Abstract
Malaxis acuminata is a terrestrial orchid that grows in shady areas of semi-evergreen to shrubby forests. It is highly valued for its medicinal properties as dried pseudo-bulbs are important ingredients of several Ayurvedic preparations. In this study, adventitious shoot buds were induced from internodal explants of M. acuminata grown on Murashige and Skoog (MS) medium supplemented with different concentrations of 6-benzyladenine (BA), kinetin (Kn), and thidiazuron (TDZ). Of the three cytokinins used, TDZ at 3 mg l−1 induced the highest frequency (82%) of organogenic explants. However, all responding explants produced only a single adventitious shoot irrespective of the type and concentration of the cytokinin. Adding 0.5 mg l−1 α naphthaleneacetic acid (NAA) to the medium enhanced adventitious shoot formation. In the presence of 3 mg l−1 TDZ and 0.5 mg l−1 NAA, frequency of organogenesis was 96% with a mean number of 6.1 shoots per explant. Prolonged culture or subculture on the same medium did not promote further shoot production. However, transfer of these cultures to MS medium supplemented with 3 mg l−1 TDZ and 0.5 mg l−1 NAA and various concentrations of different polyamines (PAs), including spermine, spermidine, and putrescine, significantly increased mean shoot number per explant. The highest frequency of shoot induction (100%) and mean shoot number per explant (14.6) was observed on MS medium with 3 mg l−1 TDZ, 0.5 mg l−1 NAA, and 0.4 mM spermidine. Regenerated shoots were excised and subcultured on an elongation medium consisting of MS medium with 3 mg l−1 BA. Moreover, the highest frequency of rooting (96%) and mean number of roots per shoot (3.3) was observed on MS medium with 4 mg l−1 indole-3-butyric acid (IBA) and 1.5 mg l−1 activated charcoal (AC). Almost 90% of rooted shoots were successfully acclimatized and established ex vitro.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Orchids are one of the largest and most diverse groups among angiosperms. According to one estimate, the family Orchidaceae includes 800 genera and 25,000 species (Stewart and Griffiths 1995). These ornamental plants are widely distributed, cultivated for their beautiful flowers, and are of economic importance. In addition to their ornamental value, orchids are also well known for their medicinal usage especially in the traditional folk medicine. The medicinal use of orchids was first reported by the Chinese (Bulpitt 2005). The Chinese pharmacopoeia, ”the Sang Nung Pen Tsao Ching”, illustrated Dendrobium as a source of tonic, astringent, analgesic, and anti-inflammatory compounds as far back as 200 BC (Singh and Tiwari 2007), and since the Vedic period in India (Singh and Tiwari 2007). Orchids are used in traditional medicine as they are rich in active compounds including several alkaloids (Okamoto et al. 1966; Lawler and Slaytor 1969; Elander et al. 1973; Nurhayati et al. 2009). In the Ayurvedic branch of traditional medicine, a group of eight drugs, known as “Ashtavarga”, provide important ingredients for different types of tonics. Dried pseudo-bulbs of Malaxis acuminata serve as important sources of Astavarga utilized in the preparation of the Ayurvedic tonic ‘Chyavanprash’. The latter is one of the most widely used Ayurvedic preparations for promoting human health and preventing disease (Uniyal 1975; Govindarajan et al. 2007).
Malaxis acuminata is a small, medium-sized terrestrial orchid, up to 30 cm in length, with pseudo-bulbs at the base, and with fibrous roots. Leaves have sheathing leaf base and new plants grow along the vicinity of the decaying mother plant. Flowers, in terminal racemes, are small, pale yellowish-green in color, but with a purple tinge.
Acute habitat destruction has resulted in the disappearance of this orchid from some areas of its natural habitat. Orchids are among the most vulnerable of plant families (Pridgeon 1996) with almost all orchid species forming a strong association with mycorrhizal fungi for development (Zettler 1997). Due to the economic importance of pseudobulbs of orchids, plants have been harvested excessively and beyond sustainable levels.
Tissue culture provides an alternate method for large-scale propagation of threatened and endangered plants, including orchid micropropagation using various explants (Tokuhara and Mii 2001; Chen et al. 2002a, 2005, 2009; Ket et al. 2004; Kauth et al. 2006; Thomas and Michael 2007). In this study, we report on an efficient shoot organogenesis system for M. acuminata using internodal explants.
Materials and methods
Plant material
Three- to four-year-old potted plants of M. acuminata growing at the Botanical Garden of St. Thomas College, Pala, were used as donor plants. Internodal stem segments, 1 cm in length, were excised from these donor plants and washed under running water for 30 min. Explants were then immersed in an aqueous solution of 4% (v/v) liquid detergent (Laboline; Qualigens, Mumbai, India) for 10 min, and rinsed three times with distilled water. Then, they were surface-sterilized with an aqueous solution of 0.1% (w/v) HgC12 for 8 min and rinsed three times with sterile distilled water.
Culture medium and growth conditions
Cut ends of internodal stem segments were trimmed before these were placed in culture tubes (one explant per tube) containing 12 ml Murashige and Skoog (1962) medium (MS) containing 100 mg l−1 (w/v) myo-inositol, 3% (w/v) sucrose (Qualigens), and solidified with 8 g l−1 agar (Bacteriological grade; Hi Media, Mumbai, India). This medium was supplemented with various plant growth regulators, including 1.0–4.0 mg l−1 6-benzyladenine (BA), 1.0–4.0 mg l−1 thidiazuron (TDZ), 1.0–10.0 mg l−1 kinetin (Kn), either separately or in combination with 0.5 mg l−1 α naphthaleneacetic acid (NAA). The pH of the medium was adjusted to 5.8 with 0.1 N NaOH or HCl prior to autoclaving. All medium-containing culture vessels were autoclaved at 104 kPa and 121°C for 20 min.
For each treatment, at least 24 explants were used, and all experiments were repeated three times. Each culture period lasted 8 weeks. All cultures were maintained at 25 ± 2°C, 70% relative humidity, and 16 h photoperiod of 35–50 μmol m−2 s−1 irradiance provided by cool-white fluorescent tubes (Phillips, Mumbai, India).
Data on number of organogenic explants and number of developing shoots per explant were recorded.
Effects of various polyamines on shoot organogenesis
The adventitious shoots from the media supplemented with BA (1–4 mg l−1), TDZ (1–4 mg l−1) or Kn (1–4 mg l−1) in combination with NAA (0.5 mg l−1) were randomly selected and transferred to MS medium containing 3 mg l−1 TDZ and 0.5 mg l−1 NAA, supplemented with various concentrations (0.2–1.0 mM) of polyamines, including spermine, spermidine, and putrescine.. Numbers of explants, replications, and culture conditions were as described above. The average adventitious shoot number was calculated at the time of culture as well as at the 4th and 8th weeks after culture.
Shoot elongation
Adventitious shoots were excised from explants and subcultured on MS medium supplemented with 3 mg l−1 BA for shoot elongation. All media were prepared as described above, and cultures were grown under conditions as described above.
Rooting of shoots, acclimatization, and field transfer
Individual shoots with two to three expanded leaves were transferred to half-strength MS medium supplemented with 2–8 mg l−1 of indole-3-butyric acid (IBA) or NAA (2–8 mg l−1).
Rooted shoots, with 2–3 roots, were removed from culture tubes, and washed thoroughly in running tap water for 5–6 min to remove traces of agar. These were then planted in plastic cups (6 cm in diameter) containing a potting mixture of charcoal chips and soil (1:1). Plantlets were initially covered with a polythene sheet for 1 month to maintain high relative humidity (90%). These were irrigated every other day with half-strength MS liquid medium. The number of surviving plants was recorded after 12 weeks. All surviving acclimatized plants were eventually transferred to the field.
Data analysis
All data were subjected to analysis of variance using SAS, and means were compared using Duncan’s multiple range test (Duncan 1955).
Results
When explants were cultured on MS basal medium supplemented with BA or TDZ or Kn alone, explants were organogenic, and each explant developed single shoots (Table 1). None of the explants incubated on a medium lacking any plant growth regulator (PGR) were organogenic. Among all three cytokinins tested, TDZ induced the highest frequency of shoot organogenesis, and this was followed by Kn. Among the different levels of TDZ evaluated, 3.0 mg l−1 TDZ resulted in 82% organogenic explants (Table 1). Explants produced a single small white protuberance after 4 weeks of culture. After another 4 weeks, this single protuberance directly differentiated into a well-defined shoot without any intervening callus or protocorm-like-body formation and was about 1.0 cm in length. Further elongation of the solitary shoot did not take place in the same medium. The explant turned dark or brown in color after 8 weeks of culture in most media tested.
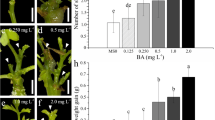
When explants were grown on different cytokinin-containing media, but supplemented with 0.5 mg l−1 NAA, a higher organogenic response was observed on all PGR treatment combinations. The highest frequency of organogenic explants (96%) and number of adventitious shoots per explant (6.1) were observed on MS medium supplemented with 3 mg l−1 TDZ and 0.5 mg l−1 NAA (Table 1; Fig. 1a, b). Among Kn levels used, 2 mg l−1 Kn in combination with 0.5 mg l−1 NAA induced 88% of organogenic explants with 5.4 shoots per explant (Table 1).
Various stages of adventitious shoot bud induction, shoot elongation and rooting of shoots from internodal explants of M. acuminata. a Adventitious shoot induction on explants grown on MS medium supplemented with 3 mg l−1 TDZ and 0.5 mg l−1 NAA after 4 weeks of culture. An average number of about six shoots were produced per explant. Bar 0.8 cm. b Same as in (a) after 8 weeks of culture. Bar 0.8 cm. c Adventitious shoot formation on MS medium fortified with 3 mg l−1 TDZ, 0.5 mg l−1 NAA and 0.4 mM spermidine 4 weeks after subculture from MS with 3 mg l−1 TDZ and 0.5 mg l−1 NAA. A mean number of 14.6 shoots was observed. Bar 1 cm. d Same as in (c) 8 weeks after culture. Adventitious shoots have developed further. Bar 1 cm. e Shoots on elongation medium which consists of MS medium supplemented with 3 mg l−1 BA. Shoots were 1.8 cm in 8 weeks. Bar 0.8 cm. e Rooted shoots taken out from MS medium supplemented with 4 mg l−1 IBA and 1.5 g l−1 AC 8 weeks after culture. Bar 1 cm
Extended culture on the same medium or subculture on a fresh medium with the same composition did not improve shoot yield. Therefore, three polyamines (PAs) were tested to evaluate their role on adventitious shoot formation. Spermidine and putrescine produced more number of shoots than spermine (Table 2). All cultures were responded in four different concentrations of three PAs. However, the average shoot number per culture varied with the type and concentration of PA. Spermidine at 0.4 mM and putrescine at 0.4 and 0.8 mM concentrations gave a three-fold increase in shoot number after 8 weeks of culture (Table 2). Optimum response (9.8 and 14.6 shoots after 4 and 8 weeks of culture) was obtained on MS medium supplemented with TDZ (3 mg l−1), NAA (0.5 mg l−1) along with 0.4 mM spermidine (Table 2; Fig. 1c, d).
The whitish green shoots originated from the internodal explants did not show elongation and leaf development on shoot induction medium, furthermore the average shoot length remained almost similar to earlier experiments. Therefore, for further elongation of shoots, the shoot clumps were detached from the explant and transferred to MS medium supplemented with 3 mg l−1 BA. On this medium, the shoots attained an average height of 1.8 cm with expanded leaves in 8 weeks (Fig. 1e).
For rooting, the shoots were transferred to MS medium containing IBA (2–8 mg l−1) and NAA (2–8 mg l−1). However, there was no rooting on this medium despite reducing the concentration of medium to half strength. Thus, 1.5 mg l−1 activated charcoal (AC) was added to promote rooting. The shoots failed to root on lower concentrations (0.2–1.0 mg l−1) of IBA and NAA even in the presence of AC (data not shown). However, in higher concentrations (2.0–8.0 mg l−1), rooting efficiency was significantly improved in presence of AC. Maximum response (96%) was observed on MS medium supplemented with 4 mg l−1 IBA and 1.5 mg l−1 AC (Table 3; Fig. 1f). On this medium, an average number of 3.3 roots per shoot were observed after 8 weeks. Comparatively, IBA gave better result than NAA in terms of percent cultures responding and number of roots per shoot (Table 3).
The plants were transferred to the greenhouse after 4 weeks, where they have acclimatized. The survival percentage was 90% after 12 weeks in the greenhouse. No phenotypic variation was observed among the in vitro raised plants.
Discussion
The objective of this study was to develop an efficient in vitro multiple shoot induction system, which will allow large-scale multiplication of the primary induced sterile shoots. Since the plants are medicinally useful and threatened, the use of an efficient micropropagation system as a means to multiply for controlled production of the desired plants will take the pressure off the wild populations.
In our work, TDZ was more beneficial in inducing shoots than other cytokinins. The induction rate varied with type and concentration of growth regulators. Of the various auxin–cytokinin combinations meant for shoot induction, 3 mg l−1 TDZ and 0.5 mg l−1 NAA induced maximum response with 96% cultures responding with an average number of 6.1 shoots per explant. Thus, in this study, TDZ played a central role for shoot induction in M. acuminata. After the first report of cytokinin-like activity of TDZ by Mok et al. (1982), TDZ has been successfully used to induce adventitious shoot formation in numerous systems, particularly woody plants, and is reported to be more efficient than purine-type cytokinins (BA or Kn) even at extremely low concentrations (Huetteman and Preece 1993).
TDZ has been effectively utilized in orchids to induce direct somatic embryogenesis in Oncidium (Chen et al. 1999; Chen and Chang 2001), shoot regeneration in Phalaenopsis, Doritaenopsis, and epiphytic Cymbidium (Ernst 1994; Chen and Piluek 1995; Nayak et al. 1997), embryogenic callus induction in Cymbidium ensifolium and Oncidium sp. (Chang and Chang 1998; Chen and Chang 2000a, b), induction of protocorm like bodies (PLB) from flower stalk of Epidendrum radicans (Chen et al. 2002b), and multiple shoot induction in Rhynchostylis retusa (Thomas and Michael 2007).
Our investigation confirmed the positive role of PAs in inducing adventitious shoot induction. Spermidine and putrescine produced enhanced results compared with spermine when tested with TDZ and NAA. The significantly high shoot number was observed when spermidine at 0.4 mM and putrescine at 0.4 and 0.8 mM concentrations were employed in the medium.
Polyamines (PAs) are molecules that are responsible for different plant developmental processes (Silveira et al. 2006; Tun et al. 2006). They control several cellular processes including DNA replication, cell division, protein synthesis, flower development, in vitro flower induction, fruit development, senescence, abiotic and biotic stress responses, and secondary metabolism (Kumar et al. 1997; Bagni and Tassoni 2001; Bais and Ravishankar 2002; Kuznetsov et al. 2006; Tun et al. 2006). PAs are extensively used for various purposes in plant tissue culture including somatic embryogenesis (Feirer 1995; Minocha et al. 1999; Kevers et al. 2002; Rajesh et al. 2003; Bertoldi et al. 2004; Silveira et al. 2006; Santa-Catarina et al. 2007; Venkatachalam and Bhagyalakshmi 2008) root induction (Biondi et al. 1990; Heloir et al. 1996; Grigoriadou et al. 2002; Couee et al. 2004), androgenesis, and gynogenesis (Tiainen 1992; Rajyalakshmi et al. 1995; Martinez et al. 2000; Ashok Kumar et al. 2004; Chiancone et al. 2006). Similarly, polyamine induced callus regeneration in sugar beet (Hagege et al. 1994) and direct shoot regeneration in Brassica campestris (Chi et al. 1994) and Chinese radish (Pua et al. 1996) has also been reported. In orchids like Vanilla planifolia and Dendrobium, PAs promoted in vitro propagation (Thyagi et al. 2001; Saiprasad et al. 2004).
Root induction was observed only in the presence of AC irrespective of auxins used. AC at 1.5 mg l−1 was optimum for root induction with IBA (2.0–8.0 mg l−1) and NAA (2.0–8.0 mg l−1). In orchids like Cypripedium flavum, 100% rooting was observed on medium containing AC (0.6 g l−1) alone. This is probably due to the partial darkness created by the AC in the medium which is similar to the underground environment of C. flavum habitats (Yan et al. 2006). AC-stimulated rooting has also been reported in other orchids like Renanthera imschootiana (Seeni and Latha 1992), Anoectochilus formosanus (Ket et al. 2004), Cymbedium faberi (Chen et al. 2005), and Dendrobium hybrid (Martin and Madassery 2006).
In conclusion, this simple and efficient method for micropropagating large number of plantlets via internodal adventitious shoots could be used for large-scale propagation and ex situ conservation of the medicinal terrestrial orchid M. acuminata.
Abbreviations
- AC:
-
Activated charcoal
- BA:
-
6-Benzyladenine
- IBA:
-
Indole-3-butyric acid
- Kn:
-
Kinetin
- MS:
-
Murashige and Skoog
- NAA:
-
α Naphthaleneacetic acid
- PAs:
-
Polyamines
- TDZ:
-
Thidiazuron
References
Ashok Kumar HG, Ravishankar BV, Murthy HN (2004) The influence of polyamines on androgenesis of Cucumis sativus L. Eur J Hort Sci 69:201–205
Bagni N, Tassoni A (2001) Biosynthesis, oxidation and conjugation of aliphatic polyamines in higher plants. Amino Acids 20:301–317
Bais HP, Ravishankar GA (2002) Role of polyamines in the ontogeny of plants and their biotechnological applications. Plant Cell Tissue Organ Cult 69:1–34
Bertoldi D, Tassoni A, Martinelli L, Bagni N (2004) Polyamines and somatic embryogenesis in two Vitis vinifera cultivars. Physiol Plant 120:657–666
Biondi S, Diaz T, Iglesias I, Gamberini G, Bagni N (1990) Polyamines and ethylene in relation to adventitious root formation in Prunus avium shoot cultures. Physiol Plant 78:474–483
Bulpitt CJ (2005) The uses and misuses of orchids in medicine. QJM: An Int J Medicine 98:625–631
Chang C, Chang WC (1998) Plant regeneration from callus culture of Cymbidium ensifolium var. misericors. Plant Cell Rep 17:251–255
Chen JT, Chang WC (2000a) Efficient plant regeneration through somatic embryogenesis from callus cultures of Oncidium (Orchidaceae). Plant Sci 160:87–93
Chen JT, Chang WC (2000b) Plant regeneration via embryo and shoot bud formation from flower-stalk explants of Oncidium ‘Sweet Sugar’. Plant Cell Tissue Organ Cult 62:95–100
Chen JT, Chang WC (2001) Effects of auxins and cytokinins on direct somatic embryogenesis from leaf explants of Oncidium ‘Gower Ramsey’. Plant Growth Regul 34:229–232
Chen Y, Piluek C (1995) Effects of thidiazuron and N6-benzylamino-purine on shoot regeneration of Phalaenopsis. Plant Growth Regul 16:99–101
Chen JT, Chang C, Chang WC (1999) Direct somatic embryogenesis on leaf explants of Oncidium Gower Ramsey and subsequent plant regeneration. Plant Cell Rep 19:143–149
Chen LR, Chen JT, Chang WC (2002a) Multiple shoot formation and plant regeneration from stem explants of Paphiopedilum orchids. In Vitro Cell Dev Biol-Plant 38:595–597
Chen LR, Chen JT, Chang WC (2002b) Efficient production of protocorm like bodies and plant regeneration from flower stalk explanted of the sympodial orchid Epidendrum radicans. In Vitro Cell Dev Biol-Plant 38:441–445
Chen Y, Liu X, Liu Y (2005) In vitro plant regeneration from the immature seeds of Cymbidium faberi. Plant Cell Tissue Organ Cult 81:247–251
Chen WH, Tang CY, Kao YL (2009) Ploidy doubling by in vitro culture of excised protocorms or protocorm-like bodies in Phalaenopsis species. Plant Cell Tissue Organ Cult 98:229–238
Chi GL, Lin WS, Lee JEE, Pua EC (1994) Role of polyamines on de novo shoot morphogenesis from cotyledons of Brassica campestris ssp. pekinensis (Lour.) Olsson in vitro. Plant Cell Rep 13:323–329
Chiancone B, Tassoni A, Bagni N, Germana MA (2006) Effect of polyamines on in vitro anther culture of Citrus clementina Hort. ex Tan. Plant Cell Tissue Organ Cult 87:145–153
Couee I, Hummel I, Sulmon C, Gouesbet G, El Amrani A (2004) Involvement of polyamines in root development. Plant Cell Tissue Organ Cult 76:1–10
Duncan DB (1955) Multiple range and multiple F test. Biometrics 11:1–42
Elander M, Leander K, Rosenbloom J, Ruusa E (1973) Studies on orchidaceae alkaloids. XXXII. Crepidine, crepidamine and dendrocrepine from Dendrobium crepidatum Lindl. Acta Chem Scand 27:1907–1913
Ernst R (1994) Effect of thidiazuron on in vitro propagation of Phalaenopsis and Doritaenopsis (Orchidaceae). Plant Cell Tissue Organ Cult 39:273–275
Feirer RP (1995) The biochemistry of conifer embryo development: amino acids, polyamines, and storage proteins. In: Jain SM, Gupta PK, Newton RJ (eds) Somatic embryogenesis in woody plants, vol 1. Kluwer, Dordrecht, pp 317–336
Govindarajan R, Singh DP, Rawat AKS (2007) High-performance liquid chromatographic method for the quantification of phenolics in ‘Chyavanprash’ a potent Ayurvedic drug. J Pharm Biomed Anal 43:527–532
Grigoriadou K, Miltiadis V, Eleftherios PE (2002) In vitro propagation of the Greek olive cultivar ‘Chondrolia Chalkidikis’. Plant Cell Tissue Organ Cult 71:47–54
Hagege D, Kevers C, Genus J, Gaspar T (1994) Ethylene production and polyamine content of fully habituated sugar beet calli. J Plant Physiol 143:722–725
Heloir MC, Kevers C, Hausman JF, Gaspar T (1996) Change in the concentration of auxins and polyamines during rooting of in vitro propagated walnut shoots. Tree Physiol 16:515–520
Huetteman CA, Preece JE (1993) Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tissue Organ Cult 33:105–119
Kauth PJ, Vendrame WA, Kane ME (2006) In vitro seed culture and seedling development of Calopogon tuberosus. Plant Cell Tissue Organ Cult 85:91–102
Ket NV, Hahn EJ, Park SY, Chakrabarty D, Paek KY (2004) Micropropagation of an endangered orchid Anoectochilus formosanus. Biol Plant 48:339–344
Kevers C, Gaspar T, Jacques D (2002) The beneficial role of different auxins and polyamines at successive stages of somatic embryo formation and development of Panax ginseng in vitro. Plant Cell Tissue Organ Cult 70:181–188
Kumar A, Altabella T, Taylor MA, Tiburcio AF (1997) Recent advances in polyamine research. Trend Plant Sci 2:124–130
Kuznetsov V, Radyukina NL, Shevyakova NI (2006) Polyamines and stress: biological role, metabolism and regulation. Rus J Plant Physiol 53:583–604
Lawler LJ, Slaytor M (1969) The distribution of alkaloids in New South Wales and Queensland Orchidaceae. Phytochem 8:1959–1962
Martin KP, Madassery J (2006) Rapid in vitro propagation of Dendrobium hybrids through direct shoot formation from foliar explants, and protocorm-like bodies. Sci Hort 108:95–99
Martinez LE, Aguero CB, Lopez ME, Glamarini CR (2000) Improvement of in vitro gynogenesis induction in onion (Allium cepa L.) using polyamines. Plant Sci 156:221–226
Minocha R, Dale RS, Cathie R, Steele KD, Minocha SC (1999) Polyamine levels during the development of zygotic and somatic embryos of Pinus radiata. Physiol Plant 105:155–164
Mok MC, Mok DWS, Armstrong DJ, Shudo K, Isogai Y, Okamoto T (1982) Cytokinin activity of N-phenyl-N0-(1, 2, 3-thiadiazol-5-yl)-urea (thidiazuron). Phytochem 21:1509–1511
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–479
Nayak NR, Rath SP, Patnaik S (1997) In vitro propagation of three epiphytic Cymbidium Aloifolium (L.) SW, Dendrobium aphyllu (Roxb) Fisch and Dendrobium moschatum (Buchham) SW through thidiazuron-induced high frequency shoot proliferation. Sci Hort 71:416–426
Nurhayati N, Gondé D, Ober D (2009) Evolution of pyrrolizidine alkaloids in Phalaenopsis orchids and other monocotyledons: identification of deoxyhypusine synthase, homospermidine synthase and related pseudogenes. Phytochem 70:508–516
Okamoto T, Natsume M, Onaka T, Uchmaru F, Shimizu M (1966) The structure of dendramine (6-oxydendrobine) and 6-oxydendroxine. The fourth and fifth alkaloid from Dendrobium nobile. Chem Pharm Bull 14:676–680
Pridgeon AM (1996) Orchids—status survey and conservation action plan. IUCN, Cambridge, UK
Pua EC, Sim GE, Chi GL, Kong LF (1996) Synergistic effect of ethylene inhibitors and putrescine on shoot regeneration from hypocotyl explants of Chinese radish (Raphanus sativus L. var. longipinnatus Bailey) in vitro. Plant Cell Rep 15:685–690
Rajesh MK, Radha E, Karun A, Parthasarathy VA (2003) Plant regeneration from embryo-derived callus of oil palm—the effect of exogenous polyamines. Plant Cell Tissue Organ Cult 75:41–47
Rajyalakshmi K, Chowdhry CN, Maheshwari N, Maheshwari SC (1995) Anther culture response in some Indian wheat cultivars and the role of polyamines in induction of haploids. Phytomorp 45:139–145
Saiprasad GVS, Raghuveer P, Khetarpal S, Chandra R (2004) Effect of various polyamines on production of protocorm-like bodies in orchid—Dendrobium ‘Sonia’. Sci Hort 100:161–168
Santa-Catarina C, Silveira V, Scherer GFE, Segal Floh EI (2007) Polyamine and nitric oxide levels relate with morphogenetic evolution in somatic embryogenesis of Ocotea catharinensis. Plant Cell Tissue Organ Cult 90:93–101
Seeni S, Latha PG (1992) Foliar regeneration of the endangered Red Vanda, Renanthera imschootiana Rolfe (Orchidaceae). Plant Cell Tissue Organ Cult 29:167–172
Silveira V, Santa-Catarina C, Tun NN, Scherer GFE, Handro W, Guerra MP, Floh EIS (2006) Polyamine effects on the endogenous polyamine contents, nitric oxide release, growth and differentiation of embryogenic suspension cultures of Araucaria angustifolia (Bert.) O. Ktze. Plant Sci 171:91–98
Singh AKR, Tiwari C (2007) Harnessing the economic potential of Orchids in Uttaranchal. ENVIS Bull Hima Ecol 14:1–3
Stewart J, Griffiths M (1995) Manual of orchids. Timber Press, Portland, Oregon
Thomas TD, Michael A (2007) High-frequency plantlet regeneration and multiple shoot induction from cultured immature seeds of Rhynchostylis retusa Blume., an exquisite orchid. Plant Biotech Rep 1:243–249
Thyagi RK, Yusuf A, Jeyaprakash P, Poonam D (2001) Effects of polyamines on in vitro conservation of Vanilla planifolia (Salisb.) Ames. Ind J Plant Genet Res 14:300–302
Tiainen T (1992) The role of ethylene and reducing agents on anther culture response of tetraploid potato (Solanum tuberosum L.). Plant Cell Rep 10:604–607
Tokuhara K, Mii M (2001) Induction of embryogenic callus and cell suspension culture from shoot tips excised from flower stalk buds of Phalaenopsis (Orchidaceae). In Vitro Cell Dev Biol Plant 37:457–461
Tun NN, Santa-Catarina C, Begum T, Silveira V, Handro W, Segal Floh EI, Scherer GFE (2006) Polyamines induce rapid biosynthesis of nitric oxide (NO) in Arabidopsis thaliana seedlings. Plant Cell Physiol 47:346–354
Uniyal MR (1975) Astavarga. Sandigdha Vanaushadhi. Dhanwantri Partrika. Sri Jwala Ayurevd Bhawan Aligarh, India
Venkatachalam L, Bhagyalakshmi N (2008) Spermine induced morphogenesis and effect of partial immersion system on the shoot cultures of banana. Appl Biochem Biotechnol 151:502–511
Yan N, Hu H, Huang J, Xu K, Wang H, Zhou Z (2006) Micropropagation of Cypripedium flavum through multiple shoots of seedlings derived from mature seeds. Plant Cell Tissue Organ Cult 84:113–117
Zettler LW (1997) Terrestrial orchid conservation by symbiotic seed germination: techniques and perspectives. Selbyana 18:188–194
Acknowledgments
We thank the Principal, St. Thomas College, Pala, for providing with necessary laboratory facilities. T.D.T. acknowledges the financial assistance from the Indian National Science Academy (INSA) and the Japan Society for the Promotion of Science (JSPS) in the form of bilateral exchange programme (No. IA/JSPS/2009-2010).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheruvathur, M.K., Abraham, J., Mani, B. et al. Adventitious shoot induction from cultured internodal explants of Malaxis acuminata D. Don, a valuable terrestrial medicinal orchid. Plant Cell Tiss Organ Cult 101, 163–170 (2010). https://doi.org/10.1007/s11240-010-9673-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-010-9673-0