksono, Litz RE, Grosser JW (1998) Isolation, culture and regeneration of avocado (Persea americana Mill.) protoplasts. Plant Cell Rep 18:235–242
Xu CH, Xia GM, Zhi DY, Xiang FN, Chen HM (2003) Integration of maize nuclear and mitochondrial DNA into the wheat genome through somatic hybridization. Plant Sci 165:1001–1008
Xu X, Lu J, Dalling D, Jittayasothorn Y, Grosser JW (2007) Isolation and culture of grape protoplasts from embryogenic suspension cultures and leaves of Vitis vinifera and Vitis rotundifolia. Acta Hortic 738:787–790
Acknowledgments
Much of the research reported herein was supported by grants from the Florida Citrus Production Research Advisory Council, NVDMC (The New Varieties Development and Management Corporation through the Florida Dept. of Citrus) USDA/CSREES, and by the Florida Agricultural Experiment Station.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grosser, J.W., Gmitter, F.G. Protoplast fusion for production of tetraploids and triploids: applications for scion and rootstock breeding in citrus. Plant Cell Tiss Organ Cult 104, 343–357 (2011). https://doi.org/10.1007/s11240-010-9823-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-010-9823-4