Abstract
Chromosome doubling was induced in vitro in a diploid hybrid of Rosa rugosa Thunb. using oryzalin as the spindle inhibitor. Nodal sections, 2 mm long, were exposed to 2.5 or 5 μM oryzalin and 10 mm nodal sections were exposed to 5 μM oryzalin for 0 (controls), 6, 12, 24 and 48 h. The ploidy of the emergent shoots was determined by flow cytometry. The frequency of tetraploid and mixoploid leaves that developed from 2 mm nodal sections exposed to 5 μM oryzalin peaked at 12 h exposure, when 35% of the leaves were tetraploid, but fell after longer exposures. Fewer tetraploid and mixoploid leaves were found when 2 mm nodes were exposed to 2.5 μM oryzalin for 6 and 12 h, indicating that it took longer for a spindle inhibiting concentration of oryzalin to build up in the meristem. However, the frequencies of tetraploid and mixoploid leaves continued to rise after 12 h and were highest at 48 h, when 44% were tetraploid. In treatments with 5 μM oryzalin, the frequencies of tetraploid and mixoploid leaves were lower, at equivalent exposure times, in 10 mm nodes than 2 mm nodes. This suggests that oryzalin diffused to the meristem mainly via the cut surfaces and that access via the epidermis and cuticle was impeded.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chromosome numbers of roses are based on multiples of seven. They range from 2n = 2x = 14 to 2n = 8x = 56 and aneuploidy is rare (Darlington and Wylie 1955; Crane and Byrne 2003). Chromosome doubling of diploid species and cultivars facilitates their use in breeding programs with tetraploid recurrent flowering cultivars. It has been achieved by topical application of colchicine (Fagerlind 1958; Semeniuk and Arisumi 1968; Basye 1992) or trifluralin (Zlesak et al. 2005) to the apex of seedlings. However, in order to double the chromosome number of rare or sterile hybrids, a method is needed that can be applied to vegetative tissues of clonally propagated plants. That was achieved by exposing in vitro shoot tissues to colchicine (Ma et al. 1997) or oryzalin (Kermani et al. 2003). Oryzalin inhibits spindle formation at approximately one thousandth of the concentration of colchicine. Kermani et al. (2003) preferred oryzalin to colchicine because it is safer to use, cheaper to apply and less likely to cause chromosome aberrations in plant tissues. When they treated shoot tips (10 mm long) of a Rosa rugosa Thunb. hybrid, “Thérèse Bugnet” (2n = 2x = 14), to 5 μM oryzalin, tetraploids were found after exposures of 14 days but not after exposures of 7 days. However, an exposure of only 24 h was sufficient to induce chromosome doubling in nodal sections (1 mm long). They concluded that the epidermis and cuticle are impervious to oryzalin and that oryzalin reached the meristem via the two cut surfaces of 1 mm nodes more rapidly than the single cut surface of 10 mm shoot tips.
Following the demonstration by Kermani et al. (2003) that chromosome doubling could be induced rapidly by applying oryzalin to nodal sections of roses, our present objectives were to investigate the effect of the following variables on the efficiency of chromosome doubling: (1) the length of nodal sections, (2) the concentration of oryzalin and (3) the period of exposure to oryzalin. The cultivar was chosen for chromosome doubling because it is resistant to black spot disease and tetraploid plants were needed for breeding purposes.
Materials and methods
Plant material
The rose used in this investigation was a diploid hybrid between two cultivars “Martin Frobisher” and “Mistress Quickly” supplied by David Austin Roses Ltd, Albrighton, UK. Both of these parental cultivars are themselves hybrids of R. rugosa Thunb. (Cairns 2003). Petroselinum crispum (Mill.) Nyman ex A.W. Hill “Champion Moss Curled”, obtained from Thompson and Morgan Ltd, Ipswich, UK, was used as an internal standard for flow cytometry.
Tissue culture methods
Single nodes (approx. 10 mm long) were excised from the stems of greenhouse-grown plants and the leaves were removed. The nodal sections were then surface sterilised as follows: they were washed in running tap water for one hour, dipped for 10 s in 70% ethanol, immersed for 15 min in solution of sodium hypochlorite (0.5% available chlorine), then washed in three changes of sterile (autoclaved) distilled water, each of 15 min. After the cut ends had been trimmed, the nodes were cultured on multiplication medium that contained VS salts and vitamins (van der Salm et al. 1994) and 6-benzylamino purine (BAP; 1.0 μM). On this medium, lateral shoots were produced which were excised and further multiplied by subculture to fresh medium at intervals of 5 weeks. Nodal sections from these shoots were cultured on the axillary bud development medium of Dubois and de Vries (1996) that contains thidiazuron (10 μM). When oryzalin (British Greyhound Chromatography and Allied Chemicals, Birkenhead, UK) was included in this medium, it was added from a stock solution (50 mM) in dimethoxysulphoxide (which acts both as solvent and sterilant) after the autoclaved medium had cooled to 60°C. Shoots with 3–4 opened leaves were rooted on a medium that contained half strength VS salts and vitamins, naphthaleneacetic acid (0.25 μM) and indolebutyric acid (0.25 μM). Cultures on both shoot multiplication and rooting media were maintained on 50 ml of medium in glass honey jars (300 ml capacity) with screw-capped transparent plastic lids and nodal sections were cultured in Petri dishes (90 mm diameter) containing 25 ml of medium. All culture media contained 30 gl−1 sucrose and were adjusted to pH 5.8, solidified with 2.5 g l−1 Phytagel (Sigma-Aldrich Co. Ltd., Poole, UK). Rooted plantlets were acclimatized for 3–4 weeks in culture vessels (109 mm2 × 95 mm high, with lids that incorporated an air-permeable filter of 40 mm diameter; Osmotek Ltd, Rehovot, Israel) containing 300 ml of potting compost moistened with half strength VS salts and vitamins, and were then transferred to soil in a humid greenhouse for assessment of their morphology, chlorophyll content and pollen fertility. The culture media, empty honey jars (before the addition of culture media) and Osmotek vessels containing potting compost were sterilized by autoclaving for 15 min at 120°C. All cultures were maintained at 25 ± 2°C. Nodal sections in Petri dishes were maintained in darkness but all other cultures, including rooted plantlets in Osmotek vessels, were illuminated by high-pressure metal halide lamps (HQI-T, Osram, Germany; PPFD 60 μmol m−2 s−1 at the plant surface) on a 16/8 h light/dark cycle.
Oryzalin treatments
Mid-stem nodes were taken from in vitro shoots on multiplication medium 6 weeks after they had been subcultured. They were prepared by cutting the stem symmetrically above and below the mid point of the node to give 2 or 10 mm nodal sections (Fig. 1). Three treatments were investigated, 2 mm nodes exposed to 2.5 μM oryzalin, 2 mm nodes exposed to 5 μM oryzalin and 10 mm nodes exposed to 5 μM oryzalin. In each treatment, the nodes were immersed horizontally in the semi-solid medium. For each type of node and concentration of oryzalin, exposure times of 6, 12, 24, 48 h were tested using 45 nodal sections per treatment. One set of controls (0 h exposure) consisting of 45 nodes was prepared for 2 mm nodes and another set of 45 nodes was prepared for 10 mm nodes. Each batch of 45 nodes was arranged with 15 nodes per Petri dish. After exposure to oryzalin or oryzalin-free medium (controls) in axillary bud development medium, the nodal sections from each Petri dish were washed in sterile distilled water and transferred to a fresh Petri dish containing oryzalin-free axillary bud development medium. For a total of one week from the start of the oryzalin treatment, the Petri dishes were maintained in darkness. The nodes were then transferred to honey jars (five per jar) containing shoot multiplication medium. After the axillary buds had elongated into shoots with at least 10 leaves, the survival percentages for the different treatments were recorded and the ploidy of each of the three youngest leaves on each shoot was assessed. These leaves were chosen because they were initiated after exposure of nodes to oryzalin and relatively unlikely to contain sectorial chimeras (Kermani et al. 2003). Furthermore, the ploidy of each leaf and its subtended axillary bud could be expected to correspond as they would have been initiated from the same meristem initials.
Chromosome counts and the assessment of ploidy
Chromosome counts were prepared from root-tip squashes of control shoots following the procedures of Yokoya et al. (2000).
The ploidy of each rose leaf tested was determined from its nuclear DNA amount which was estimated by flow cytometry following the procedures of Yokoya et al. (2000). This involved preparing suspensions of nuclei by chopping together young leaves of the rose and an internal standard of known DNA amount, P. crispum “Champion Moss Curled”, in an isolation buffer. Staining solution containing propidium iodide was then added and the fluorescence intensity of the nuclei was determined with a Partec CA-III flow cytometer (Partec GmbH). The 2C DNA amount of the rose leaf was estimated by dividing the fluorescence intensity of the G1 peak of the rose by that of the G1 peak of P. crispum, and multiplying by the 2C DNA amount of P. crispum (4.46 pg; Yokoya et al. 2000).
Assessment of morphology, chlorophyll content and pollen viability
Some nodes on shoots arising from oryzalin-treated and control nodes, identified as tetraploid or diploid by the ploidy of their leaves, were sub-cultured and the ploidy of the induced shoots was confirmed by flow cytometry. Branch shoots were then rooted, acclimatized and transferred to soil in a greenhouse. After an inflorescence had developed on each of these shoots, various morphological characters (defined in Table 1) were assessed on one mid-stem leaf, the inflorescence and the terminal flower of the inflorescence. The chlorophyll content of leaves was estimated by the method of Roberts et al. (1990b). Pollen viability was assessed as the percentage of grains stained with fluorescein diacetate that fluoresced under UV light (Kermani et al. 2003). These assessments were carried out on 12 tetraploid plants which consisted of four plants originating from each of three different oryzalin-treated nodes and 12 diploid plants derived from 12 different control nodes.
Statistical methods
Analyses of variance were carried out on the measurements of tetraploid shoots to determine whether or not variation was greater amongst than within the three groups that had been separately induced. The significance of differences between means of measurements of diploid and tetraploid plants were tested with Student t tests. Differences between the frequencies of mixoploid + tetraploid leaves (versus diploid leaves) after two different exposure times were tested using 2 × 2 contingency χ2 tests and differences between frequencies of viable pollen (versus inviable pollen) in four samples were compared using a 2 × 4 contingency χ2 test. These methods are described by Sokal and Rolfe (1995).
Results
Chromosome counts on root-tip cells of the R. rugosa hybrid “Martin Frobisher” × “Mistress Quickly” indicated that it was diploid (2n = 14). Furthermore, the average 2C DNA amount determined by flow cytometry in a sample of 50 in vitro leaves taken from “control” shoots was 1.06 (SD ± 0.03) pg which is only slightly greater than the 2C DNA amount of 0·98 (SD ± 0·08) pg recorded by Yokoya et al. (2000) for diploid R. rugosa var. alba.
The percentage of surviving shoots declined in relation to the time that nodes were exposed to oryzalin, falling from 67–73% amongst the controls to 18–27% in the 48 h treatments (Fig. 2).
The percentages of oryzalin-treated and control nodes of Rosa “Martin Frobisher” × “Mistress Quickly” that survived to produce shoots with 10 nodes in relation to time that nodes were exposed to oryzalin a 2 mm nodes treated with 2.5 μM oryzalin; b 2 mm nodes treated with 5 μM oryzalin; c 10 mm nodes treated with 5 μM oryzalin
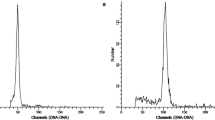
Tetraploid and, infrequently, hexaploid and octoploid leaves on oryzalin-treated shoots were identified by the presence of peaks with fluorescence intensities that were multiples of those of the diploid peaks. Flow cytometry histograms of diploids and polyploids indicated that most leaf nuclei were at the G1 stage of the cell cycle and G2 peaks were small or undetectable (Fig. 3a, b). Mixoploid leaves with both diploid and tetraploid cells were also found. These were identified by the presence of two peaks of similar height corresponding in fluorescence intensity to the G1 peaks for, respectively, diploid and tetraploid leaves (Fig. 3c) and were interpreted as periclinal chimeras. The ratio of tetraploid to mixoploid leaves, over all treatments, was 1:0.75.
Flow cytometry histograms showing numbers of nuclei per channel of Rosa “Martin Frobisher” × “Mistress Quickly” and the internal calibration standard, Petroselinum crispum, in relation to channel numbers (which are linearly proportional to DNA amount). A G1 peaks of nuclei of the diploid rose (a) and the standard (b). B G1 peaks of a tetraploid rose (a) and the standard (b). C G1 peaks of the diploid (a1) and tetraploid (a2) nuclei of a mixoploid rose, and the standard (b)
Only diploid leaves were found in the controls for the treatments of 2 mm nodes, but amongst the controls for 10 mm nodes, 3% of leaves tested (all three leaves on a single shoot) were mixoploid. Trends in the frequencies of diploid to tetraploid conversions in relation to time of exposure to oryzalin are shown Fig. 4. In order to use all data that are indicative of diploid to tetraploid conversions, the frequencies of tetraploid and mixoploid leaves, (based on three leaves per surviving shoot) were combined. The frequencies of pure tetraploids are indicated adjacent to the data points for 12–48 h. After the treatment of 2 mm nodes with 5 μM oryzalin, the frequencies of tetraploid + mixoploid leaves rose to 11% at 6 h and 40% at 12 h (35% pure tetraploids) but then fell to 17% in the 24 h treatment and 25% in the 48 h treatment. A 2 × 2 contingency chi-square test showed that the proportion of tetraploid + mixoploid to diploid leaves was significantly smaller in the 24 h treatment than in the 12 h treatment (χ2 = 11.11; df = 1; P < 0.001). After the treatment of 2 mm nodes with 2.5 μM oryzalin, the frequencies of tetraploid + mixoploid leaves were lower over the first 12 h than at corresponding exposure times for 2 mm nodes to 5 μM oryzalin but rose to 67% at 48 h (44% were tetraploid). A 2 × 2 contingency chi-square test showed that the proportion of tetraploid + mixoploid to diploid leaves was significantly greater in the treatment of 2 mm nodes with 2.5 μM oryzalin for 48 h than of 2 mm nodes with 5 μM oryzalin for 12 h (χ2 = 11.72; df = 1; P < 0.001). After the treatment of 10 mm nodes with 5 μM oryzalin, the frequencies of tetraploid + mixoploid leaves were lower than for equivalent exposure times in either of the treatments with 2 mm nodes.
Percentage of tetraploid + mixoploid leaves amongst surviving shoots that developed from the oryzalin-treated and control nodes of Rosa “Martin Frobisher” × “Mistress Quickly” in relation to time that nodes were exposed to oryzalin (a 2 mm nodes treated with 2.5 μM oryzalin; b 2 mm nodes treated with 5 μM oryzalin; c 10 mm nodes treated with 5 μM oryzalin. The numbers adjacent to data points for exposures of 12–48 h indicate the percentages of leaves that were tetraploid)
When 2 mm nodes were treated with 2.5 μM oryzalin for 48 h, 8% of the leaves (all three tested on a single shoot) were octoploid and when 10 mm nodes were treated with 5 μM oryzalin for 12 h, 4% of all leaves (all three tested on a single shoot) were hexaploid. The shoots on which the hexaploid and octoploid leaves were found were very slow growing and barely viable.
Three nodal sections with tetraploid leaves were taken from shoots derived from different oryzalin-treated nodes and recultured. Leaves on the resultant shoots showed no deviation from tetraploidy. Four shoots derived from each of these 3 tetraploid shoots and 12 diploid shoots were rooted, transferred to compost, then grown under greenhouse conditions. Their morphology and chlorophyll content were investigated at the time of first flowering (Table 1). Analysis of variance showed that the tetraploids derived from separately induced nodes did not differ significantly (F test probability > 0.05) in any of these characters. The total chlorophyll per unit leaf area of diploid and tetraploid plants did not differ significantly (P > 0.05) but differences between diploid and tetraploid leaves were highly significantly (P < 0.001) for all other characters (Table 1). The tetraploid leaves were longer and heavier than diploid leaves, and had significantly fewer leaflets per leaf. The terminal leaflets were significantly longer and wider, and their width to length ratio was greater (Fig. 5). The tetraploids had fewer flowers per inflorescence than the diploids but their flower diameters and petal numbers were both greater (Fig. 6). The viability of pollen grains was assessed in samples of 450 pollen grains in three tetraploid plants of different origin and a diploid plant. The viability of pollen was 35.0 (SE ± 2.2)% in the diploid and 38.9 (SE ± 2.2), 35.8 (SE ± 2.1) and 33.9 (SE ± 2.1)% in the tetraploid plants. A 4 × 2 contingency χ2 test showed that the proportions of viable to non-viable grains did not differ significantly in the four plants tested (χ2 = 3.41; df = 3; P > 0.1).
Discussion
The frequency of tetraploid + mixoploid leaves on shoots arising from 2 mm nodes exposed to 5 μM oryzalin peaked at 12 h then declined (Fig. 4). Roberts et al. (1990a) estimated the cell cycle time of the fast cycling cells in root meristems of a diploid rose (R. wichurana) to be 10 h and found that the highest frequency of tetraploid cells was induced after a 12 h exposure to colchicine. If the cell cycle times in the shoot meristems of “Martin Frobisher” × “Mistress Quickly” correspond to those in the root meristems of R. wichurana, 12 h would correspond to the time needed for all the fast cycling cells of the meristem to pass through a spindle-inhibited mitosis. DNA synthesis is not blocked by oryzalin and meristematic cells can go through several cycles of endoreduplication (Grandjean et al. 2004). Thus it can be expected that a 24 h exposure would be sufficient for these cells to go through a second spindle-inhibited mitosis, to produce octoploid cells and that further reduplication might occur during a 48 h exposure. The poor viability of the few hexaploid and octoploid shoots that were recovered suggests that these high ploidy levels adversely affect growth. If the proliferation of meristematic cells of high ploidy is retarded, diploid cells that were slow cycling when the oryzalin was applied may become established as the main proliferating cells of the meristem. Consequently, there would be an apparent decline in the frequency of conversion to tetraploidy when 2 mm nodes were exposed to 5 μM oryzalin for longer than 12 h.
A comparison of treatments of 2 mm nodes with 2.5 and 5 μM oryzalin shows that the 2.5 μM treatment gave lower frequencies of tetraploid + mixoploid leaves after exposures of 6 and 12 h, presumably because a spindle inhibiting concentration in the meristem took longer to achieve. The frequency of tetraploids + mixoploids induced by a 48 h exposure to 2.5 μM oryzalin (67%) was higher than that induced by a 12 h exposure to 5 μM oryzalin. It is suggested that over 48 h, 2.5 μM oryzalin reached a greater proportion of meristematic cells, before significant reduplication of chromosomes, than in nodes exposed to 5 μM oryzalin for 12 h.
The frequencies of tetraploid + mixoploid leaves were lower, for corresponding exposures to 5 μM oryzalin, in shoots arising from 10 mm than 2 mm nodes (Fig. 4). It is proposed that the passage of oryzalin to the meristem in the 10 mm nodes was delayed because diffusion via the cut surface, rather than via the cuticle and epidemis, is the primary route of access to the meristem. Kermani et al. (2003) came to a similar conclusion when they observed that tetraploidy could be induced more rapidly in nodal sections than in shoot tips. The presence, in low frequency, of some tetraploid + mixoploid leaves after short exposures may result partly from an underlying presence of tetraploid cells in the nodes used for this batch of treatments, as indicated by the mixoploid shoot found amongst the controls.
The occurrence of octoploid cells can readily be explained by endoreduplication of tetraploid cells but the origin of hexaploid cells is more obscure. Possibly cells with DNA amounts corresponding to those expected of a hexaploid were formed at mitosis by partial spindle inactivation leading to an unbalanced segregation of chromosomes. When Zlesak et al. (2005) applied the spindle inhibitor trifluralin to diploid R. chinensis, triploids were formed in addition to tetraploids, presumably likewise, by an unbalanced segregation of chromosomes.
In each of the treatments, the frequency of surviving nodes declined in relation to time (Fig. 2). However, differences between treatments were less marked than might be expected if loss of viability could be attributed only to chromosome doubling in the meristem. This suggests that viability is also determined by the effects of oryzalin on cells closer to the cut surface, possibly by interactions between oryzalin and the tubulin of the cytoskeleton.
Topical application of spindle inhibitors to the apex of seedlings in vivo (Fagerlind 1958; Semeniuk and Arisumi 1968; Basye 1992; Zlesak et al. 2005) is relatively simple because it does not involve in vitro culture, but no successful attempt to induce chromosome doubling in clonally propagated rose cultivars by an in vivo method has been published. Only two previously published reports describe chromosome doubling of diploid roses using in vitro methods and in both, shoot tips and nodal sections were tested. In one, Ma et al. (1997) obtained their highest frequency of tetraploids (13%) when nodal sections (unspecified length) of R. wichurana × roxburghii were exposed to 2.5 mM colchicine for two days. In the other, Kermani et al. (2003) obtained their highest frequency of tetraploids (66% of survivors) when nodal sections (1 mm) of R. rugosa “Scabrosa” were obtained after exposure to 5 M oryzalin for 1 day. The latter study involved the exposure of only 30 treated nodes to a single concentration of oryzalin for a single exposure time and the present investigation was intended to extend this work. Chromosome doubling with oryzalin and colchicine have been contrasted in several genera, without a clear consensus emerging as to which is the more effective. For example, Blakesley et al. (2002) working with Acacia and Petersen et al. (2003) working with Miscanthus found that colchicine was more effective than oryzalin. However, Vainola (2000) working with Rhododendron and Grzebelus and Adamus (2004) working with Allium found that oryzalin was the more effective. The present investigation shows that the interactions between concentration of the spindle inhibitor and time of exposure have profound effects on the efficiency of chromosome doubling. Consequently, misleading conclusions might easily be drawn from experiments involving limited ranges of spindle inhibitor concentration and exposure times.
Significant differences (Table 1) were observed between diploids and tetraploids in the morphology of leaf and floral characters (Figs. 5, 6). These included increases in the length of leaves, the breadth to length ratio of leaflets and the number of petals per flower which were also observed by Kermani et al. (2003) in another R. rugosa hybrid, “Thérèse Bugnet”. A pronounced increase in the width to length ratio of leaflets, which was also observed by Kermani et al. (2003) in all diploid to tetraploid conversions, could be used as a provisional indicator of successful conversion from diploidy to tetraploidy if a flow cytometer is unavailable.
Chromosome doubling did not affect the proportions of viable to non-viable pollen grains which suggests that other factors, besides chromosome homology, are responsible for the non-viability of most pollen grains. However, the viability of 35–39% of the pollen in the induced tetraploids indicates that they can be used for breeding at the tetraploid level.
The following conclusions regarding the efficiency of chromosome doubling, based on the in vitro application of oryzalin to a diploid rose in this study, may assist in the planning of chromosome doubling with other spindle inhibitors and in other genera:
-
1.
The time taken for oryzalin to achieve a spindle inhibiting concentration in the meristem is related primarily to the closeness of the cut surface to the meristem.
-
2.
The exposure times should be long enough to maximize the population of meristematic cells that are affected by the spindle inhibitor but short enough to minimize the proportion of cells with redoubled chromosome numbers.
-
3.
If the concentration of the spindle inhibitor is too great, chromosome redoubling may occur in some cells before the spindle inhibitor fully permeates the meristem.
References
Basye RE (1992) The future of the rose. Amer Rose Ann 31:62–63
Blakesley D, Allen A, Pellny T, Roberts AV (2002) Natural and induced polyploidy in Acacia dealbata Link. and Acacia mangium Willd. Ann Bot 90:391–398. doi:10.1093/aob/mcf200
Cairns T (2003) Modern roses XI. Academic, San Diego
Crane YM, Byrne DH (2003) Karyology. In: Roberts AV, Debener T, Gudin S (eds) Encyclopedia of rose science. Elsevier, Oxford, pp 267–273
Darlington CD, Wylie AP (1955) Chromosome atlas of flowering plants. 2nd edn. Allen & Unwin, London
Dubois LAM, de Vries DP (1996) The direct regeneration of adventitious buds on leaf explants of glasshouse-grown cut rose cultivars. Acta Hortic 424:327–329
Fagerlind F (1958) Hip and seed formation in newly formed Rosa polyploids. Acta Hortic Berg 17:229–256
Grandjean O, Vernoux T, Laufs P, Belcram K, Mizukami Y, Traas J (2004) In vivo analysis of cell division, cell growth and differentiation at the shoot apical meristem in Arabidopsis. Plant Cell 16:74–87. doi:10.1105/tpc.017962
Grzebelus E, Adamus A (2004) Effect of anti-mitotic agents on development and genome doubling of gynogenic onion (Allium cepa L.) embryos. Plant Sci 167:569–574. doi:10.1016/j.plantsci.2004.05.001
Kermani MJ, Sarasan V, Roberts AV, Yokoya K, Wentworth J, Sieber VK (2003) Oryzalin-induced chromosome doubling in Rosa and its effect on plant morphology and pollen viability. Theor Appl Genet 107:1195–1200. doi:10.1007/s00122–003–1374–1
Ma Y, Byrne DH, Chen J (1997) Amphidiploid induction from diploid rose interspecific hybrids. HortSci 32:292–295
Petersen KK, Hagberg P, Kristiansen K (2003) Colchicine and oryzalin mediated chromosome doubling in different genotypes of Miscanthus sinensis. Plant Cell Tissue Organ Cult 73:137–146. doi:10.1023/A:10223854303371
Roberts AV, Lloyd D, Short KC (1990a) In vitro procedures for the induction of tetraploidy in a diploid rose. Euphytica 49:33–38. doi:10.1007/BF00024128
Roberts AV, Smith EF, Mottley J (1990b) The preparation of micropropagated plantlets for transfer to soil without acclimatization. In: Pollard JW, Walker JM (eds) Methods in molecular biology, plant cell tissue culture, vol 6. Humana Press, Totowa, pp 226–237
Semeniuk P, Arisumi T (1968) Colchicine-induced tetraploid and cytochimeral roses. Bot Gaz 129:190–193
Sokal RR, Rolfe FJ (1995) Biometry. WH Freeman, New York
Vainola A (2000) Polyploidization and early screening of Rhododendron hybrids. Euphytica 112:239–244. doi:10.1023/A:1003994800440
Van der Salm TPM, Van der Toorn CJG, ten Cate CHH, Dubois LAM, De Vries DP, Dons HJM (1994) Importance of the iron chelate formula for micropropagation of Rosa hybrida L. ‘Moneyway’. Plant Cell Tissue Organ Cult 37:73–77. doi:10.1007/BF00048120
Yokoya K, Roberts A V, Lewis R, Mottley J, Brandham PE (2000) Nuclear DNA amounts in roses. Ann Bot 85:557–561. doi:10.1006/ambo.1999.1102
Zlesak DC, Thill CA, Anderson NO (2005) Trifluralin-mediated polyploidization of Rosa chinensis minima (Sims) Voss seedlings. Euphytica 141:281–290. doi:10.1007/s10681–005–7512-x
Acknowledgments
The authors thank David Austin Roses Ltd. for support throughout this investigation and Carl Bennett and Colin Curtiss for their help.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Merkle.
Rights and permissions
About this article
Cite this article
Allum, J.F., Bringloe, D.H. & Roberts, A.V. Chromosome doubling in a Rosa rugosa Thunb. hybrid by exposure of in vitro nodes to oryzalin: the effects of node length, oryzalin concentration and exposure time. Plant Cell Rep 26, 1977–1984 (2007). https://doi.org/10.1007/s00299-007-0411-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-007-0411-y