Abstract
Availability of explants with adequate embryogenic competence is one of the most important limitations for the development of regenerable cell suspensions in banana. To increase the number and ease of accessibility to potentially embryogenic explants, a novel methodology is described by which young male flower clusters isolated from adult plants are induced to form new flower buds and proliferate in vitro. Different concentrations of the plant growth regulator thidiazuron (TDZ) induced inflorescence proliferation, which could be maintained over time as a continuous source of young flower buds. Intensity of proliferation was evaluated during successive subcultures. At the third cycle of proliferation, the highest multiplication rate (2.89) was obtained on the medium containing 5 μM TDZ. Newly generated floral tissues were assessed for embryogenic competence, resulting in an average embryogenic frequency of 12.5%. The observed embryogenic capacity, together with the recurrent availability of immature flowers, allowed for the direct initiation of cell suspensions from bulked explant cultures. Regular observation and regeneration tests during the development of suspended cell cultures confirmed their embryogenic condition. Produced embryos successfully matured and germinated to regenerate hundreds of somatic in vitro plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bananas, and their close relative plantains, are perennial herbaceous monocots that belong to the Musa genus of the Musaceae family. They are cultivated worldwide in tropical and subtropical areas, contributing over 20% of the total fresh fruit produced in the world (Faostat 2005).
Regardless of the existence of more than 1,000 different varieties, most domesticated types are highly sterile polyploids. Sterility has historically hindered conventional breeding programs, as it has similarly affected plant propagation and germplasm management. Recently, the development of different methods for somatic embryogenesis has considerably assisted in vitro technologies for these species. Diverse procedures have been originally described for somatic embryo induction in banana and differ depending on the type of explant employed, to include zygotic embryos (Cronauer-Mitra and Krikorian 1988), foliar bases and corm slices (Novak et al. 1989), in vitro-cultured meristems (Dhed’a et al. 1991), and immature male (Escalant et al. 1994) and female (Grapin et al. 2000) flowers. Despite the many options, the most widely applicable methodologies for the establishment of regenerable embryogenic cell suspensions from valuable seedless cultivars have been restricted to the use of either in vitro multiple meristems (Xu et al. 2005; Kulkarni et al. 2006; Strosse et al. 2006) or immature flowers (Côte et al. 1996; Grapin et al. 1996; Ganapathi et al. 1999; Wong et al. 2006) as starting material. Nevertheless, somatic embryogenesis in banana is still far from being considered a routine technology and has not even been successfully applied to some cultivars.
To overcome some of the limitations found in currently available methodologies (reviewed by Schoofs et al. 1999), this paper describes the use of in vitro proliferating inflorescences as an alternative pathway towards the induction of somatic embryogenesis and plant regeneration from immature banana flowers. The proposed method includes an initial phase of inflorescence proliferation for a fast and profuse generation of young flower buds. Newly generated, immature floral tissues with proven embryogenic capacity are subsequently used for the direct development of cell suspension cultures, from which plants are finally regenerated via somatic embryogenesis.
Materials and methods
Plant material
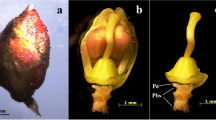
Male flower bunches were obtained from adult, field-grown banana (Musa acuminata Colla, AAA group, Cavendish subgroup, cv. ‘Dwarf Cavendish’) plants, 10 days after inflorescence emission. Distal, 10 cm-long inflorescence bunch portions containing immature male flowers were surface-sterilized in 70% ethanol for 30 min and washed three times in distilled, sterile water. Outer protective bracts and corresponding groups of male flowers (hands) were sequentially removed from the rachis and discarded, until reaching the first one to be used as a primary explant, which measured approximately 25 mm width. Explant extraction continued along the rachis towards the bunch tip. The last excised hand corresponded to a size of approximately 6 mm width. A total of 24 hands per individual bunch were used as primary explants. Protective bracts individually covering each flower cluster were removed before explant establishment. A typical primary explant after excision and processing is shown in Fig. 1a.
a Primary explant (hand) used to induce inflorescence proliferation, showing two rows of male flowers surrounded by the base of a protective bract. b Emergence and c development of new flower buds into flower clusters, after 6 and 10 weeks, respectively, on medium containing 5 μM TDZ. d Cauliflower-like clumps of flower buds produced from excised flower clusters on the same medium, 4 months after induction. Bars 2 mm
Inflorescence proliferation
Semi-solid inflorescence proliferation media (IPM) consisted of the mineral salts and vitamin mixture of Murashige and Skoog (1962), supplemented with sucrose at 87 mM and thidiazuron (TDZ) at concentrations of 0, 0.1, 2.5 or 5 μM. Primary explants were transferred to fresh media every 2 weeks until formation of young flower clusters. Newly formed inflorescences or curds (as referred to in this work due to their similarity with the central clusters of flower buds in cauliflower) were maintained under proliferation by regular subculturing on a monthly basis.
Somatic embryogenesis and plant regeneration
Curds used as secondary explants to initiate experiments on somatic embryogenesis were excised from proliferating cultures, maintained in 5 μM TDZ-containing medium, beyond the third cycle of division. A model type curd is shown in Fig. 4a. To determine their embryogenic potential, tiny curds measuring 3–4 mm were individually placed in 60 mL culture tubes containing 15 mL of semi-solid embryogenesis induction M1 medium (Escalant et al. 1994) and incubated without media refreshment. The embryogenic potential was visually determined, based on the presence of somatic embryos on curd-derived calli after 3 months of culture.
To develop embryogenic cell suspensions, sets of 20 curds were cultured in 50 mL Erlenmeyer flasks containing 5 mL of the above-mentioned liquid M1 medium, following a procedure adapted from Chong-Pérez et al. (2005). Briefly, half the volume of the medium was routinely refreshed every 15 days. After 3 months from initiation, cultures were sieved through a mesh with 500 μm pore size and the filtrates transferred to M2 medium (Côte et al. 1996). Generated suspensions were similarly refreshed with M2 medium every 15 days for maintenance. Samples were routinely taken for microscopic observation. For embryo development, 200 μL suspended cell samples were transferred to solid M3 medium (Côte et al. 1996). Germination of embryos was carried out on solid M4 medium (Côte et al. 1996). Finally, plantlets developed on regeneration (REG) medium (Strosse et al. 2006).
Media preparation and culture conditions
Prior to sterilization, the pH of the media was adjusted to 5.8 for IPM, M3, M4, and REG; to 5.3 for M2; and to 5.7 for M1. The heat-labile compounds TDZ and zeatin were added after autoclaving. Gelled media included Gelrite™ at 2 g L−1, except for the M3 and M4 media, which contained 4 and 3 g L−1, respectively. All media were autoclaved at 120 C for 20 min. Cultures for induction and maintenance of inflorescence proliferation were kept in the dark. Liquid cultures were incubated in the dark at 90 rpm shaking speed. Germination of embryos and plantlet development were carried out under a 16-h photoperiod using Lumilux L 840 fluorescent tubes (Osram), providing 20 μmol m−2 s−1 photosynthetic photon flux. All cultures were maintained at a temperature of 27 ± 1°C.
Microscopy
Unprocessed samples were observed under a Leica MZFLIII microscope. For histological observation, samples were fixed in a formalin–acetic acid–alcohol (FAA) solution, sequentially dehydrated in a tertiary butylic alcohol series, embedded in paraffin wax, sectioned at 10 μm and stained with Gerlach´s stain (Gerlach 1969). Samples of suspended cell cultures were stained with fluorescein diacetate (FDA) to test cell viability (Widholm 1972). Stained sections and cell samples were observed using a Leica DM2500 microscope equipped for fluorescence.
Data collection and analysis
Experiments for inducing inflorescence proliferation were initiated from a total of 360 explants excised from 15 flower bunches (24 hands per bunch). These were grouped by size into three classes as follows: “small”, between 6 and 10 mm; “medium”, between 10 and 15 mm; and “large”, between 15 and 25 mm width; each class comprising eight explants. Induction frequencies per size class were based on neoformation of floral buds and determined 2 months after culture initiation. Determination of inflorescence multiplication rates was based on the number of initially responding explants per processed flower bunch and resulting amounts of curds produced after each of the first three monthly subcultures under proliferation. A total of 424 curds were used for the establishment of four independent tests on semi-solid medium in experiments to induce somatic embryogenesis (264 curds) and eight individual liquid cultures in experiments to initiate suspended cell cultures (160 curds). Mean values and standard errors shown correspond to the average of at least three replicates, unless otherwise stated. Separation of means was carried out using Duncan’s Multiple Range Test (Duncan 1955).
Results and discussion
Immature banana male flowers showed in vitro organogenic competence in the presence of TDZ. Flower clusters (Fig. 1a) initially responded to TDZ by generating new groups of flower buds that became visible 6 weeks after establishment and appeared at the insertion site of the protective bract surrounding each individual explant (Fig. 1b). The whole axillary region showed profuse inflorescence neoformation that overgrew original explants after four more weeks in culture (Fig. 1c). This type of response shows similarities with the flower bud-like structures observed by Verron et al. (1995) in the monocot lily of the valley. As in our case, the addition of a cytokinin resulted essential for the production of buds from immature flowers, while its concentration appeared to have little influence. Indeed, organogenesis occurred at all TDZ concentrations tested, though the quality of generated clusters varied.
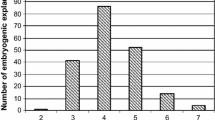
Explants cultured on basal medium lacking TDZ did not show any signs of organogenesis after 2 months incubation (Fig. 2). Comparable results have been reported for bamboo and ginseng explants, which have been shown to flower and thereof proliferate inflorescences in vitro in medium containing TDZ (Lin et al. 2003; 2004). In banana, a concentration as low as 0.1 μM resulted sufficient to induce the organogenic response on explants smaller than 15 mm width. Higher concentrations, i.e., 2.5 and 5 μM, were otherwise required for the formation of new flower buds on larger explants (Fig. 2). Despite the TDZ concentration, the highest induction frequencies, with average values in the range of 62.5–93.8%, corresponded to explants of the “small” class, while “medium” and “large” size explants resulted in induction frequencies of 8.3–34.4% and 0–3.1%, respectively (Fig. 2).
In vitro-produced flower bud clusters retained the ability to further proliferate when excised from the original primary explants and cultured individually (Fig. 1d). The intensity of proliferation depended on the TDZ concentration. While at 0.1 μM TDZ, the production of curds remained stable after the first subculture, at concentrations over 1.0 μM, the average number of flower clusters obtained per bunch increased sequentially, reaching a maximum of 162 curds after the third cycle of division at 5.0 μM TDZ (Fig. 3). According to the corresponding monthly multiplication rates, highest values resulted after the first cycle of subcultures at TDZ concentrations as low as 0.1 and 1 μM (Fig. 3). However, during the following two cycles, the tendency of multiplication reversed to directly correlate with TDZ concentration, reaching a second maximum peak of 2.89 for the third subculture at 5.0 μM TDZ.
Effect of TDZ concentration on inflorescence proliferation during three successive subcultures from initially established flower bunches. The total number of flower clusters produced at each cycle and corresponding multiplication rates are shown. Data represent average values from three independent experiments. Multiplication rate values followed by the same letters are not significantly different at P < 0.05
Inflorescence proliferation in banana could be sustained in time and hence allowed for the mass production of young floral tissues to be used as secondary explants for embryogenesis induction. In this study, we sequentially investigated their embryogenic potential and plant regeneration capacity. Proliferating flowers resemble both the clusters of multiple meristems and the immature male/female flowers that have been widely used for initiating banana embryogenic cultures (Strosse et al. 2003). Vascular initiations, evidenced in a histological section of a curd (Fig. 4b), have been previously described for the highest quality proliferation class of banana meristems, being related to the origin of the so-called meristematic globules and derived embryogenic cells (Dhed’a et al. 1991; Schoofs 1997). On the other hand, the reproductive nature and underdeveloped state of proliferating inflorescences certainly share intrinsic characteristics with immature banana flowers from adult plants, even displaying their normal in-row arrangement of individual flowers (Fig. 4a).
Morphology of a secondary explant and thereof derived structures during the process of somatic embryogenesis and plant regeneration. a Curd from a proliferating inflorescence culture showing the in-row disposition of flower buds. Bar 1 mm. b Longitudinal histological section of a meristematic region in a curd revealing rounded, vascular initiations (arrows) within submeristematic, parenchymatous tissue. Bar 0.1 mm. c, d Released, compact cell aggregate observed after FDA staining under visible (c) and UV-light (d), 12 weeks after induction in liquid medium. Bars 50 μm. e Embryogenic response of a cell suspension sample after 4 weeks on solid M3 medium. Bar 0.5 mm. f Embryo germination after 5 weeks on M4 medium. Bar 1.5 mm. g Germinated embryo individually cultured, 10 days later. Bar 4 mm. h Embryo-derived plantlets developing on REG medium 4 months from initiating regeneration tests. Bar 15 mm
Preliminary tests carried out to determine the capacity of curds for initiating an embryogenic response showed the occurrence of small, translucent embryos at initial stages of development, arising either individually or in small groups on the surface of whitish, embryogenic calli. Embryogenesis was allowed to proceed for 90 days under inducing conditions and revealed an average embryogenic frequency of 12.5%, from a total of 264 cultured explants (Table 1). This process also showed similarities to common events and elapsed reaction times observed for meristematic cultures and immature flowers, including the sequential formation of round-shaped meristematic globules and embryogenic callus bearing translucent proembryos (Strosse et al. 2003). Differences in plant genotype, explant nature and media composition make it difficult to compare embryogenic frequencies (EF) as obtained from inflorescence proliferation, meristematic cultures and male/female flower-based methods. However, our results using a Cavendish banana are in accordance with overall EFs reported in the literature for cultivars within the AAA group, irrespective of the technology used. Accordingly, Xu et al. (2005) obtained 10.8% EF for the “Williams” dessert banana when embryogenesis was induced from multiple meristem cultures, while Strosse et al. (2006) reported 3.8% EF on average for tested Cavendish cultivars. Escalant et al. (1994), however, reported that 37% of processed ‘Grande Naine’ immature inflorescences resulted embryogenic, i.e., an EF of 4.8% for explanted flower clusters.
Inflorescence proliferation allows for an exponential production of explants, which, when combined with satisfactory embryogenic frequencies facilitates the implementation of new approaches for the establishment of regenerable cell suspensions. Chong-Pérez et al. (2005) proposed the initiation of cell suspensions by direct bulked cultivation of immature flowers in liquid, reducing the total time required for their establishment. Adapting this new methodology to the use of proliferating inflorescence-derived curds, it has been possible to complete the process of plant regeneration. Once again, in vitro produced flower buds behaved similarly to male flowers explanted from adult plants during the establishment of liquid cell cultures. Meristematic globules developed on established explants and cell aggregates of different sizes and in variable quantities (Fig. 4c) were released after 90 days of incubation. These aggregates appeared to be composed of cells with dense cytoplasm and evident nuclei, intensely fluorescent under UV-light after FDA-staining (Fig.4d), and comparable to the embryogenic type II aggregates described by Georget et al. (2000) in a banana embryogenic cell suspension of the cv. ‘Grande Naine’. Spontaneous embryo formation was recorded for 25% of initiated suspensions after 140 days in liquid culture. Their embryogenic status was further confirmed in the embryogenesis test, where first embryos appeared on day 190 (Fig. 4e; Table 1). Subsequent maturation and germination occurred after approximately two more months (Fig. 4f, g). As a result, 504 somatic plantlets could be regenerated from one in eight (12.5%) initially established suspension cultures (Table 1). Young plantlets, showing the first one to three leaves at this stage, were individually transferred to culture tubes approximately 1 month after embryo germination (Fig. 4h). Regenerated plants were further transferred to the nursery and were being evaluated for clonal stability at the moment of writing this manuscript. Although none of them showed symptoms of abnormal morphology during the in vitro stage, the incidence of somaclonal variation will have to be determined in field plantation.
Research on somatic embryogenesis in banana has been widely and traditionally based on either multiple meristem cultures or field-derived inflorescences. However, the use of in vitro proliferating inflorescences for the establishment of embryogenic cultures has not been reported in the literature for any plant species. The methodology described here using a Cavendish banana therefore offers new possibilities for somatic embryogenesis in plants, particularly in cases of limited accessibility to embryogenesis competent tissues, as in highly recalcitrant banana and plantain varieties. Similarly, the process of inflorescence proliferation itself could provide a new source of explants to be combined with currently available protocols for the establishment of banana embryogenic cell suspensions (Strosse et al. 2003).
The use of proliferating inflorescence cultures for the direct initiation of cell suspensions could also contribute to overcome a number of acknowledged shortcomings to the production of embryogenic banana cell cultures (Schoofs et al. 1999). Indeed, compared to available methodologies, (1) difficulty of accessibility to field inocula would not directly affect embryogenic competence, (2) explant excision is neither as laborious as whole flower bud processing, nor requires optical equipment, (3) fewer flower buds would initially be necessary to attempt embryogenesis on a large scale, (4) explant production does not entail intensive subculturing to improve the quality of starting material, and (5) the time length from establishment of primary explants to plant regeneration could be considerably reduced.
References
Côte FX, Domergue R, Monmarson S, Schwendiman J, Teisson C, Escalant JV (1996) Embryogenic cell suspensions from the male flower of Musa AAA cv. ‘Grand Nain’. Physiol Plant 97:285–290. doi:10.1034/j.1399-3054.1996.970211.x
Cronauer-Mitra SS, Krikorian AD (1988) Plant regeneration via somatic embryogenesis in the seeded diploid banana Musa ornata Roxb. Plant Cell Rep 7:23–25. doi:10.1007/BF00272970
Chong-Pérez B, Gómez-Kosky R, Reyes-Vega M, Bermúdez-Carballoso I, Gallardo-Colina J, Freire-Seijo M, Posada-Pérez I, Herrera-O’Farril I, Swennen R (2005) New methodology for the establishment of cell suspensions of ‘Grande Naine’ (AAA). InfoMusa 14:13–18
Dhed’a D, Dumortier F, Panis B, Vuylsteke D, De Langhe E (1991) Plant regeneration in cell suspension cultures of the cooking banana cv. ‘Bluggoe’ (Musa spp. ABB group). Fruits 46:125–135
Duncan DB (1955) Multiple range and multiple F test. Biometrics 11:1–42
Escalant JV, Teisson C, Côte F (1994) Amplified somatic embryogenesis from male flowers of triploid banana and plantain cultivars (Musa spp.). In Vitro Cell Dev Biol Plant 30:181–186
Faostat (2005) Food and Agricultural Organization of the United Nations Statistical Database. http://faostat.fao.org
Ganapathi TR, Suprasanna P, Bapat VA, Kulkarni VM, Rao PS (1999) Somatic embryogenesis and plant regeneration from male flower buds in banana. Curr Sci 76:1228–1231
Georget F, Domergue R, Ferrière N, Côte FX (2000) Morphohistological study of the different constituents of a banana (Musa AAA, cv. ‘Grande Naine’) embryogenic cell suspension. Plant Cell Rep 19:748–754. doi:10.1007/s002999900188
Gerlach D (1969) A rapid safranin-crystal violet-light green staining sequence for paraffin sections of plant materials. Stain Technol 44:210–211
Grapin A, Schwendiman J, Teisson C (1996) Somatic embryogenesis in plantain banana. In Vitro Cell Dev Biol Plant 32:66–71
Grapin A, Ortíz JL, Lescot T, Ferrière N, Côte FX (2000) Recovery and regeneration of embryogenic cultures from female flowers of False Horn Plantain. Plant Cell Tissue Organ Cult 61:237–244. doi:10.1023/A:1006423304033
Kulkarni VM, Suprasanna P, Bapat VA (2006) Plant regeneration through multiple shoot formation and somatic embryogenesis in a commercially important and endangered Indian banana cv. ‘Rajeli’. Curr Sci 90:842–846
Lin CS, Chen CT, Lin CC, Chang WC (2003) A method for inflorescence proliferation. Plant Cell Rep 21:838–843. doi:10.1007/s00299-003-0571-3
Lin CS, Vidmar J, Chang WC (2004) Effects of growth regulators on inflorescence proliferation of Bambusa edulis. Plant Growth Regul 43:221–225. doi:10.1023/B:GROW.0000045980.48099.7c
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco cultures. Physiol Plant 15:473–497
Novak FJ, Afza R, Van Duren M, Perea-Dallos M, Conger BV, Xiaolang T (1989) Somatic embryogenesis and plant regeneration in suspension cultures of dessert (AA and AAA) and cooking (ABB) bananas (Musa spp.). Bio/Technol 7:154–159. doi:10.1038/nbt0289-154
Schoofs H (1997) The origin of embryogenic cells in Musa. Ph.D. thesis, Catholic University of Leuven, Leuven
Schoofs H, Panis B, Strosse H, Mosqueda AM, Torres JL (1999) Bottlenecks in the generation and maintenance of morphogenic banana cell suspensions and plant regeneration via somatic embryogenesis therefrom. InfoMusa 8:3–7
Strosse H, Domergue R, Panis B, Escalant JV, Côte F (2003) Banana and plantain embryogenic cell suspensions. International Network for the Improvement of Banana and Plantain, Montpellier
Strosse H, Schoofs H, Panis B, Andre E, Reyniers K, Swennen R (2006) Development of embryogenic cell suspensions from shoot meristematic tissue in bananas and plantains (Musa spp.). Plant Sci 170:104–112. doi:10.1016/j.plantsci.2005.08.007
Verron P, Nard M, Cohat J (1995) In vitro organogenic competence of different organs and tissues of lily of the valley ‘Grandiflora of Nantes’. Plant Cell Tissue Organ Cult 40:237–242. doi:10.1007/BF00048129
Widholm JM (1972) The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol 47:189–194
Wong WC, Jalil M, Ong-Abdullah M, Othman RY, Khalid N (2006) Enhancement of banana plant regeneration by incorporating a liquid-based embryo development medium for embryogenic cell suspension. J Hortic Sci Biotechnol 81:385–390
Xu CX, Panis B, Strosse H, Li HP, Xiao HG, Fan HZ, Swennen R (2005) Establishment of embryogenic cell suspensions and plant regeneration of the dessert banana ‘Williams’ (Musa AAA group). J Hortic Sci Biotechnol 80:523–528
Acknowledgments
The authors would like to acknowledge the contribution of Sandra Petit for lab assistance, Mª Carmen Cid for critical suggestions, Domingo Fernández for photography, and Tony Cracknell for the final language review. This research work has been funded by the Spanish National Institute of Agricultural Research (INIA) through research project RTA03-083.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Peña.
Rights and permissions
About this article
Cite this article
Pérez-Hernández, J.B., Rosell-García, P. Inflorescence proliferation for somatic embryogenesis induction and suspension-derived plant regeneration from banana (Musa AAA, cv. ‘Dwarf Cavendish’) male flowers. Plant Cell Rep 27, 965–971 (2008). https://doi.org/10.1007/s00299-008-0509-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-008-0509-x