Abstract
In Arabidopsis, adventitious shoots are formed at a high frequency when the calli are induced from roots or hypocotyls cultured on callus induction medium (CIM) and then transferred to shoot induction medium (SIM). The prolonged duration of culture on CIM decreased the frequency of shoot regeneration. However, when 5′-azacitidine (AzaC), an inhibitor of DNA methylation, was added to CIM, the excess culturing on CIM did not decrease the frequency of shoot regeneration. The level of methyl cytosine was up-regulated when hypocotyl explants were cultured on CIM for 2 weeks. We examined the expression patterns of genes that are involved in the formation or regeneration of shoots. Prolonged duration of culture on CIM up-regulated the CUC1, CLV1, CLV3, ESR1, and WUS mRNA levels, and the addition of AzaC to CIM reduced their expression levels. Our results suggest that an increase in DNA methylation decreased the shoot-forming ability and that AzaC can partially recover this ability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many plant species can regenerate adventitious shoots, roots and embryos from somatic cells, even after they have already differentiated into several specialized tissues or organs (Preil 2003). These competences are called totipotency, which suggests plants can initiate developmental programs even after they have fully developed. Among them, adventitious shoot formation in tissue culture is the means by which many plant species are commercially micropropagated, especially ornamental plants. It is also the means by which many transgenic plants are produced, i.e., by regenerating shoots from transformed cells or tissues (Tzfira and Citovsky 2006). Thus, totipotency is an important property of plants especially in horticulture and breeding of crops.
Shoot regeneration of plants have been well characterized physiologically using regeneration experiments of tobacco, Arabidopsis and the other species (Hicks 1994; Sugiyama 1999; Zhao et al. 2008). In general, in the first phase, cells of developed tissue acquire the competence to change their developmental fate (dedifferentiation) and to be committed to shoot organogenesis. In the next phase, shoot formation (redifferentiation) is undertaken. Although experimental procedures for organogenesis in vitro vary among species, in Arabidopsis tissue culture the first phase is initiated by culturing on a callus induction medium (CIM) containing high concentration of synthetic auxin, 2,4-D (Valvekens et al. 1988; Akama et al. 1992). Then, the explants are cultured on a shoot induction medium (SIM) that contains a specific ratio of auxin and cytokinin. Plant cells acquire the competence to form adventitious shoots during exposure to auxin. Simultaneously, in tobacco, calli are usually induced from somatic tissues in the presence of high concentration of auxin (Skoog and Miller 1957). When they are transferred onto media containing high concentrations of cytokinin, many shoots are formed from the callus. Thus, phytohormones, especially auxin and cytokinin, play very important roles in acquisition of the competence to form adventitious shoots.
However, long duration of culture on CIM which containing high concentration of auxin results in losing the competence to regenerate shoots from calli (Murashige and Nakano 1967; Valvekens et al. 1988). The cells of calli that have been grown on CIM for a long time may change the response to phytohormones, compared to the cells which have been cultured on CIM for short duration. It is still unknown what causes these physiological changes during culture on CIM. Genetic or epigenetic changes can result in these modifications of physiological state or developmental fate. In Arabidopsis, on the one hand, a transposable element affects the development by disruption of the gene involved in biosynthesis of a phytohormone, brassinosteroid (Miura et al. 2001). On the other hand, the level in DNA methylation itself also affects the flowering time or the development of floral organs (Burn et al. 1993; Kakutani 1997). However, there is no report of the effects of DNA methylation on shoot regeneration of Arabidopsis or other plants.
In this study, we examined the effects of 5′-azacytidine (AzaC) on the regeneration of shoots in Arabidopsis. We also evaluated the change of cytosine methylation in callus during culture on CIM. Moreover, we investigated the expression pattern of genes involved in the formation of shoot meristem or in DNA methylation. From the results, we discuss the role of DNA methylation in shoot regeneration.
Materials and methods
Plant growth and tissue culture
Plant tissue culture was carried out as described by Valvekens et al. (1988). Seeds of Arabidopsis thaliana ecotype Landsberg erecta (Ler) were surface-sterilized for 2 min in 70% EtOH, transferred to 5% NaOCl/0.1% Triton X-100 for 5 min, rinsed five times with sterile distilled water, and placed on Petri dishes containing Murashige and Skoog medium (Murashige and Skoog 1962) supplemented with 0.05% 2-Morpholinoethanesulfonic acid (MES), 0.25% gellan gum, and 1% sucrose at pH 5.7 to germinate. Plants were grown at 22°C in a 16-h light/8-h dark cycle. The same growth-room conditions were used for tissue culture procedures.
For callus formation, hypocotyl segments (5 mm long) were excised from germinated seedling at 10 days after sowing. Explants were cultured on Petri dishes containing CIM [B5 medium (Gamborg et al. 1968) supplemented with 0.5 mg/l 2,4-D, 0.1 mg/l kinetin, 0.05% MES, 0.25% gellan gum, 1% sucrose at pH 5.7] for 7–21 days. Then, the cultured explants (calli) were transferred onto SIM [B5 medium supplemented with 0.15 mg/l indoleacetic acid, 0.5 mg/l N6-(Δ2-isopentenyl)-adenine, 0.05% MES, 0.25% gellan gum, 1% sucrose at pH 5.7] for shoot regeneration. The same conditions were used when 50 μM azacitidine was supplemented in any of these mediums.
Histology
Plant tissues were fixed by immersing in FAA solution (formaldehyde: acetic acid: ethanol: distilled water; 1:1:18:20) for 24 h and dehydrated by ethanol series. Then they were embed in Technovit 8100 resin (Kulzer). Transverse and longitudinal sections were cut at 5 mm thick by a RM2125RT microtome (Leica) and stained with Toluidine blue solution.
DNA extraction and analysis of methyl cytosine
Total cellular DNA was extracted from explants or cultured calli by Plant DNAzol reagent (Invitrogen) as described in the manufacturer’s protocol. Quantification of methyl cytosine was performed according to Demeulemeester et al. (1999). Approximately 40 μg DNA was hydrolyzed to bases in 50 μl of 70% perchloric acid (100°C for 1 h). The pH was adjusted to between 3 and 5 with KOH. The resulting KClO4 precipitate was washed twice with 200 μl distilled water and the total hydrolysate reduced to dryness in a speedvac before high pressure liquid chromatography (HPLC) analysis. The bases from the hydrolysis of 40 μg DNA were redissolved in 250 μl sodium acetate (10 mmol, pH 4). After 8 min centrifugation at 10,000g, the supernatant was filtered through a 4-mm nonsterile syringe filter with pore size 0.2 μm (GL chromato disc; Kurabou, Japan). An aliquot of the filtrate was injected on the HPLC using a 100-μl sample loop. The bases were separated on a Partisil 10 SCX (4.6 × 250 mm; Chemco, Japan) column at 60°C with sodium acetate as eluent and a flow rate of 2 ml/min. The absorbance of the bases was measured at 280 nm with a UV/VIS detector (Shimazu, Japan). The standard was a mixture of the five bases: thymine, guanine, adenine, cytosine and methyl cytosine (mC) in a concentration of 2 nM/100 μl each and was used for all measurements. By comparing peak areas of similar retention times, the unknown concentrations of cytosine and mC in a sample were calculated and the percentage of mC was calculated as (concentration of mC × 100)/(concentration of mC + concentration of cytosine). All analyses were repeated three times (from three separate DNA extractions).
RNA extraction and quantitative RT-PCR
Total RNA was extracted from hypocotyl explants or cultured calli by SDS-phenol method (Ozeki et al. 1990). cDNA was synthesized from 1 μg of total RNA using Superscript II reverse transcriptase (Invitrogen) and p(dT)15 primer (Roche) in a 60-μl reaction. Real-time quantitative PCR (RT-PCR) assays were performed in a 7300 Real Time PCR System (ABI) coupled with an Absolute QPCR SYBR Green ROX Mix (AB gene). Gene-specific standard curve was generated from serial dilutions of the cDNA. Each sample had three replicates. Relative expression level was shown as the ratio of the each gene to the value of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene (AT1G13440). Conditions and primers used for PCR of each gene were as described in Table 1.
Results
Effects of the duration of culture on CIM on shoot regeneration
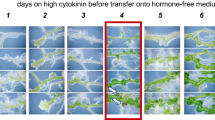
Shoots can regenerate on hypocotyl explants when they are cultured on SIM after they have been pre-cultured on CIM for 2–8 days (Akama et al. 1992). To examine the effect of the excess culturing on CIM on shoot regeneration, we cultured hypocotyl explants from Arabidopsis seedlings on CIM for 1–3 weeks and then transferred them onto SIM. Preincubation of explants for 1 week on CIM resulted in the shoot regeneration at high frequency on SIM (Fig. 1). The sizes of the calli formed on the hypocotyls were dependent on the duration of culture on CIM (Figs. 1a and 3). The calli formed on hypocotyls cultured on CIM for 3 weeks were much larger than that cultured on CIM for 1 week. Seven days after transferring onto SIM, green spots appeared on the explants that had been cultured on CIM for 1 and 2 weeks, but not on the calli cultured on CIM for 3 weeks. On day 14 on SIM, many shoots were formed from the green spots on the explants that had been cultured on CIM for 1 week. A few small shoots were formed on some explants that had been cultured on CIM for 2 weeks. The calli formed on explants that had been cultured on CIM for 3 weeks turned brown until day 28 after transferring to SIM. The adventitious shoots were formed on 100% of the explants on SIM when the explants had been cultured on CIM for 1 week (Fig. 1b). However, shoots regenerated on less than 30% of the hypocotyls pre-cultured on CIM for 2 weeks even after they were cultured on SIM for 35 days. No shoots were formed on the hypocotyl explants that had been cultured on CIM for 3 weeks. These results indicate that prolonged duration of preculture on CIM result in the reduction of competence of shoot regeneration.
Effect of the duration of culture on CIM on shoot regeneration from hypocotyl. Hypocotyls were excised from seedlings of Arabidopsis and cultured for 1, 2 and 3 weeks (CIM1w, CIM2w, CIM3w, respectively). These calli were transferred onto SIM and cultured for 35 days. a Photo images of calli cultured on SIM. Bars 5 mm. b Percentage of shoot regeneration on calli (shoot forming calli/total calli). Bars standard deviations (n = 5)
Effects of 5′-azacytidine on regeneration of Arabidopsis shoots
Excessed duration of culture on CIM decreased the frequency of shoot regeneration on hypocotyl explants. To investigate the possible role of DNA methylation in losing competence to regenerate shoots, we examined the effects of 5′-azacytidine (AzaC), an inhibitor of DNA methylation, on the formation of adventitious shoots from hypocotyl. When hypocotyl explants were cultured on CIM supplemented with 50 μM AzaC for 1 week, there were no inhibitory or promoting effects on shoot formation after transferring onto SIM (Fig. 2b, opened circles). In the absence of AzaC, culturing explants on CIM for 2 weeks resulted in the reduction of the frequency of shoot regeneration (Fig. 1b, black triangles). However, when hypocotyls had been cultured on CIM supplemented with 50 μM AzaC for 2 weeks, adventitious shoots were formed on almost all the hypocotyls on SIM until day 35 (Fig. 2b, black triangles). There were no indications of shoot regeneration on the explants that had been cultured on CIM for 3 weeks (Fig. 1a). However, green spots appeared on the explants cultured on CIM supplemented with AzaC for 3 weeks and then transferred onto SIM (Fig. 2a). When hypocotyl explants were cultured on CIM containing AzaC for 3 weeks with renewing the medium every week, they formed adventitious shoots at high frequency (30/30 hypocotyls, data not shown) after transferring onto SIM. In contrast, shoots were not regenerated from the explants that had been cultured on CIM without AzaC for 3 weeks with renewal of the medium every week (0/30 hypocotyls, data not shown). These results indicate that AzaC can avoid reduction of competence to regenerate shoots even the explants are cultured on CIM for 3 weeks. From the histological study of cultured hypocotyls, the calli cultured on CIM for 14 days were larger than those cultured for 7 days (Fig. 3). Toluidine blue stains cytoplasm and nuclei of cells blue and does not stain vacuoles. The proliferated cells in the central region of the calli cultured on CIM for 14 days were not stained blue, it means the tissue contain more vacuolated cells. The calli cultured on CIM with AzaC for 14 days were large and contained fewer vacuolated cells than the calli cultured on CIM without AzaC for the same duration. They were similar to the tissue cultured on CIM without AzaC for 7 days.
Effect of application of azacitidine to CIM on shoot regeneration from hypocotyl. Hypocotyls were excised from seedlings of Arabidopsis and cultured for 1, 2 and 3 weeks on CIM supplemented with AzaC (CIM1w + AzaC, CIM2w + AzaC, CIM3w + AzaC, respectively). These explants were transferred onto SIM without AzaC and cultured for 35 days. a Photo images of calli cultured on SIM. Bars 5 mm. b Percentage of shoot regeneration on calli. Bars standard deviations (n = 5)
Shoots can regenerate not only from hypocotyls segments but also from root explants in Arabidopsis (Valvekens et al. 1988). We examined the effect of AzaC on shoot regeneration from roots (Fig. 4). In the absence of AzaC, adventitious shoots formed on almost all the root explants on SIM after pre-culturing on CIM for 1 or 2 weeks. Culture on CIM for 3 weeks resulted in the reduction of the frequency of shoot regeneration. Shoots were formed on less than 20% of hypocotyls. There were no differences in the frequency of shoot regeneration between with or without AzaC in CIM. This suggests that AzaC cannot recover the loss of competence to regenerate shoots from root explants. The time course of shoot regeneration from root explants precultured on CIM was similar to that from hypocotyl explants on CIM with AzaC (Figs. 2, 4).
Effects of the duration of culture on CIM and of application of azacytidine to CIM on shoot regeneration from root. Roots were excised from seedlings of Arabidopsis and cultured for 1, 2 and 3 weeks on CIM supplemented with (opened circles) or without AzaC (black triangles). These calli were transferred onto SIM without AzaC and cultured for 35 days. a Shoot regeneration on root calli cultured on CIM for a week. b Shoot regeneration on root calli cultured on CIM for 2 weeks. c Shoot regeneration on root calli cultured on CIM for 3 weeks. Bars standard deviations (n = 3)
DNA methylation during culture on CIM
The rate of mC and total cytosine was determined by HPLC analysis of hydrolyzed DNA from explants (Fig. 5). Before culture on CIM, the percentage of mC in DNA of hypocotyl explants was about 4%. Almost the same level of mC existed in explants cultured on CIM for 7 days. However, the percentage of mC in explants was increased to 7% after culture on CIM for 2 weeks. Supplementation of azacitidine inhibited this increase.
Expression profile of genes involved in formation of shoot meristem
The application of AzaC in CIM could avoid the reduction of the frequency of shoot regeneration on SIM when the explants had been cultured on CIM for a long time. Before shoot regeneration, shoot meristem has to be formed on the explant. To investigate the effects of duration of culture on CIM and application of AzaC in CIM on the formation of shoot meristem, we examined the mRNA level of some of shoot meristem-related genes, i.e. CLV1 (Clark et al. 1997), CLV3 (Fletcher et al. 1999), CUC1 (Takada et al. 2001), ESR1 (Banno et al. 2001), STM (Long et al. 1996) and WUS (Mayer et al. 1998) in hypocotyl explants cultured on CIM and after being transferred on SIM by RT–PCR (Fig. 6). In CIM, mRNA level of all of these genes were highest in the explants cultured on CIM for 2 weeks. Among these genes, the expression patterns of CUC1, CLV1 and STM were similar. CUC1, CLV1 and STM mRNA levels were higher in explants of 2w CIM than in those of 1w CIM and 2w CIM + AzaC. Moreover, CUC1, CLV1 and STM mRNA levels were up-regulated when transfered onto SIM after culture on CIM for 1 week. However, in the explants of 2w CIM and 2w CIM + AzaC, CUC1, CLV1 and STM mRNA levels were not changed when transfered onto SIM. Their mRNA level in SIM was lowest in 2w CIM + AzaC compared with the others. On the other hand, the expression patterns of CLV3, ESR1 and WUS were also similar (Fig. 6). CLV3, ESR1 and WUS mRNA levels were higher in explants of 2w CIM than in those of 1w CIM and 2w CIM + AzaC. The mRNA levels of CLV3, ESR1 and WUS were similar in 1w CIM and 2w CIM + AzaC.
Effect of azacitidine on mRNA level of genes involved in shoot meristem formation during shoot regeneration. Hypocotyls were excised from seedling and cultured for 1 or 2 weeks with or without AzaC (1w CIM, 2w CIM, 2w CIM + AzaC, respectively) on CIM, and then transferred on SIM. mRNA level was quantified by RT-PCR and normalized by compairing with the level of GAPDH gene. Black column the expression level of each gene in explants at the end of CIM culture. Grey column the expression level of each gene in explants cultured on SIM for 1 week. Bars standard deviations (n = 3)
Discussion
Excessed culture on CIM results in the decline of shoot regeneration from hypocotyls
The optimum duration of culture on CIM for shoot regeneration is 2–8 days when using hypocotyl explants (Akama et al. 1992). In the present study, we examined the effects of prolonged culture on CIM on shoot regeneration on SIM. We found that excessive duration of culture on CIM resulted in the decline of shoot regeneration on SIM (Fig. 1). There were no shoots on the hypocotyl explants that had been cultured on CIM for 3 weeks. The mass of callus formed on the hypocotyls was much larger in CIM3w culture than in CIM1w, depending on the duration of culture on CIM. However, the frequency of shoot regeneration was inversely proportional to the period of incubation on CIM. From our histological study, the tissue of explants that was cultured on CIM for 14 days contained many vacuolated cells (Fig. 3). These show that the cells in the explants lost their competence to form adventitious shoots during prolonged culture on CIM, though they still had the ability to proliferate. We suppose that the decline of shoot regeneration by prolonged culture on CIM does not result from the inhibition of cell division. The reduction of the frequency of shoot regeneration was not a result of the exhaustion of nutrition in the medium, because shoots were not formed on the hypocotyl explants that had been cultured on CIM for 3 weeks with renewal of the medium every week (data not shown). Some physiological changes might occur in the cells of explants during long durations of culture on CIM via genetic events or epigenetic modifications.
5′-Azacytidine can rescue the reduction of competence to regenerate shoots
Though excessive duration of culture on CIM decreased the frequency in forming adventitious shoots on hypocotyl explants, supplementation of a hypomethylating drug AzaC in CIM resulted in the partial recovery of the frequency of shoot formation on SIM (Fig. 2). This effect was clearer when the medium containing AzaC was renewed every week (data not shown). mC in DNA increased when the hypocotyls explants were cultured on CIM for 2 weeks and the supplementations of AzaC inhibited methylation of genomic DNA (Fig. 5). These may indirectly suggest the involvement of DNA methylation in the loss of the competence to regenerate shoots. Ruiz-Garcia et al. (2005) reported that the level of DNA methylation increased during development of Arabidopsis. Recently, Baranek et al. (2010) observed changes in the methylation pattern of DNA in grapevine somaclones after in vitro culture. The change in DNA methylation in Protocorm-like bodys of Cymbidium was reported by Chen et al. (2009). These might suggest that DNA methylation level in plants change during in vitro culture in general. AzaC is an inhibitor of DNA methylation, and it can awake silenced genes. Chang and Pikaard (2005) showed that AzaC could affect pattern of DNA methylation and expression pattern of genes in Arabidopsis. AzaC could contribute to prevent the reduction of the competence to form adventitious shoots from hypocotyl when they are cultured on CIM for long durations by inhibiting DNA methylation and gene silencing. We expected that application of AzaC kept awake the genes involved in regeneration of shoots. From the histological study, prolonged culture of hypocotyl explants on CIM resulted in the increase of mass of the callus and of vacuolated cells (Fig. 3). AzaC seemed to inhibit proliferation or vacuolation of cells in the explants cultured on CIM for a long time. In general, meristematic cells contain few vacuoles, but developed cells are occupied by large vacuoles. These cytological change might be important for competence of shoot regeneration.
Excessive culture on CIM also resulted in the inhibition of shoot regeneration from root explants, as hypocotyls. However, the reduction of the frequency of shoot regeneration from root was not recovered by supplementation of AzaC in CIM (Fig. 4). This might reflect the difference of cell state between hypocotyls and roots. Preculture with bromodeoxyuridine, an analog of thymidine, in CIM can inhibit shoot regeneration from hypocotyl explants but not from roots on SIM (Sugiyama and Imamura 2006). The methylation of DNA occurring in root explants might be irreversible and not be recovered by AzaC.
AzaC affects the expression of shoot meristem genes
Preceding regeneration of shoots on an explant, it is indispensable to reconstruct shoot meristems on it. To investigate the effects of duration of culture on CIM and application of AzaC in CIM on the formation of shoot meristem, we examined the mRNA expression profiles of some shoot meristem-related genes. From the results, we found that the expression patterns of these genes were perturbed by prolonged culture on CIM. These genes can be divided into two groups according to their pattern of expression. One consisted of CUC1, CLV1 and STM, and the other was CLV3, ESR and WUS. The mRNA levels of CLV3, ESR and WUS were similar in 1w CIM and 2w CIM + AzaC, and lower than in 2w CIM, even after being transferred onto SIM (Fig. 6). ESR1 encodes an AP/EREBP transcription factor, and is involved in shoot regeneration via cytokinin signaling (Banno et al. 2001; Kirch et al. 2003). WUS is a homeobox gene involved in the production of stem cells in shoot meristem (Mayer et al. 1998). Enhanced expression of WUS has been shown to promote shoot regeneration or somatic embryogenesis in some species (Zuo et al. 2002; Che et al. 2007; Arroyo-Herrera et al. 2008; Solis-Ramos et al. 2009). In the explants cultured on CIM for 2 weeks, mRNA levels of ESR1 and WUS were higher than the other conditions. These should lead to enhancement of shoot regeneration. However, the frequency of shoot regeneration was low in this condition. CLV3 encodes a small secretory peptide which is a negative regulator of meristem size (Fletcher et al. 1999). CLV3 mRNA level was also up-regulated by 2 weeks of culture on CIM. It may not reflect the inhibition of enlargement of the meristem size but the increased number of them. STM is one of KNOX homeobox genes that are involved in the formation and maintenance of shoot meristem (Long et al. 1996). CUC1 encodes a NAC domain transcription factor involved in the shoot formation via regulation of the STM gene (Aida et al. 1999; Takada et al. 2001). CLV1 encodes a receptor kinase which can act as the receptor of CLV3 peptide (Clark et al. 1997). The expression levels of CUC1, CLV1 and STM was higher in the explants of 2w CIM than in those of 2w CIM + AzaC, either on CIM and SIM (Fig. 6). This might suggest that meristem formation itself was not inhibited when the explants were cultured on CIM for 2 weeks. Enhanced expression of these genes in the explants might cause the increase of meristem number when they are cultured on CIM for a long time.
Comparing the explants cultured on CIM for 1 and 2 weeks, prolonged duration of culture on CIM resulted in the reduction of shoot regeneration and in the increase of methyl cytosine (Fig. 5). However, the expression level of meristem-related genes was higher in 2w CIM than in 1w CIM and 2w CIM + AzaC. DNA methylation causes the inhibition of transcription, in general. This may suggest that these genes are not the direct target of methylation that causes inhibition of shoot regeneration during long culture on CIM. Culture on CIM for a long duration may inhibit rediferentiation such as formation of leaves and stems. The target of methylation may be the genes involved in this step.
In this study, we found that the long duration of culture on CIM reduced the competence to regenerate shoots, and it could be recovered by application of AzaC into CIM. The application of AzaC may be useful for conserving the competence for regeneration. Excessive culture on CIM caused the changes in expression pattern of genes involved in the formation of shoot meristem. Supplementation of AzaC in CIM partially normalized the expression pattern of these genes. These results indirectly suggested the involvement of DNA methylation in obliteration of competence of shoot regeneration during prolonged culture on CIM. Though we have revealed the change of DNA methylation level during CIM culture, we have not yet confirmed the certain target genes. We are now investigating the direct targets of DNA methylation.
References
Aida M, Ishida T, Tasaka M (1999) Shoot apical meristem and cotyledon formation during Arabidopsis embryogenesis: interaction among the CUP-SHAPED COTYLEDON and SHOOT MERISTEMLESS genes. Development 126:1563–1570
Akama K, Shiraish H, Ohta S, Nakamura K, Okada K, Shimura Y (1992) Efficient transformation of Arabidopsis thaliana: comparison of the efficiencies with various organs, plant ecotypes and Agrobacterium strains. Plant Cell Rep 12:7–11
Arroyo-Herrera A, Gonzalez AK, Moo RC, Quiroz-Figueroa FR, Loyola-Vargas VM, Rodriguez-Zapata LC, Dhondt CB, Suarez-Solis VM, Castano E (2008) Expression of WUSCHEL in Coffea canephora causes ectopic morphogenesis and increases somatic embryogenesis. Plant Cell Tissue Organ Cult 94:171–180
Banno H, Ikeda Y, Niu QW, Chua NH (2001) Overexpression of Arabidopsis ESR1 induces initiation of shoot regeneration. Plant Cell 13:2609–2618
Baranek M, Krizan B, Ondrusikova E, Pidra M (2010) DNA-methylation changes in grapevine somaclones following in vitro culture and thermotherapy. Plant Cell Tissue Organ Cult 101:11–22
Burn JE, Bagnall DJ, Metzger JD, Dennis ES, Peacock WJ (1993) DNA methylation, vernalization, and the initiation of flowering. Proc Natl Acad Sci USA 90:287–291
Chang S, Pikaard CS (2005) Transcript profiling in Arabidopsis reveals complex responses to global inhibition of DNA methylation and histone deacetylation. J Biol Chem 280:796–804
Che P, Lall S, Howell SH (2007) Developmental steps in acquiring competence for shoot development in Arabidopsis tissue culture. Planta 226:1183–1194
Chen X, Ma Y, Chen F, Song W, Zhang L (2009) Analysis of DNA methylation patterns of PLBs derived from Cymbidium hybridium baced on MSAP. Plant Cell Tissue Organ Cult 98:67–77
Clark SE, Williams RW, Meyerowitz EM (1997) The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell 89:575–585
Demeulemeester MAC, Van Stallen N, De Proft MP (1999) Degree of DNA methylation in chicory (Cichorium intybus L.): influence of plant age and vernalization. Plant Sci 142:101–108
Fletcher JC, Brand U, Running MP, Simon R, Meyerowitz EM (1999) Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science 283:1911–1914
Gamborg OLR, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Hicks GS (1994) Shoot induction and organogenesis in vitro: a developmental perspective. In Vitro Cell Dev Biol 30:10–15
Kakutani T (1997) Genetic characterization of late-flowering traits induced by DNA hypomethylation mutation in Arabidopsis thaliana. Plant J 12:1447–1451
Kirch T, Simon R, Grünewald M, Werr W (2003) The DORNROSCHEN/ENHANCER OF SHOOT REGENERATION1 gene of Arabidopsis acts in the control of meristem ccll fate and lateral organ development. Plant Cell 15:694–705
Long JA, Moan EI, Medford JI, Barton MK (1996) A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 379:66–69
Mayer KF, Schoof H, Haecker A, Lenhard M, Jurgens G, Laux T (1998) Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95:805–815
Miura A, Yonebayashi S, Watanabe K, Toyama T, Shimada H, Kakutani T (2001) Mobilization of transposons by a mutation abolishing full DNA methylation in Arabidopsis. Nature 411:212–214
Murashige T, Nakano R (1967) Chromosome complement as a determinant of the morphogenic potential of tobacco cells. Amr J Bot 54:963–970
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Ozeki Y, Matsui K, Sakuta M, Matsuoka M, Ohashi Y, Kano-Murakami Y, Yamamoto N, Tanaka Y (1990) Differential regulation of phenylalanine ammonia-lyase genes during anthocyanin synthesis and by transfer effect in carrot suspension cultures. Physiol Plant 80:379–387
Preil M (2003) Micropropagation of ornamental plants. In: Laimer M, Rucker W (eds) Plant tissue culture 100 years since Gottlieb Haberlandt. Springer, New York, pp 115–133
Ruiz-García L, Cervera MT, Martínez-Zapater JM (2005) DNA methylation increases throughout Arabidopsis development. Planta 222:301–306
Skoog F, Miller CO (1957) Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol 54:118–130
Solis-Ramos LY, Gonzalez-Estrada T, Nahuath-Dzib S, Zapata-Rodriguez LC, Castano E (2009) Overexpression of WUSCHEL in C. chinese causes ectopic morphogenesis. Plant Cell Tissue Organ Cult 96:279–287
Sugiyama M (1999) Organogenesis in vitro. Curr Opin Plant Biol 2:61–64
Sugiyama M, Imamura K (2006) Dose-, time-, and tissue-dependent effects of 5-bromo-2′-deoxyuridine on the in vitro organogenesis of Arabidopsis thaliana. Plant Cell Tissue Organ Cult 87:17–25
Takada S, Hibara K, Ishida T, Tasaka M (2001) The CUP-SHAPED COTYLEDON1 gene of Arabidopsis regulates shoot apical meristem formation. Development 128:1127–1135
Tzfira T, Citovsky V (2006) Agrobacterium-mediated genetic transformation of plants: biology and biotechnology. Curr Opin Biotechnol 17:147–154
Valvekens D, Montagu MV, Lijsebettens MV (1988) Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc Natl Acad Sci USA 85:5536–5540
Zhao XY, Su YH, Cheng ZJ, Zhang ZS (2008) Cell fate switch during in vitro plant organogenesis. J Integr Plant Biol 50:816–824
Zuo JR, Niu QW, Frugis G, Chua NH (2002) The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. Plant J 30:349–359
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tokuji, Y., Takano, S., Tonomura, M. et al. Influence of 5′-azacitidine on promoting recovery of cell competence for shoot organogenesis in Arabidopsis . Plant Cell Tiss Organ Cult 106, 289–297 (2011). https://doi.org/10.1007/s11240-011-9920-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-011-9920-z