Abstract
Somatic embryogenesis in Crambe abyssinica, an important industrial oilseed species, was investigated. Cotyledon, hypocotyl and root explants from 8-day-old seedlings of C. abyssinica cv. Prophet were cultured with levels of 1-naphthaleneacetic acid (NAA) and 2,4-dichlorophenoxyacetic acid (2,4-D) ranging from 2.2 to 39.0 μM, combined with 6-benzyladenine (BA) to achieve an auxin:cytokinin ratio of 20:1, and callus formation assessed. Callus formation frequency for cotyledon and hypocotyl explants was 100% for levels of 2,4-D from 4.5 to 33.9 μM. The response was similar with NAA levels of 13.0 to 39.0 μM. Root explants were less responsive. When calluses were transferred to a medium containing 0.56 μM each of thidiazuron and BA with 1.0 μM indole-3-butyric acid (IBA), somatic embryos were induced. Moreover, embryos were induced from calluses grown on media containing either 11.3 μM 2,4-D or 13.0 μM NAA, or higher. On a medium without plant growth regulators, embryos were induced but at a much lower frequency. For all three explants, 22.6 μM 2,4-D and 26.0 μM NAA was optimal for embryogenic callus induction. Hypocotyl-derived calluses were superior to cotyledon- and root-derived calluses for embryo induction. The best embryo formation response was with medium containing 5.0–6.0% sucrose. The highest average number of embryos per callus (36) was obtained from hypocotyl calluses from medium with 22.6 μM 2,4-D. Somatic embryos germinated best on half-strength B5 or MS medium with 3% sucrose, and plantlets were successfully established under greenhouse conditions. The results indicate that high levels of auxins are required for the induction of embryogenic calluses from explants of C. abyssinicia, while cytokinins are critical for somatic embryo formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crambe abyssinica is the most economically important (Mulder and Mastebroek 1996; Wang et al. 2000) of the c.34 species of the genus Crambe, a member of the Brassicaceae (Prina and Martinez-Laborde 2008). Its seeds contain up to 35% oil, with erucic acid accounting for 55–60% of the fatty acids (Mulder and Mastebroek 1996). This is a highly valuable product with industrial and pharmaceutical applications in the manufacture of coatings, detergents, slip agents, cosmetics, lubricants and nylon (Cooke and Konstant 1991; Capelle and Tittonel 1999; Bondioli et al. 1998; Vargas-Lopez et al. 1999). In addition, the meal from the de-hulled C. abyssinica seed contains 45–50% protein, and is considered of value for animal feed and protein recovery (Carlson et al. 1996; Carlson and Tookey 1983; Massoura et al. 1998). Although C. abyssinica is widely grown for its seed oil, it lacks sufficient genetic variation for important agronomic traits and this has hampered improvement through conventional plant breeding (Mulder and Mastebroek 1996; Mastebroek and Lange 1997; Warwick and Gugel 2003). Attempts have been made to incorporate agronomically useful traits from C. hispanica into C. abyssinica by interspecific hybridization (Mulder and Mastebroek 1996). However, as has been demonstrated with Brassica species (Cardoza and Stewart 2004), the combination of tissue culture with genetic transformation techniques could be a valuable complement to conventional plant breeding for improvement of C. abyssinica. A key requirement for the application of biotechnology to crop improvement is development of a tissue culture system to facilitate plant regeneration from cultured cell and tissues. In spite of the importance of C. abyssinica as an oilseed crop, there are only a few reports of plant regeneration from cultured cells and tissues. Gao et al. 1998 reported a plant regeneration frequency of 45% from single cell cultures of C. abyssinica. In addition, haploid embryos were recovered from isolated microspore culture (Ferrie and Keller 2007). Transformation studies with hypocotyl explants of C. abyssinica cv. Galactica resulted in the recovery of transformed plants by adventitious shoot regeneration (Li et al. 2009), but the reported shoot regeneration frequency was quite variable with an average of 43%. There have been no published reports of somatic embryogenesis in C. abyssinica. Plant regeneration by somatic embryogenesis is generally preferred to regeneration by shoot formation as such embryos usually germinate into seedlings without the need for an additional rooting phase. The objective of the present study was to evaluate the potential of seedling explants of C. abyssinica cv. Prophet for somatic embryo formation and plant regeneration.
Materials and methods
Plant material
Seeds of Crambe abyssinica, cv. Prophet were obtained from the Agriculture and Agri-Food Canada (AAFC) research center in Saskatoon, Canada. They were washed for 10 min with distilled water containing a few drops of Tween 20, followed by treatment with a solution of 1.0 mg l−1 each of streptomycin sulfate and penicillin G for 30 min. Seeds were then immersed in 70% ethanol for 1 min, rinsed with distilled water and immersed in 25% commercial Javex (active ingredient 5.25% sodium hypochlorite) for 25 min. After being washed four times with sterilized distilled water, the seeds were placed on sterile paper towels and maintained under sterile conditions, and then germinated to provide seedling explants.
Germination medium
Half-strength Murashige and Skoog (MS) (Murashige and Skoog 1962) basal medium with 1.5% sucrose but without plant growth regulators (PGRs) was used for seed germination. The medium was adjusted to pH 5.8 with NaOH or HCl and 0.8% w/v agar added before autoclaving at 121°C for 25 min. The medium was dispensed in 100 × 25 mm Petri dishes and allowed to solidify, after which 8–10 seeds distributed on each dish. Dishes were sealed with strips of Saran Wrap and incubated at 24°C in darkness for 6 days. They were then transferred to a photoperiod of 16 h and light intensity of 40 μE m−2 s−1 at the same temperature for 1 or 2 days before use.
Explanting
Seedlings were transferred to sterile Petri dishes and root and cotyledons carefully removed with a scalpel. Most of the petiole was included with the cotyledon. The apical and basal portions (approximately 2 mm) of the hypocotyl were discarded and the remainder cut into 5–7 mm transverse segments. The upper 2 mm of the root, at the junction of the hypocotyls, was discarded and the rest of the root cut into 2–3 cm sections and placed on the culture medium. Where lateral roots occurred these were not removed from the explant.
Callus induction medium
MS basal medium with B5 vitamins (Gamborg et al. 1968) was supplemented with 250 mg 1−1 l-proline and 500 mg l−1 casein hydrolyaste and the sucrose concentration was increased to 5%. For PGRs, five different concentrations of 2,4-dichlorophenoxyacetic acid (2,4-D) ranging from 2.2 to 33.9 μM and 1-naphthalene acetic acid (NAA) concentrations ranging from 2.6 to 39 μM were added separately to the medium. In addition, either thidiazuron (TDZ) or benzyladenine (BA) was added at the appropriate level, to achieve an auxin to cytokinin ratio of 20:1. All PGRs were added to the medium before autoclaving. The pH of the medium was adjusted to 5.8 with either NaOH or HCl and 0. 8% agar w/v added. After autoclaving at 121°C for 25 min, the medium was dispensed in sterile 100 × 25 mm Petri plates. Explants were distributed on the medium and the dishes sealed with strips of Saran Wrap. Each culture dish contained 15–20 hypocotyl segments, 10–12 cotyledons or 10 root segments. Cotyledonary petioles were not embedded in the medium; instead, the abaxial surface of the cotyledon was placed in contact with the medium. The cultures were incubated at 24°C and a photoperiod of 16 h at an intensity of 50 μE m−2 s−1. Callus development was monitored over a period of 21 days and the percentage of explants with callus recorded. Explants were then transferred to fresh medium of the same composition and maintained under the same conditions. After a further 1–2 weeks, the frequency of callus formation was again recorded.
Embryogenic callus determination
To evaluate the embryogenic potential of the callus, all explants with callus (28 days old) were transferred for embryo induction to B5 medium (Gamborg et al. 1968) with 5% sucrose and l-proline and casein hydrolysate as described above for callus induction. In addition, 0.5 μM each of TDZ and BA and 1.0 μM indole-3-butyric acid (IBA) were added. The medium was prepared as described above and the cultures maintained under the same conditions used for callus induction. The number of explants with embryos and the number of embryos per explant were recorded at intervals of 7 days. Occasionally, some cultures tended to turn brown and had to be transferred to fresh medium; otherwise, the callus was not sub-cultured. The final embryo count and frequency was recorded after 28 days. Only embryos where the cotyledons could be seen with a low power stereomicroscope were counted. To determine whether the somatic embryos were capable of synthesizing erucic acid, fatty acid analysis was conducted on embryos and callus by gas chromatography as described by Katavic et al. (2001).
Effect of sucrose levels on the frequency of embryogenic calluses
For this experiment, the culture medium used for embryogenic callus determination was modified to include sucrose levels of 3.0, 6.0 or 10.0%. The medium was inoculated with 28-day-old calluses from explants cultured on 26.0 μM NAA or 22.6 μM 2,4-D. The cultures were maintained under the same callus induction conditions described above. The number of calluses with embryos was recorded after 28–30 days.
Effect of BA levels on the frequency of embryogenic calluses
For this experiment, levels of BA ranging from 0.0 to 4.0 μM were incorporated into the embryo induction medium and both IBA and TDZ were omitted. Calluses originating from media containing 26.0 μM NAA or 22.6 μM 2,4-D were used to inoculate the medium and cultures were maintained under the same conditions used for the determination of embryogenic callus frequency. The percentage of calluses with embryos was assessed after 28–30 days.
Embryo germination
Embryos for this study were derived from calluses of all three explants cultured with 22.6 μM 2,4-D and 26.0 μM NAA treatments. Twenty-eight-day-old embryos were pooled and used for germination studies. No attempt was made to identify the explant origin or the auxin treatment (NAA or 2,4-D) from which embryos were derived. The culture medium was full- or half-strength B5 or MS medium, without PGRs but with 3.0% sucrose, and solidified with 0.8% w/v agar. The pH of the medium was adjusted to 5.7, sterilized and dispensed in 100 × 25 mm Petri plates. Ten embryos were placed on each plate, which was sealed with Saran Wrap and incubated at 24°C and a photoperiod of 16 h at light intensity of 50 μE m−2 s−1. After 21–28 days, the percentage germination was recorded and rooted seedlings transferred to Magenta boxes containing the same medium and incubated under the same conditions, for further development. Germination was assessed as embryos with developed root and shoot axes; where there was only shoot or root, this was not regarded as germination. The survival rate was determined after 20 days and rooted plantlets potted in 6-inch pots containing Sunshine Mix #3 to which 5.0 g of Nutricote controlled release fertilizer (13:13:13, Sun Gro Horticulture, Canada) had been added. Plantlets were covered with inverted Magenta boxes for 4 days, to maintain high relative humidity, and kept under greenhouse conditions with daylight supplemented with high-pressure sodium halide bulbs. The greenhouse temperature varied between 24 and 27°C. After an additional 10 days, the survival rate was determined and plants were allowed to mature and set seeds.
Unless otherwise indicated, there were three replicates of all experiments and each experiment was performed three times. Except for the germination and survival rate of embryos, all data were analyzed by one-way analysis of variance and the means compared by Tukey’s test.
Results
Callus development
Auxins are critical for the induction of somatic embryos (Jimenez 2005; Raghavan 2004). In this study, we investigated the influence of NAA and 2,4-D on the induction of embryogenic calluses. Initial experiments were designed to assess callus formation in response to auxin treatment. Exposure of dark-germinated seedlings to light before explanting minimized tissue damage during explanting but had no effect on callus formation. At all levels of both NAA and 2,4-D, the callus formation frequency of root explants was lower than that of hypocotyl and cotyledon explants (Table 1). Concentrations of NAA and 2,4-D ranging from 11.3 to 39.0 μM resulted in callus formation frequency of 100% for hypocotyl and cotyledon explants, but not for the root explants (Table 1). Compared to 5.2 μM NAA, 4.5 μM 2,4-D induced a significantly higher frequency of calluses on hypocotyl and cotyledon explants (Table 1). However, with root explants both auxins appeared equally effective in inducing calluses, regardless of concentration. No callus was observed on any of the explants in control cultures without PGRs. In addition, TDZ and BA levels (0.05–1.7 μM), which were used to achieve an auxin to cytokinin ratio of 20:1, did not by themselves induce callus formation on any of the explants (results not shown). Furthermore, there was no difference in auxin-induced callus formation when either TDZ or BA was used as the cytokinin. While the addition of both l-proline and casein hydrolysate had a beneficial effect on callus formation by reducing browning (data not shown), they could be omitted from the culture medium without affecting the frequency of callus formation.
Calluses developed more slowly on root explants compared to the other explants. At high levels of auxin (22.6–39.0 μM), all explants had a brownish appearance with healthy callus development from the cut surfaces of explants. This callus was also more nodular in comparison to that from explants on auxin levels of 2.2–5.2 μM. Callus growth was not quantified but visual observation indicated a reduction in growth at auxin levels of 22.6–39.0 μM compared to lower levels. At levels of 2.6 and 5.2 μM NAA, root initiation occurred on some explants but this was seldom observed with similar levels of 2,4-D.
Embryogenic callus determination
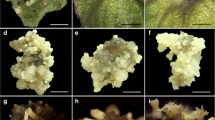
Although calluses which developed in the presence of high auxin concentrations had a distinctly nodular appearance, no embryos were observed up to 35 days in culture. Figure 1a and b shows 28-day-old calluses on hypocotyl and cotyledonary petiole explants, respectively. When calluses from these auxin treatments were transferred to a medium for embryo formation, calluses from low auxin treatments continued to produce nodular structures but no embryos were evident up to 28 days (Table 2). However, calluses from higher auxin treatments (11.3–39.0 μM) initiated embryos at frequencies ranging from 7.4 to 42.2% in the case of NAA, and 10.3–47.3% for 2,4-D (Table 2). For both auxins, the hypocotyl had the highest frequency of embryogenic callus induction, while the root callus was the lowest. Embryos appeared as greenish structures on the surface of the callus and some had normal cotyledons. Figure 1d and e shows somatic embryos from hypocotyl callus, while Fig. 1c shows globular structures on hypocotyl callus derived from a culture medium containing 11.3 μM 2,4-D. Somatic embryos were also induced on a medium without PGRs, but at a much lower frequency (5.5–8.7%). Some embryos were normal with shoot and root axes (Fig. 1d), but some lacked root axis and had multiple cotyledons (Fig. 1f).
Somatic embryo development in Crambe abyssinica. Twenty-eight-day-old callus from hypocotyl (a) and cotyledonary petiole (b) respectively, cultured on 5.0 mg l−1 NAA. c Globular embryos from hypocotyl explant callus cultured on embryo induction medium. Cotyledonary embryos with the arrow pointing to the root axes (d); cluster of embryos from hypocotyl callus (e); embryos with undeveloped root axes (f); multiple shoot and root formation from embryos on half-strength B5 medium (g)
Embryo production
Embryo number per callus was a function of both the type and concentration of auxin in the callus formation medium. Calluses derived from cultures containing 22.6 μM 2,4-D and 26.0 μM NAA had a significantly higher average number of embryos per callus compared to the other auxin levels (Table 3). The highest average number of embryos (36) was from hypocotyl calluses formed on a medium with 22.6 μM 2,4-D, whereas with calluses from 26.0 μM NAA the average was 23.7. While there was little difference in the average number of embryos produced on calluses derived from 13.0 to 30.0 μM NAA for all three explants, with 11.3 μM 2,4-D the average embryo number for hypocotyl callus was much higher (22.4), compared to cotyledon (9.7) and root (8.4) callus. With some embryos there was no distinct root axis and some were joined together (Fig. 1e).
Effect of sucrose levels on embryo formation
Sucrose concentration influenced the embryogenic response of the callus. With 3.0% sucrose in the embryo formation medium, the percentage of calluses with embryos ranged from 2.7 for NAA to 7.5% 2,4-D-derived callus (Table 4). With 6.0% sucrose in the medium, there was an approximately five to sevenfold increase in the frequency of embryogenic callus formation, depending on the type of explant and type of auxin from which the callus was derived (Table 4). Among explants, the highest embryo induction frequencies (47.2 and 38.1%) were recorded for 2,4-D- and NAA-derived hypocotyl callus, respectively. Compared to 6.0% sucrose, 10.0% sucrose significantly reduced the frequency of embryo induction, which ranged from 5.3 to 17.2% across explants (Table 4). In addition, cotyledon calluses from both NAA and 2,4-D treatments had a higher embryo induction frequency compared to the other explants. Only calluses derived from 22.6 μM 2,4-D and 26.0 μM NAA were used in this study.
Effect of BA on embryo formation frequency
On a medium without BA, 2,4-D-derived root and hypocotyl calluses initiated embryo at frequencies of 5.8 and 11.2%, respectively, compared to frequencies of 5.9 and 9.7% for NAA-derived callus from these two explants (Table 5). Cotyledon-derived calluses from both auxin treatments had approximately the same response. With 0.8 μM BA in the culture medium, there was a significant increase in embryo formation frequency for callus of all explant types when compared with the control. Hypocotyl callus was more responsive than callus from the other explants. The frequency of embryo formation in response to 0.8 μM BA was higher (28.4%) for 2,4-D-derived cotyledon callus than for NAA-derived cotyledon callus (21.7%) (Table 5). This was also the case for hypocotyl callus but not for root callus, where the response was similar for both auxin treatments. At higher BA levels (2.0 and 4.0 μM) there was a significant reduction in the frequency of embryo formation for calluses from both auxin treatments. In cultures with 4.0 μM BA there was a tendency towards development of nodular structures on the callus but these did not produce embryos or shoots. With similar levels of TDZ, the embryo formation frequency response was approximately the same (data not shown).
Embryo germination and plant recovery
Not all the embryos were defined bipolar structures (Fig. 1d, e); some were joined at the root axis while others lacked a defined root pole (Fig. 1f). The presence of erucic acid in tissues is regarded as a marker for embryonic tissues of Brassica (De la Roche and Keller 1977), and therefore somatic embryos and non-embryogenic tissues were analyzed qualitatively for the presence of erucic acid. Significant levels of erucic acid were found in the somatic embryos but not in the non-embryogenic calluses. Thus, regardless of the heterogeneity of the embryo population, they appear to be functional embryos. When 28-day-old embryos were cultured on hormone-free full strength MS or B5 medium with 3.0% sucrose for 21 days, the germination frequency was 33 and 42.3% for MS and B5 media, respectively (Table 6). When these plantlets were transferred to the same medium contained in Magenta boxes for a further 20 days, only 10 and 17% of the original embryos on MS and B5 medium, respectively, were recovered as plantlets with root and shoot axes. Many turned brown and developed roots but not shoots. Embryos cultured on hormone-free half-strength B5 and half-strength MS medium with 3.0% sucrose germinated at rates of 67.5 and 45%, respectively (Table 6), and plantlet survival rate after 20 days on the same media in Magenta boxes was 43.9 and 24.3%, respectively. Plantlet elongation was better in media of half strength compared to full strength. Figure 2a shows a seedling germinated on half-strength B5 medium for 21 days while Fig. 1g shows clusters of plantlets on the same medium. When plantlets were allowed to develop further on the same media in Magenta boxes, plant development was better on half-strength B5 compared to half-strength MS or full-strength MS and B5. Figure 2b shows a representative cluster of plantlets cultured on half-strength B5 medium in Magenta boxes for 20 days. Plantlets were transferred to soil and established under greenhouse conditions, where plant recovery was approximately 70% (data not shown). The germination medium, MS or B5, had no apparent effect on this process. Plants were fertile and set seeds (Fig. 2c).
Discussion
The literature on somatic embryogenesis in plant cells is extensive, and this method of plant regeneration is viewed as preferable to organogenesis, as somatic embryos have fully developed root and shoot axes and can germinate into seedlings (Namasivayam 2007; Feher et al. 2002, 2003). Somatic embryos can either be formed directly from cells of cultured tissues, or be induced indirectly from cells arising from calluses (Williams and Maheswaran 1986). While embryogenic competence can be induced, the process is under genetic control (Pinto et al. 2008; Rose and Nolan 2006). Induction of somatic embryos on explants or cultured cells generally requires an auxin, and 2,4-D and NAA are the most often used (Sharma et al. 2007; Rose et al. 1999; Jimenez 2005; Raghavan 2004).
However, somatic embryogenesis can occur in the absence of exogenous growth regulators (Loh and Loh 2000); immature seeds from rapid cycling Brassica responded to low pH with somatic embryo production from seedling hypocotyls. Although calluses could be induced on all explants of C. abyssinica by a wide range of auxin concentrations (Table 1), embryo formation was only observed with 2,4-D and NAA at levels ranging from 11.3 to 39.0 μM (Table 2). The optimum level of auxin appears to be 22.6 μM of 2,4-D and 26.0 μM of NAA in combination with a cytokinin to give an auxin to cytokinin ratio of 20:1. A sucrose level of 5.0% in the callus induction medium was required, as lower levels reduced the formation of embryogenic callus (data not shown). While the addition of casein hydrolysate and l-proline did not affect the frequency of callus formation, they were nevertheless beneficial to embryo formation. Amino acids have been shown to exert beneficial effects on somatic embryo formation and development by providing a ready source of reduced nitrogen (Mohamed et al. 2004; Bela and Shetty 1999). High levels of sucrose may exert an osmotic stress effect which is conducive to the somatic embryo induction process (Gaj 2004; Lou and Kako 1995). In the present study, embryo formation was indirect, as they only appeared after callus formation and required transfer of callus to a medium with lower PGR levels (Table 2). Embryo formation from calluses of C. abyssinica was also a function of sucrose concentration, as both 3.0 and 10.0% sucrose reduced embryo formation frequency, compared to 5 and 6% (Tables 2, 4). With 10.0% sucrose, embryos appeared smaller and the callus tended to senesce more rapidly compared to cultures with lower sucrose. High and low levels of sucrose also reduced somatic embryo formation in carnation petals (Karami et al. 2006).
Calluses induced with 22.6 μM 2,4-D and 26.0 μM NAA produced embryos when cultured on a medium without PGRs (Table 5). However, the addition of low levels of BA enhanced the frequency of embryo formation several-fold (Table 5); a similar response was obtained with TDZ. When IBA alone was used, the frequency of embryo formation was about the same as the control (data not shown). Other auxins were not evaluated but the findings suggest that cytokinins are essential to the embryo formation process. Similar observations were reported for embryo formation of other species (JayaSree et al. 2001; Tejavathi et al. 2007; Rose et al. 1999). Embryogenic callus induction in Musa spp. was positively influenced by TDZ (Perez-Hernandez and Rosell-Garcia 2008). Even though putative embryogenic callus was induced in Blihga sapida, embryo formation was still dependent on TDZ (Webster et al. 2006). However, cytokinins sometimes inhibit the embryo development process (Kaparakis and Alderson 2008). In the present study, reduction of embryo formation frequency at higher levels of BA was observed but these levels appear sub-optimal and not inhibitory, since the response was equal or above the control (Table 5). Relatively high levels of auxin were required for embryogenic callus induction on C. abyssinica explants, as calluses from lower levels were non-embryogenic (Tables 1, 2). The auxin concentration required for induction of embryogenic callus may depend on plant species, since with soybean, levels of 2,4-D exceeding 100 μM were required (Tomlin et al. 2002). Both 2,4-D and NAA appear equally effective for the induction of embryogenic calluses from C. abyssinica explants. We did not observe shoot formation in our study even when calluses were cultured with increased levels of BA or TDZ. However, 3-day-old seedlings of C. abyssinica cv. Galatica regenerated shoots in response to TDZ and NAA (Li et al. 2009). In addition, a shoot regeneration frequency of 45% was reported for single cell cultures of this species (Gao et al. 1998). The difference in explant age, which in our study was 8 days, and culture protocol may account for the failure to observe shoot formation in our experiments. However, in our study, the frequency of embryo formation was as high as 47%, depending on explant and culture conditions (Table 4), comparable to the shoot regeneration frequency reported by Gao et al. (1998).
Embryo number per explant followed a similar pattern to that of embryogenic frequency (Table 3). However, the average number was a function of the type of explant, with hypocotyl callus having the highest and root callus the lowest (Table 3). While the average number varied with experiments, the numbers were approximately the same at the highest levels of NAA and 2,4-D (Table 3). Calluses from hypocotyl explants cultured with 26.0 μM NAA were generally smaller than cotyledon calluses from the same medium (Fig. 1a, b, respectively). In view of the higher number of embryos per hypocotyl callus compared to root and cotyledon callus, it is likely that there were more cells competent for embryogenesis in hypocotyl tissues.
Full-strength MS and B5 media were not effective for embryo germination and survival and resulted in a high mortality rate (Table 6). On these media, some embryos usually turned brown and deteriorated after about 14 days. Addition of IBA (0.5 μM) to the medium did not improve the survival response. However, with half-strength MS and half-strength B5, germination and survival were better (Table 6). It can be concluded that embryos were sensitive to the high levels of salts in these media. Figure 2a shows a seedling germinating on half-strength B5 medium. Multiple shoots were evident in some cultures (Fig. 1g) and this was related to embryos with undeveloped root axis and, perhaps, secondary embryos. There may have been embryo-like structures and embryoids among the somatic embryo population, but the fact that they accumulated erucic acid, which is regarded as a marker for zygotic embryos of Brassica spp. (De la Roche and Keller 1977), indicated that these structures were functional embryos. In addition, erucic acid was not detected in non-embryogenic calluses of C. abyssinica.
No specific treatments, such as low-temperature incubation, use of activated charcoal or polyethylene glycol were used during the embryo maturation phase of C. abyssinica embryos, although such treatment has been shown to improve embryo germination and survival rate in other species (Walker and Parrott; 2001; Andrade and Merkle 2005). In some experiments a large percentage of the plated embryos developed roots while shoots atrophied (data not shown). Establishment of plantlets transferred from Magenta boxes to soil was close to 70%. Fertile plants were recovered. Figure 2c shows a cluster of somatic embryos-derived plants with a number of siliques, while Fig. 2b shows plantlets on half-strength B5 medium for 28 days. The leaf tip browning was generally observed in most cultures but this was not restricted to somatic embryo-derived plantlets, as a similar response was observed with shoot cultures established from seedlings.
Conclusion
In this report we have provided evidence for plant regeneration by somatic embryogenesis from cultured seedling explants of C. abyssinica cv. Prophet. Induction of embryogenic callus was achieved with relatively high levels of auxin combined with low levels of cytokinin. Only NAA and 2,4-D were used, but other types of auxins may prove suitable. All three types of explants formed embryogenic callus, with hypocotyl the most responsive. Embryo formation from callus was enhanced by the addition of low levels of cytokinin. Although seedling explants yielded embryogenic callus, other explants such as immature zygotic embryos may be suitable. In addition, preliminary studies indicated that leaf explants from in vitro shoot cultures of C. abyssinica could be induced to undergo callus formation and somatic embryogenesis in response to 2,4-D and NAA, but compared to seedlings the embryo induction process was more protracted. Explant selection is likely to be a key factor for successful expression of genes involved in various phases of the somatic embryogenesis program (Thakare et al. 2008; Nolan et al. 2003; Braybrook et al. 2006). Since genotype is an important factor for the expression of somatic embryogenesis in culture (Rose et al. 1999; Rose and Nolan 2006) other genotypes of C. abyssinica should be investigated. In terms of plant recovery, conditions for embryo germination and survival remain to be optimized.
Abbreviations
- NAA:
-
Naphthaleneacetic acid
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- BAP:
-
Benzylaminopurine
- TDZ:
-
Thidiazuron
- IBA:
-
Indole-3-butyric acid
- PGR:
-
Plant growth regulator
References
Andrade GM, Merkle SA (2005) Enhancement of American chestnut somatic seedling production. Plant Cell Rep 24:326–334
Bela J, Shetty K (1999) Somatic embryogenesis in anise (Pimpinella anisum L.): the effect of proline on embryogenic callus formation and ABA on advanced embryo development. J Food Biochem 23:17–32
Bondioli P, Folegatti L, Lazzeri L, Palmieri S (1998) Native Crambe abyssinica oil and derivatives as renewable lubricants: an approach to improve its quality by chemical and biotechnological processes. Ind Crops Prod 7:231–238
Braybrook SA, Stone SL, Park S, Bui AQ, Le BH, Fisher RL, Goldberg RB, Harada JJ (2006) Genes directly regulated by LEAFY COTYLEDON2 provide insight into the control of embryo maturation and somatic embryogenesis. Proc Natl Acad Sci USA 103:3468–3473
Capelle A, Tittonel ED (1999) Crambe, a potential non-food oil crop. 1: production. Agro Food Ind High Tech 10:22–27
Carlson KD, Tookey HL (1983) Crambe meal as a protein source for feed. J Am Oil Chem Soc 60:1979–1985
Carlson KD, Gardner JC, Anderson VL, Hanzel JJ (1996) Crambe: new crop success. In: Janick J (ed) Progress in new crops. ASHS, Alexandria, pp 306–322
Cooke L, Konstant DA (1991) What’s new in oilseeds? Check out Crambe 1. Agric Res Serv 39:16–17
De la Roche AI, Keller WA (1977) The morphogenetic control of erucic acid synthesis in Brassica campestris. Z Pflanzenzuecht 78:319–326
Feher A, Pasternak T, Otovos K, Miskilczi P (2002) Induction of embryogenic competence in somatic plant cells: a review. Biologia 57:5–12
Feher A, Pasternak TP, Dudits D (2003) Transition of somatic plant cells to an embryogenic state. Plant Cell Tiss Organ Cult 74:201–228
Ferrie AMR, Keller WA (2007) Optimization of methods for using polyethylene glycol as a non-permeating osmoticum for the induction of microspore embryogenesis in the Brassicaceae. In Vitro Cell Dev Biol Plant 43:348–355
Gaj MD (2004) Factors influencing somatic embryogenesis induction and plant regeneration with particular dereference to Arabidopsis thaliana (L.) Heynh. Plant Growth Regul 43:27–47
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of cultures of soybean root cells. Exp Cell Res 50:151–158
Gao HB, Wang Y, Gao F, Luo P (1998) Studies on the plant regeneration from single cell culture of Crambe abyssinica. Hereditas (Beijing) 20(suppl):50–52
JayaSree T, Pavan U, Ramesh M, Rao AV, Jagan Mohan Reddy K, Sadanandam A (2001) Somatic embryogenesis from leaf cultures of potato. Plant Cell Tiss Organ Cult 64:13–17
Jimenez V (2005) Involvement of plant hormones and growth regulators on in vitro somatic embryogenesis. Plant Growth Regul 47:91–110
Kaparakis G, Alderson PG (2008) Role of cytokinins in somatic embryogenesis of pepper (Capsicum annuum L.)? J Plant Growth Regul 27:110–114
Karami O, Deljou A, Esna-Ashari M, Ostad-Ahmadi P (2006) Effect of sucrose concentrations on somatic embryogenesis in carnation (Dianthus caryophyllus L.). Sci Hortic 110:340–344
Katavic V, Friesen W, Barton BL, Gossen KK, Giblin EM, Luciw T, An J, Zou J-T, MacKenzie SL, Keller WA, Males D, Taylor DC (2001) Improving erucic acid content in rapeseed through biotechnology: what can the Arabidopsis FAE1 and SLC1 genes contribute. Crop Sci 41:739–747
Li X, Ahlman A, Yan X, Lindgren H, Zhu L-H (2009) Genetic transformation of the oilseed crop Ceambe abyssinica. Plant Cell Tiss Organ Cult 100:149–156
Loh WL, Loh CS (2000) Direct somatic embryogenesis, plant regeneration and in vitro flowering in rapid cycling Brassica napus. Plant Cell Rep 19:1177–1183
Lou H, Kako S (1995) Role of sugar concentration in inducing somatic embryogenesis from cucumber cotyledons. Sci Hort 64:11–20
Massoura E, Vereijken JM, Kolster P, Derksen JTP (1998) Proteins from Crambe abyssinica oilseed. 11. Biochemical and functional properties. J Am Oil Chem Soc 75:329–335
Mastebroek HD, Lange W (1997) Progress in a Crambe breeding programme. Ind Crops Prod 6:221–227
Mohamed SV, Wang CS, Thiruvengadam M, Jayabalan N (2004) In vitro plant regeneration via somatic embryogenesis through cell suspension cultures of horsegram [Macrotyloma uniflorum (Lam) Verdc]. In Vitro Cell Dev Biol Plant 40:284–289
Mulder JH, Mastebroek HD (1996) Variation for agronomic characteristics in Crambe hispanica, a wild relative of Crambe abyssinica. Euphytica 89:267–278
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Namasivayam P (2007) Acquisition of embryogenic competence during somatic embryogenesis. Plant Cell Tiss Organ Cult 90:1–8
Nolan KE, Irwanto RR, Rose RJ (2003) Auxin up-regulates Mt ESRK1 expression in both Medicago truncatula root-forming and embryogenic cultures. Plant Physiol 133:218–230
Perez-Hernandez JB, Rosell-Garcia P (2008) Inflorescence proliferation for somatic embryogenesis induction and suspension-derived plant regeneration from banana (Musa AAA, cv’Dwarf Cavendish’) male flowers. Plant Cell Rep 27:965–971
Pinto G, Park YS, Neves L, Araujo C, Santos C (2008) Genetic control of somatic embryogenesis induction in Eucalyptus globulus Labill. Plant Cell Rep 27:1093–1101
Raghavan V (2004) Role of 2,4-dichlorophenoxyacetic acid (2,4-D) in somatic embryogenesis on cultured zygotic embryos of Arabidopsis: cell suspension, cell cycling and morphogenesis during continuous exposure of embryos to 2,4-D. Am J Bot 91:1743–1756
Rose RJ, Nolan KE (2006) Genetic regulation of somatic embryogenesis with particular reference to Medicago truncatula. In Vitro Cell Dev Biol Plant 42:473–481
Rose RJ, Nolan KE, Bicego L (1999) The development of highly regenerable seed line jemalong 2HA for transformation of Medicago truncatula—implications for regenerability via somatic embryogenesis. J Plant Physiol 155:788–791
Sharma SK, Bryan GJ, Millam S (2007) Auxin pulse treatment holds the potential to enhance efficiency and practicability of somatic embryogenesis in potato. Plant Cell Rep 26:945–950
Tejavathi DH, Rajanna MD, Sowmya R, Gayathramma K (2007) Induction of somatic embryos from cultures of Agave vera-cruz Mill. In Vitro Cell Dev Biol Plant 43:423–428
Thakare D, Tang W, Hill K, Perry SE (2008) The MADA-domain transcriptional regulator AGAMOUS-LIKE15 promotes somatic embryo development in Arabidopsis and soybean. Plant Physiol 146:1663–1672
Tomlin ES, Branch SR, Chamberlain D, Gabe H, Wright Ms, Stewart CN Jr (2002) Screening of soybean. Glycine max (L.) Merrill, lines for somatic embryo induction and maturation capability from immature cotyledons. In Vitro Cell Dev Biol Plant 38:543–548
Vargas-Lopez JM, Wiesenborn D, Tostenson K, Cihacek L (1999) Processing of Crambe oil and isolation of erucic acid. J Am Oil Chem Soc 76:801–809
Walker DR, Parrott WA (2001) Effect of polyethylene glycol and sugar alcohols on soybean somatic embryo germination and conversion. Plant Cell Tiss Organ Cult 64:55–62
Warwick SI, Gugel K (2003) Genetic variation in the Crambe abyssinica-C. hispanica-C. glabrata complex. Genet Resour Crop Evol 50:291–300
Webster SA, Mitchell SA, Reid WA, Ahmad MH (2006) Somatic embryogenesis from leaf and zygotic embryo explants of Bligha sapida ‘Cheese’ ackee. In Vitro Cell Dev Biol Plant 42:467–472
Williams EG, Maheswaran G (1986) Somatic embryogenesis: factors influencing coordinated behavior of cells as an embryonic group. Ann Bot 57:443–462
Acknowledgments
The authors wish to acknowledge the technical assistance provided by Keith Pahl and Prakash Venglat and to Lisa Kloeble and Holly Kemp for their help in the preparation, typing and formatting of this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Don Palmer, C., Keller, W.A. Somatic embryogenesis in Crambe abyssinica Hochst. ex R.E. Fries using seedling explants. Plant Cell Tiss Organ Cult 104, 91–100 (2011). https://doi.org/10.1007/s11240-010-9808-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-010-9808-3