Abstract
For promoting the maturation of two embryogenic cell lines of Abies cephalonica Loud., the effect of two sucrose concentrations (87.6 and 175.2 mM) applied alone (Control) or in combination with activated charcoal (AC) (1 week) or with polyethylene glycol (PEG1 and PEG4) (1 or 4 weeks) was studied. The effect of each maturation medium was tested with four concentrations of abscisic acid (4, 8, 16 and 32 μM ABA). AC supplement significantly increased the percentage of embryogenic cell masses (ECMs) with cotyledonary embryos but did not affect the average number of embryos per 1 g of ECMs fresh weight. The highest percentages of ECMs (77.5%) with cotyledonary somatic embryos and the highest average number of somatic embryos (36.5 ± 13.4) were found on media with PEG for 4 weeks. The maturation media with 87.6 mM sucrose significantly increased the number of ECMs able to form cotyledonary embryos (66.8%) when PEG was included in the maturation medium, but did not affect the average number of somatic embryos. Overall correlation between proliferation diameter during maturation phase and the number of ECMs with somatic embryos (r = −0.40) as well as all significant correlations within individual experimental variants were consistently negative after 8 weeks of maturation, meaning that proliferation growth hampers the formation of somatic embryos. The highest germination percentages 21.6% and 18.2% were obtained when somatic embryos were maturated on media with or without AC supplement, respectively. Lowest germination percentages, 9.1% and 4.4%, were obtained on maturation media with PEG4 and PEG1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aussenac (2002) considering a climatic scenario, proposed that Mediterranean firs (e.g., Abies cephalonica Loud.) could be in danger in some parts of the present range, but, on the other hand, could also replace other species in more northern zones with temperate humid climate. In these climate zones it could replace silver fir (Abies alba Mill.) which is suffering dieback in a large part of its distribution range (Larsen 1986). As fir species are generally highly productive and therefore important for commercial forestry, they have traditionally been involved in conventional tree improvements programmes including interspecific hybridization (Greguss et al. 1994). In Central Europe, among the most promising parental combinations were those where A. cephalonica is the mother tree (Greguss and Longauer 1996). Quite a lot of effort has been put into the development of vegetative propagation methods for firs, in order to rapidly gain the benefits of traditional breeding to be utilized in reforestation (Greguss et al. 1994). Somatic embryogenesis, with its potential for mass multiplication has become a useful technique for large scale propagation of many coniferous species (Timmis 1998), and in combination with cryopreservation somatic embryogenesis makes it possible to preserve important genotypes during field tests (reviewed by Sutton et al. 2004).
Regeneration in the genus Abies via somatic embryogenesis has been demonstrated for A. alba Miller (Hristoforoglu et al. 1995), A. balsamea (L.) Mill. (Guevin et al. 1994), A. cephalonica (Krajňáková et al. 2008), A. cilicica Carrière (Vooková and Kormut'ák 2003), A. lasiocarpa (Hook.) Nutt. (Kvaalen et al. 2005) A. nordmanniana Lk. (Nørgaard 1997), A. numidica De Lann. ex Carrière (Vooková and Kormut'ák 2002) and in several of their hybrids (Salaj et al. 2005).
A common procedure for maturation of high quality somatic embryos in conifers is the culture of embryogenic tissue on media supplemented with abscisic acid (ABA) with decreased osmotic water potential compared to the proliferation medium. This decrease is mainly achieved by increasing the concentration of sucrose (Klimaszewska and Smith 1997), gelling agents (Klimaszewska et al. 2000) or by supplementing non-plasmolyzing osmoticum (polyethylene glycol, PEG) (Attree and Fowke 1993). The effect of PEG mimics the naturally occurring water stress on seeds during the late stages of maturation. Water stress caused by PEG and/or increased concentration of sugar together with ABA is essential to somatic embryo development because it induces accumulation of storage compounds and inhibits precocious germination (reviewed by Stasolla and Yeung 2003). PEG enhanced somatic embryo maturation in several species, including Picea glauca (Moench) Voss (Attree et al. 1991), Pseudotsuga menziesi (Mir.) Franco (Gupta et al. 1993), Picea abies L. Karst. (Bozhkov and Von Arnold 1998; Svobodová et al. 1999), Pinus sylvestris L. (Keinonen-Mettälä et al. 1996; Häggman et al. 1999), P. nigra Arnold. (Salajová et al. 1999), Cryptomeria japonica D. Don (Maruyama and Hosoi 2007), Abies nordmanniana Lk. (Nørgaard 1997), A. numidica Carr. (Vooková and Kormut'ák 2002) and Abies hybrids (Salaj and Salaj 2003; Salaj et al. 2004).
Activated charcoal (AC) is commonly used in tissue culture media to darken the immediate media surroundings, adsorb inhibitory or toxic substances, and plant growth regulators (PGRs) (reviewed by Moshkov et al. 2008). Charcoal has been used in all stages of somatic embryogenesis to increase initiation frequencies of Pinus taeda L. (Pullman and Johnson 2002), induce embryogenic tissue on vegetative shoot apices of mature trees of Pinus patula (Malabadi and Van Staden 2005), improve yield and quality of somatic embryos during maturation (Capuana and Debergh 1997; Carraway and Merkle 1997; Li et al. 1997, 1998; Groll et al. 2002; Pullman et al. 2005; Lelu-Walter et al. 2006), and most frequently during germination (Vooková and Kormuák 2001; Salaj et al. 2004; Andrade and Merkle 2005).
In a previous study, we focused on the effect of carbohydrate source during the maturation of Abies cephalonica Loud. embryogenic material (Krajňáková et al. 2008). In the present work our aim was to study the effect of two sucrose concentrations, PEG and AC supplements, four ABA concentrations and their interactions on maturation. Moreover, we investigated the after-effect of maturation media on germination of somatic embryos.
Materials and methods
Plant material
Embryogenic cultures of Abies cephalonica were initiated and sub-cultured as described by Krajňáková et al. (2008). The proliferating cultures were subcultured every 2 weeks and the cultures were maintained at +24°C in the dark. The embryogenic cell lines, 6 and 8, were selected for future maturation experiments.
Maturation of embryogenic cultures
Embryogenic cell masses (ECMs) between 275 ± 25 mg in fresh weight were placed on DCR maturation medium (Krajňáková et al. 2008) with 0.05% (w/v) casein hydrolysate, 1.7 mM l-glutamine (filter sterilized and added into the cooled media), and gelled by 0.25% (w/v) PhytagelTM. pH was adjusted to 5.7. We used 8 different maturation media which are presented in Table 1. Medium A included 87.6 mM sucrose and medium B 175.2 mM sucrose but no other supplements. Media C and D were supplemented with 1% (w/v) activated charcoal (Experiment 1) and media E, F, G and H included 5% (w/v) PEG-8000 (Experiment 2). With all maturation media 4 different concentrations of racemic ABA (4, 8, 16 or 32 μM ABA) were tested. Chemicals used in all experiments were purchased from Sigma. Sub-culturing was done every 4 weeks and the maturation phase took up to 12 weeks. The cultures were monitored at the time of sub-culturing and the mature embryos were transferred to germination medium.
Germination of mature somatic embryos and transfer into ex vitro conditions
Mature somatic embryos were germinated on half-strength DCR medium supplemented with 58 mM maltose, without growth regulators and solidified using 1% (w/v) agar (Krajňáková et al. 2008) at temperature 22 ± 2°C and the light intensity was kept low for the first 2 weeks of germination (30 μE m−2 s−1, 16 h photoperiod) and then it was gradually augmented reaching up to 74 μE m−2 s−1, 16 h photoperiod.
The successfully germinated embryos were transplanted to non-fertilized horticultural peat (pH 3.8, VAPO, Finland)−perlite (2:1) mixture and they were adapted to ex vitro conditions by gradually decreasing humidity. After 1 month the plants were transplanted into commercial fertilized peat (Vapo Oy, Jyväskylä, Finland). During the growth period, the plants were fertilized once with 0.2% 5-Superex fertilizer (Superex Oy, Kekkilä, Finland).
Experimental design and data analysis
The ability of ECMs, representing cell lines 6 and 8, to produce somatic embryos were evaluated and the effect of the following maturation media components were studied: 87.6 and 175.2 mM sucrose concentrations applied alone (maturation media without supplement, Control) were compared to media with activated charcoal for 1 week (AC, Experiment 1, EXP1) or to the media with PEG for 1 or 4 weeks (PEG1 or PEG4, Experiment 2, EXP2) (Table 1). The effect of each maturation medium was tested with 4 different concentrations of ABA (4, 8, 16 or 32 μM ABA). The number of ECMs tested varied between 18 and 25 per cell line and maturation media. The whole experiment was replicated twice.
Maturing ECMs were examined three times at 4-week intervals. Four characteristics were evaluated: (1) diameter (in the following classes; <10, 10–14, 15–19, >20 mm) of ECMs which represents the proliferation growth during maturation phase; (2) presence or absence of developing somatic embryos in ECMs, i.e., response of ECMs to maturation media; (3) the existence of early and late cotyledonary embryos, and (4) the number of somatic embryos per 1 g of fresh weight of embryogenic cell mass. Diameter scores represent an ordinally scaled response variable. Mean scores were modeled using the GLM procedure (SAS 1988). The proliferation growth was monitored after 4 and 8 weeks on maturation media.
The number of ECMs with somatic embryos was counted after 4, 8 and 12 weeks on maturation medium. At week 12, the proportion between cell masses with early and late cotyledonary embryos and cell masses with non-cotyledonary embryos was determined. The response of ECM to maturation treatments (presence or absence of developing somatic embryos, presence of early and late cotyledonary or non-cotyledonary embryos) were evaluated using generalized linear models. The probabilities of forming somatic embryos were modeled as linear response functions using weighted least-square analysis (procedure CATMOD, SAS 1988). Because of a large number of sampling zeros at 12 weeks, two partial models were used for the evaluation at this date: model 1 comprising the effects of cell line and carbohydrate type and model 2 comprising the effects of carbohydrate type and ABA. Correlations between proliferation growth and responses of embryogenic cell masses to maturation media were assessed (Pearson’s correlation coefficients).
The average number of all cotyledonary somatic embryos per 1 g of fresh cell mass was determined after 12 weeks maturation. The cell masses were divided into categories 1, 2 and 3 with the total number of embryos varying from 1 to 9, from 10 to 24 and from 25 to 50 embryos, respectively. The number of somatic embryos per 1 g of fresh weight of ECM was considered a Poisson-distributed random variable. To normalize the data and to make the mean independent of variance, we used fourth-root transformation (Quinn and Keough 2002). Transformed data were analyzed using factorial ANOVA (all variants effects were considered fixed). Procedure GLM (SAS 1988) was used.
For the evaluation of germination percentages generalized linear models were used (weighted least-square analysis, procedure CATMOD, SAS 1988).
Results
Proliferation growth during maturation
Altogether, the response of 632 ECMs from cell line 6 and 775 from cell line 8 were tested. Generally, proliferation growth of the cell lines decreased during the maturation phase (data not shown). The smallest average diameter of ECMs 11.5 and 11.9 mm were observed after 8 weeks cultivation of cell line 6 on the maturation media B and H or cell line 8 on medium H, respectively. On the contrary, the largest average diameter of ECMs being 14.8 and 17.7 mm of cell lines 6 and 8 were found on media A after 8-weeks cultivation.
When media of EXP1 (C, D) were compared with control media (A, B), the main factors (cell lines, carbohydrate and ABA concentrations) affected significantly (P < 0.05) the proliferation diameter of ECMs after an 8-week lasting maturation (Table 2). The interactions among the main factors (Table 2, 6 out of 11) also affected significantly the diameter of ECMs. The proliferation diameter of cell line 6 (13.1 mm) was significantly (P < 0.05) lower than that of cell line 8 (16.5 mm). The ECM diameters on the media with 32 μM ABA (14.1 mm) were significantly (P < 0.05) smaller than the diameters found on 4, 8 or 16 μM ABA containing media. Active charcoal did not significantly affect the diameter of ECMs. The higher concentration of sucrose (175.2 mM) significantly (P < 0.05) reduced the proliferation growth (diameter 13.3 mm) compared to 87.6 mM sucrose (diameter 16.4 mm).
In the case of EXP2, (media E, F, G, H were compared with control media A and B) the main factors (cell lines, supplement type, carbohydrate and ABA concentrations) and majority of interactions among them (9 out of 11) affected significantly (P < 0.05) diameter of ECMs after 8 weeks of proliferation (Table 2). Also in this experiment, the proliferation diameter of cell line 6 (12.9 mm) was significantly (P < 0.05) lower than that of cell line 8 (15.5 mm). The lowest diameter (13.1 mm) was observed when PEG4 was used as a media supplement. Similarly to EXP1, also in EXP2, the higher concentration of sucrose (175.2 mM) significantly (P < 0.05) reduced the proliferation growth (diameter 13.0 mm) when compared to media with 87.6 mM sucrose (diameter 15.4 mm).
Capability of ECMs to produce embryos on maturation media
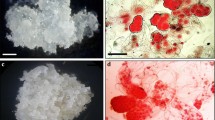
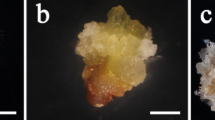
Early pre-cotyledonary embryos i.e., yellowish compact structures were observed after 4 weeks on maturation media. The cotyledonary embryos were predominantly found between the weeks 8 and 10 and onwards. For both cell lines, 6 and 8, the maturation medium H was the most successful obtaining 85% and 51% of ECMs with pre-cotyledonary and cotyledonary embryos, respectively. Maturation media A and F in the case of cell line 6 and 8, respectively, had very few ECMs with early and late cotyledonary embryos (data not shown).
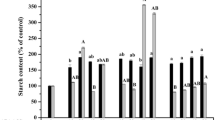
In EXP1, the capability of ECMs to produce cotyledonary embryos was significantly (P < 0.05) affected by the cell line, AC supplement, and interaction of cell line by AC supplement (Table 3, EXP1). Cell line 8 was significantly better than cell line 6 in the capacity of ECMs to produce somatic embryos, being 72.8% and 53.3%, respectively (Fig. 1a). The lowest capability of ECMs to form somatic embryos was observed on media with 4 μM ABA (Fig. 1b). AC supplement significantly increased the percentage of ECMs with cotyledonary embryos compared to control being 45.5% and 26.7%, respectively (Fig. 1c). Media with different sucrose concentrations did not significantly differ in the capability of ECMs to form somatic embryos (Fig. 1d).
Statistical evaluation of pairwise contrasts for percentage of embryogenic cell masses (ECMs) of Abies cephalonica with cotyledonary embryos on maturation media supplemented with AC. Columns with different letters are significantly different (P < 0.05). a Pairwise contrast between two embryogenic cell lines. b Pairwise contrasts among different abscisic acid (ABA) concentrations (4, 8, 16, and 32 μM). c Pairwise contrast between two different maturation supplements (none, AC). d Pairwise contrasts between two different concentrations of sucrose (86.7 and 175.2 mM)
In EXP2, cell line, PEG supplement, ABA concentration, carbohydrate concentration and interaction of cell line by supplement type and cell line by ABA concentration significantly (P < 0.05) affected the capability of ECMs to produce somatic embryos (Table 3, EXP 2). Cell line 6 was significantly better than line 8 in the capacity of ECMs to form somatic embryos (Fig. 2a). Higher concentrations of ABA (8, 16 and 32 μM) gave the significantly better number of ECMs with cotyledonary somatic embryos (Fig. 2b). Four week PEG supplement produced the highest percentage of ECMs with cotyledonary somatic embryos (Fig. 2c). On 87.6 mM sucrose medium the percentage of ECMs with cotyledonary embryos, 66.8%, was significantly higher than the corresponding percentage of ECMs, 53.7%, on medium supplemented with higher sucrose concentration (Fig. 2d).
Statistical evaluation of pairwise contrasts for percentage of embryogenic cell masses (ECMs) of Abies cephalonica with cotyledonary embryos on maturation media supplemented with PEG. Columns with different letters are significantly different (P < 0.05). a Pairwise contrast between two embryogenic cell lines. b Pairwise contrasts among different abscisic acid (ABA) concentrations (4, 8, 16, and 32 μM). c Pairwise contrasts among different maturation supplements (none, PEG1, PEG4). d Pairwise contrast between two different concentrations of sucrose (86.7 and 175.2 mM)
The analysis of the relationship between proliferation growth at the maturation phase and the capability of ECMs to produce embryos is shown in Table 4. The only significant correlation between proliferation diameter during maturation phase and the number of ECMs with somatic embryos (r = −0.47) was found at the 4 weeks time point on media with 87.6 mM sucrose (data not shown). However, the overall correlation became significant after 8 weeks of cultivation (r = −0.40), and so were also the correlations within several individual treatments (cell line 8, PEG1 and PEG4 supplements, ABA concentration 8 μM, media with 87.6 mM sucrose). Significant correlations are consistently negative, meaning that proliferation growth (increase of diameter) hampers the formation of somatic embryos.
Effects of maturation treatments on average number of somatic embryos
After 12 weeks on maturation media, the total numbers of early and late cotyledonary embryos were 1,928 and 883 in cell lines 6 and 8, respectively. The average numbers of embryos per 1 g of ECM fresh weight are presented in Table 5. The highest number of embryos per 1 g of fresh weight of ECM was obtained on the maturation media G (73.1 ± 25.6), cell line 6. In EXP1, from the main variables (cell line, AC supplement, ABA and carbohydrate concentrations), only ABA concentrations affected significantly (P < 0.05) the average number of embryos per 1 g of ECM fresh weight (Table 6, EXP1). However, the effect of supplement type seems also to depend on the combination with the other factors (see significant interactions AC supplement by ABA concentration and cell line by AC supplement and by ABA concentrations, and AC supplement type by carbohydrate concentration; Table 6, EXP1).
A different situation was observed in EXP2, where the main variables, cell line, PEG supplement, and ABA concentration affected significantly (P < 0.05) the average number of embryos per 1 g of ECM fresh weight (Table 6, EXP2). Also the majority of interactions among them were significant (8 out of 11). Carbohydrate concentrations do not exhibit any effect, not even in combination with the other factors (Table 6, EXP2). When PEG was used as a medium supplement, cell line 6 produced significantly higher average number of embryos (37.6 ± 15.2) compared to cell line 8 (10.4 ± 6.5) (Fig. 3a). Higher ABA concentrations (8, 16 and 32 μM) showed significant differences in average number of embryos for EXP2 (Fig. 3b) and 32 μM ABA was the best (29.8 ± 8.2). From the tested maturation supplements in EXP2, the highest average number of embryos per 1 g of ECM fresh weight was obtained with supplement PEG4 (36.5 ± 13.4) (Fig. 3c). The average number of embryos per 1 g of ECM fresh weight on maturation media supplemented with 87.6 mM sucrose was not significantly different compared to the average number of embryos obtained on higher sucrose media (Fig. 3d). Altogether, we obtained 1,737 cotyledonary embryos on media with a lower concentration of sucrose and 1,118 on media with higher sucrose concentration.
Statistical evaluation of pairwise contrasts for the mean number of somatic embryos per gram of embryogenic cell mass (ECM) fresh mass obtained for ECMs of Abies cephalonica on maturation media supplemented with PEG. Columns with different letters are significantly different (P < 0.05). a Pairwise contrast between two embryogenic cell lines. b Pairwise contrasts among different abscisic acid (ABA) concentrations (4, 8, 16, and 32 μM). c Pairwise contrasts among different maturation supplements (none, PEG1, PEG4). d Pairwise contrast between two different concentrations of sucrose (86.7 and 175.2 mM)
Effects of maturation treatments on germination percentage of somatic embryos
Germination percentages of somatic embryo plants ranged from 0.7% to 28.1% depending on the maturation media and cell line. In EXP1, neither cell line nor AC supplement affected the germination percentage (statistical evaluation not shown). In EXP2, cell lines, supplement type and their interaction influenced significantly (P < 0.05) the germination percentage (Table 7). With cell line 8, 14.9% embryos germinated which was significantly higher than 4.2% germinated embryos of cell line 6 (Fig. 4a). The best germination percentage was obtained with somatic embryos which maturated on media with no supplement added (germination percentage 18.3%). Significantly lower frequencies were obtained with embryos which maturated on media supplemented with PEG, being 4.7% and 4.4% for 4- and 1-week lasting application, respectively (Fig. 4b). Carbohydrate concentration did not affect germination rates (Fig. 4c).
Statistical evaluation of pairwise contrasts for percentage of germination of Abies cephalonica cotyledonary embryos which maturated on media supplemented with PEG. Columns with different letters are significantly different (P < 0.05). a Pairwise contrast between two embryogenic cell lines. b Pairwise contrasts among different maturation supplements (none, PEG1, PEG4). c Pairwise contrast between two different concentrations of sucrose (86.7 and 175.2 mM)
Discussion
The complete process of conifer plant regeneration through somatic embryogenesis encompasses a series of defined developmental steps, which require the proper execution of each step for successful completion of the next (Stasolla and Yeung 2003). Embryo development in conifers is initiated by arresting cell proliferation through the removal of auxins and cytokinins (Abies species require only the presence of cytokinins for ECM initiation) and is continued by application of abscisic acid (Stasolla et al. 2002).
In Abies species, intense proliferation is observed also during the maturation period (Find et al. 2002; Salaj et al. 2004, 2005) and represents a serious obstacle for further improvement of micropropagation technologies. In Abies nordmanniana (Stev.) Spach, PCIB [2-(p-chlorophenoxy) 2-methylpropionic acid], an auxin antagonist, was required to suppress tissue proliferation and promote embryo development. Somatic embryos were only able to mature on medium containing both PCIB and ABA (Find et al. 2002).
In the present work, somatic embryo development was negatively correlated with proliferation growth (measured as diameter of ECMs) during maturation phase. We found that 87.6 mM sucrose, maturation media with PEG (i.e., applied for 1 or 4 weeks) and 8 μM ABA significantly reduced the diameter of ECMs (i.e., proliferation growth) during maturation. Blanc et al. (2002) suggested that the slow growth of Hevea brasiliensis cultures during maturation is due to a specific physiological state of the cells entering the embryogenic pathway.
Recommended concentrations of sucrose in maturation media vary in different cultures, ranging from 1% to 6%, and the preference has been shown to be species or even cell-line specific (Garin et al. 2000; Nørgaard 1997). The beneficial effect of elevated sucrose levels on embryo maturation was firstly considered as being partly osmotic (for review see Lipavská and Konrádová 2004) but studies of Iraqi and Tremblay (2001a, b) demonstrated a developmental signalling or regulatory role of sucrose during the development of conifer somatic embryos.
The deteriorating effect of elevated sucrose concentrations on further development of somatic embryos was reported for A. nordmanniana (Nørgaard 1997) and hybrid fir Abies alba × A. numidica (Salaj et al. 2004). In our experiments, testing two different sucrose concentrations (87.6 or 175.2 mM), we have found that the differences in capability of ECMs to form cotyledonary embryos were influenced by the supplements used. Capability of ECMs to form cotyledonary embryos was higher on media with 175.2 mM sucrose, when AC was used as a supplement (Fig. 1d). On the other hand, the opposite effect of higher sucrose concentration was observed when the PEG supplements were tested (Fig. 2d). Sucrose concentrations in maturation media have not affected the average number of somatic embryos.
AC has been reported to adsorb auxins, cytokinins, vitamins, phenolic compounds, Fe-EDTA, copper and zinc ions and other media compounds (Pan and Van Staden 1998; Van Winkle et al. 2003; Pullman et al. 2005; Moshkov et al. 2008). The use of AC during maturation is mainly to adsorb plant growth regulators which were used during the proliferation period. High levels of ABA used together with AC have been reported to improve embryo yields and quality of coniferous and angiosperm species (Pullman et al. 2005; Lelu-Walter et al. 2006; Carraway and Merkle 1997; Capuana and Debergh 1997). In addition to mediating the available exogenous pulse of hormone (Pullman et al. 2005), AC can also alter endogenous hormones. Von Aderkas et al. (2002) quantified plant growth regulators during early development of somatic embryos of hybrid larch. Charcoal reduced 2,4-D concentrations of embryos and affected concentrations of five of the eight plant growth regulators quantified. In experiments of the above mentioned authors, AC did not affect embryo yield. These results are in full agreement with our experiments. AC supplement did not affect the average number of somatic embryos but the stimulatory effect of AC was noticed when the capability of ECMs to form cotyledonary embryos was considered. Among the Abies species, AC supplement during the maturation period was used only with A. alba (Hristoforoglu et al. 1995) and A. lasiocarpa (Kvaalen et al. 2005).
In recent years, PEG 4000 as a high molecular weight compound has become an excellent alternative to low molecular weight sugars and salts (Stasolla and Yeung 2003). Due to its large size, it is excluded from the symplast and can generate a long-lasting water stress (Attree and Fowke 1993). The beneficial effects of PEG were reported mainly for spruce somatic embryogenesis, where they increased embryo yield, desiccation tolerance, and accumulation of storage products (Attree and Fowke 1993; Misra et al. 1993; Kong and Yeung 1995). Recent studies focused on the effects of PEG on gene expression (Stasolla et al. 2003) and these authors reported changes in the transcript levels of many genes involved in sucrose catabolism, nitrogen assimilation and utilization, as well as the profound alterations in gene expression induced by water stress (Stasolla et al. 2004). Osmotic stress, through water depletion is viewed as an important signal in directing proper embryo development in many plant species (Von Aderkas and Bonga 2000).
The stimulatory effect of PEG on maturation process of Abies species was reported for A. nordmanniana by Nørgaard (1997), for A. numidica by Vooková and Kormut'ák (2002) and for Abies hybrids by Salaj and Salaj (2003), Salaj et al. (2004, 2005). In our experiments, the positive effect of a PEG supplement applied for 4 weeks was seen on the capability of ECMs to form cotyledonary somatic embryos (Fig. 2c) and on the average number of embryos per 1 g of ECMs fresh weight (Fig. 3c). However, the effect of supplement type seems to depend partially on the combination with other factors, especially cell line, ABA concentration and duration of PEG treatment (Table 6).
ABA is commonly used at various concentrations to improve somatic embryo development of Abies species prior to plantlet regeneration (e.g., Guevin et al. 1994; Salajová et al. 1996; Vooková and Kormut'ák 2001; Salajová and Salaj 2001). The response of ECMs to different ABA concentrations was evaluated either as the capability of ECMs to form somatic embryos or as the average number of embryos formed. Both supplements, AC and PEG, in combination with higher concentrations of ABA stimulated the capability of ECMs to form somatic embryos (Figs. 1b, 2b) and the average number of somatic embryos (Fig. 3b). The different response of tested cell lines to maturation treatments could be due to diverse ability of embryogenic tissue to utilize exogenous ABA as recently proposed by Kong and von Aderkas (2007).
Von Aderkas et al. (2002) speculated that charcoal treatment affected plantlet establishment of hybrid larch (Larix × marschlinsii Coaz), long after the charcoal treatment was stopped. Although charcoal did not improve significantly the yield or germination performance, it significantly improved plantlet establishment and reduced variability in the embryogenic response. Also Groll et al. (2002) working with somatic embryogenesis of Cassava (Manihot esculenta Crantz.) demonstrated that medium supplementation with activated charcoal had a positive effect on both differentiation and subsequent germination. We have obtained the best germination percentage with somatic embryos which matured on media supplemented with AC (21.6%).
The lowest percentages were noticed with somatic embryos regenerated on maturation supplements with PEG, being 9.1% and 4.4% for 4 and 1 week lasting application, respectively. Bozhkov and Von Arnold (1998) working with maturation of somatic embryos of Picea abies reported that, addition of 7.5% PEG to the maturation medium significantly inhibited somatic embryo germination for the vast majority of genotypes. Moreover, even after ex vitro transfer, both radicle elongation and lateral root formation were substantially suppressed in those plantlets produced from PEG-treated somatic embryos. Ramarosandratana et al. (2001a, b) argued that the development of shorter cotyledonary embryos of Pinus pinaster could not be attributed directly to the presence of PEG, but depended more on the carbohydrate source.
Salajová et al. (1996) observed low germination frequencies (12%) for fir hybrids, when the germination occurred without desiccation treatment. The positive effect of partial desiccation on the germination rate of A. numidica somatic embryos was described by Vooková and Kormut'ák (2001, 2006) increasing the germination frequencies up to 89.5%. Even if PEG has a well-known negative effect on root cap formation (Bozhkov and Von Arnold 1998; Svobodová et al. 1999), in the present study, the lower embryo germination frequencies might be due to no desiccation and cold treatments having been applied rather than to a PEG supplement.
References
Andrade GM, Merkle SA (2005) Enhancement of American chestnut somatic seedling production. Plant Cell Rep 24:326–334. doi:10.1007/s00299-005-0941-0
Attree SM, Fowke LC (1993) Embryogeny of gymnosperms—advances in synthetic seed technology of conifers. Plant Cell Tissue Organ Cult 35:1–35. doi:10.1007/BF00043936
Attree SM, Moore D, Sawhney VK, Fowke LC (1991) Enhanced maturation and desiccation tolerance of white Spruce [Picea glauca (Moench) Voss] somatic embryos—effects of a non-plasmolysing water stress and abscisic acid. Ann Bot (Lond) 68:519–525
Aussenac G (2002) Ecology and ecophysiology of circum-Mediterranean firs in the context of climate change. Ann For Sci 59:823–832. doi:10.1051/forest:2002080
Blanc G, Lardet L, Martin A, Jacob JL, Carron MP (2002) Differential carbohydrate metabolism conducts morphogenesis in embryogenic callus of Hevea brasiliensis (Mull. Arg.). J Exp Bot 53:1453–1462. doi:10.1093/jexbot/53.373.1453
Bozhkov PV, Von Arnold S (1998) Polyethylene glycol promotes maturation but inhibits further development of Picea abies somatic embryos. Physiol Plant 104:211–224. doi:10.1034/j.1399-3054.1998.1040209.x
Capuana M, Debergh PC (1997) Improvement of the maturation and germination of horse chestnut somatic embryos. Plant Cell Tissue Organ Cult 48:23–29. doi:10.1023/A:1005890826431
Carraway DT, Merkle SA (1997) Plantlet regeneration from somatic embryos of American chestnut. Can J Res 27:1805–1812. doi:10.1139/cjfr-27-11-1805
Find J, Grace L, Krogstrup P (2002) Effect of antiauxins on maturation of embryogenic tissue cultures of Nordmanns fir (Abies nordmanniana). Physiol Plant 116:231–237. doi:10.1034/j.1399-3054.2002.1160213.x
Garin E, Bernier-Cardou M, Isabel N, Klimaszewska K, Plourde A (2000) Effect of sugars, amino acids, and culture technique on maturation of somatic embryos of Pinus strobes on medium with two gellan gum concentrations. Plant Cell Tissue Organ Cult 62:27–37. doi:10.1023/A:1006402215457
Greguss L, Longauer R (1996) Survival of interspecific fir hybrids until the age of 15 years in three test plots. Lesnícky časopis 42:363–370
Greguss L, Longauer R, Krajňáková J (1994) Progress in the breeding program of specific hybridization of firs. In: Eder W (ed) Ergebnisse des 7 IUFRO Tannensymposium der WPS.1.01-08 Ökologie und Waldbau der Weißtanne. pp 144–153
Groll J, Gray VM, Mycock DJ (2002) Development of cassava (Manihot esculenta Crantz.) somatic embryos during culture with abscisic acid and activated charcoal. J Plant Physiol 159:437–443. doi:10.1078/0176-1617-00582
Guevin TG, Micah V, Kirby EG (1994) Somatic embryogenesis in cultured mature zygotic embryos of Abies balsamea. Plant Cell Tissue Organ Cult 37:205–208. doi:10.1007/BF00043618
Gupta PK, Durzan DJ (1985) Shoot multiplication from mature trees of Douglas-fir (Pseudotsuga menziesii) and sugar pine (Pinus lambertiana). Plant Cell Rep 4:643–645
Gupta PK, Pullman G, Timmis R, Kreitinger M, Carlson WC, Grob J, Welty E (1993) Forestry in the 21st Century. Bio-Technology 11:454–459
Häggman H, Jokela A, Krajňáková J, Kauppi A, Niemi K, Aronen T (1999) Somatic embryogenesis of Scots pine: cold treatment and characteristics of explants affecting induction. J Exp Bot 50:1769–1778. doi:10.1093/jexbot/50.341.1769
Hristoforoglu K, Schmidt J, Bolharnordenkampf H (1995) Development and germination of Abies alba somatic embryos. Plant Cell Tissue Organ Cult 40:277–283. doi:10.1007/BF00048134
Iraqi D, Tremblay FM (2001a) The role of sucrose during maturation of black spruce (Picea mariana) and white spruce (Picea glauca) somatic embryos. Physiol Plant 111:381–388. doi:10.1034/j.1399-3054.2001.1110316.x
Iraqi D, Tremblay FM (2001b) Analysis of carbohydrate metabolism enzymes and cellular contents of sugars and proteins during spruce somatic embryogenesis suggests a regulatory role of exogenous sucrose in embryo development. J Exp Bot 52:2301–2311. doi:10.1093/jexbot/52.365.2301
Keinonen-Mettälä K, Jalonen P, Eurola P, Von Arnold S, Von Weissenberg K (1996) Somatic embryogenesis of Pinus sylvestris. Scand J For Res 11:242–250
Klimaszewska K, Smith DR (1997) Maturation of somatic embryos of Pinus strobus is promoted by a high concentration of gellan gum. Physiol Plant 100:949–957. doi:10.1111/j.1399-3054.1997.tb00022.x
Klimaszewska K, Bernier-Cardou M, Cyr DR, Sutton BCS (2000) Influence of gelling agents on culture medium gel strength, water availability, tissue water potential, and maturation response in embryogenic cultures of Pinus strobus L. In Vitro Cell Dev Biol Plant 36(4):279–286. doi:10.1007/s11627-000-0051-1
Kong L, von Aderkas P (2007) Genotype effects on ABA consumption and somatic embryo maturation in interior spruce (Picea glauca × engelmanni). J Exp Bot 58:1525–1531. doi:10.1093/jxb/erm019
Kong LS, Yeung EC (1995) Effects of silver nitrate and polyethylene glycol on white spruce (Picea glauca) somatic embryo development—enhancing cotyledonary embryo formation and endogenous ABA content. Physiol Plant 93:298–304. doi:10.1111/j.1399-3054.1995.tb02232.x
Krajňáková J, Gömöry D, Häggman H (2008) Somatic embryogenesis in Greek fir. Can J Res 38:760–769. doi:10.1139/X07-141
Kvaalen H, Daehlen OG, Rognstad AT, Gronstad B, Egertsdotter U (2005) Somatic embryogenesis for plant production of Abies lasiocarpa. Can J Res 35:1053–1060. doi:10.1139/x05-035
Larsen JB (1986) Das Tannensterben: eine neue Hypothese zur Klärung dieser rätselhaften Komplexkrankheit der Weisstanne (Abies alba Mill.). Forstwiss Centralbl 195:381–396. doi:10.1007/BF02741747
Lelu-Walter MA, Bernier-Cardou M, Klimaszewska K (2006) Simplified and improved somatic embryogenesis for clonal propagation of Pinus pinaster (Ait.). Plant Cell Rep 25:767–776. doi:10.1007/s00299-006-0115-8
Li XY, Huang FH, Gbur EE (1997) Polyethylene glycol promoted development of somatic embryos in loblolly pine (Pinus taeda L.). In Vitro Cell Dev Biol Plant 33:184–189. doi:10.1007/s11627-997-0019-5
Li XY, Huang FH, Murphy JB, Gbur EE (1998) Polyethylene glycol and maltose enhance somatic embryo maturation in loblolly pine (Pinus taeda L.). In Vitro Cell Dev Biol Plant 34:22–26. doi:10.1007/BF02823118
Lipavská H, Konrádová H (2004) Somatic embryogenesis in conifers: the role of carbohydrate metabolism. In Vitro Cell Dev Biol Plant 40:23–30. doi:10.1079/IVP2003482
Malabadi RB, Van Staden J (2005) Somatic embryogenesis from vegetative shoot apices of mature trees of Pinus patula. Tree Physiol 25:11–16
Maruyama E, Hosoi Y (2007) Polyethylene glycol enhance somatic embryo production in Japanese cedar (Cryptomeria japonica D. Don). Propag Ornam Plants 7(2):57–61
Misra S, Attree SM, Leal I, Fowke LC (1993) Effect of abscisic acid, osmoticum, and desiccation on synthesis of storage proteins during the development of white spruce embryos. Ann Bot (Lond) 71:11–22. doi:10.1006/anbo.1993.1002
Moshkov IE, Novikova GV, Hall MA, George EF (2008) Plant growth regulators III: gibberellins, ethylene, abscisic acid, their analogues and inhibitors; miscellaneous compounds. In: George EF, Hall MA, De Klerk GJ (eds) Plant propagation by tissue culture, 3rd edn, vol 1. The background. Springer, Dordrecht, The Netherlands, pp 227–282
Nørgaard JV (1997) Somatic embryo maturation and plant regeneration in Abies nordmanniana Lk. Plant Sci 124:211–221. doi:10.1016/S0168-9452(97)04614-1
Pan MJ, Van Staden J (1998) The use of charcoal in in vitro culture—a review. Plant Growth Regul 26:155–163. doi:10.1023/A:1006119015972
Pullman GS, Johnson S (2002) Somatic embryogenesis in loblolly pine (Pinus taeda L.): improving culture initiation rates. Ann For Sci 59:663–668. doi:10.1051/forest:2002053
Pullman GS, Gupta PK, Timmis R, Carpenter C, Kreitinger M, Welty E (2005) Improved Norway spruce somatic embryo development through the use of abscisic acid combined with activated carbon. Plant Cell Rep 24:271–279. doi:10.1007/s00299-005-0933-0
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge, p 537
Ramarosandratana A, Harvengt L, Bouvet A, Calvayrac R, Paques M (2001a) Influence of the embryonal-suspensor mass (Esm) sampling on development and proliferation of maritime pine somatic embryos. Plant Sci 160:473–479. doi:10.1016/S0168-9452(00)00410-6
Ramarosandratana A, Harvengt L, Bouvet A, Calvayrac R, Paques M (2001b) Effects of carbohydrate source, polyethylene glycol and gellan gum concentration on embryonal-suspensor mass (Esm) proliferation and maturation of maritime pine somatic embryos. In Vitro Cell Dev Biol Plant 37:29–34. doi:10.1007/s11627-001-0006-1
Salaj T, Salaj J (2003) Somatic embryo formation on mature Abies alba × Abies cephalonica zygotic embryo explants. Biol Plant 47:7–11. doi:10.1023/A:1027312410957
Salaj T, Matúšová R, Salaj J (2004) The effect of carbohydrates and polyethylene glycol on somatic embryo maturation in hybrid fir Abies alba × Abies numidica. Acta Biol Crac Ser; Bot 46:159–167
Salaj T, Vooková B, Salaj J (2005) Protocols for somatic embryogenesis of hybrid firs. In: Jain SM, Gupta PK (eds) Protocol for somatic embryogenesis in woody plants. Springer, Netherlands, pp 483–496
Salajová T, Salaj J (2001) Somatic embryogenesis and plantlet regeneration from cotyledon explants isolated from emblings and seedlings of hybrid firs. J Plant Physiol 158:747–755. doi:10.1078/0176-1617-00278
Salajová T, Jásik J, Kormuť'ák A, Salaj J, Hakman I (1996) Embryogenic culture initiation and somatic embryo development in hybrid firs (Abies alba × Abies cephalonica, and Abies alba × Abies numidica). Plant Cell Rep 15:527–530
Salajová T, Salaj J, Kormut'ák A (1999) Initiation of embryogenic tissues and plantlet regeneration from somatic embryos of Pinus nigra Arn. Plant Sci 145:33–40. doi:10.1016/S0168-9452(99)00067-9
SAS (1988) SAS/STAT® user’s guide, release 6.03 edition. SAS Institute, Cary
Stasolla C, Yeung EC (2003) Recent advances in conifer somatic embryogenesis: improving somatic embryo quality. Plant Cell Tissue Organ Cult 74:15–35. doi:10.1023/A:1023345803336
Stasolla C, Kong LS, Yeung EC, Thorpe TA (2002) Maturation of somatic embryos in conifers: morphogenesis, physiology, biochemistry, and molecular biology. In Vitro Cell Dev Biol Plant 38:93–105
Stasolla C, Van Zyl L, Egertsdotter U, Craig D, Liu WB, Sederoff RR (2003) The effects of polyethylene glycol on gene expression of developing white spruce somatic embryos. Plant Physiol 131:49–60. doi:10.1104/pp.015214
Stasolla C, Belmonte MF, Van Zyl L, Craig D, Liu W, Yeung EC, Sederoff R (2004) The effect of reduced glutathione on morphology and gene expression of white spruce (Picea glauca) somatic embryos. J Exp Bot 55:695–709. doi:10.1093/jxb/erh074
Sutton BCS, Attree SM, El-Kassaby YA, Grossnickle SC, Polonenko DR (2004) Commercialisation of somatic embryogenesis for plantation forestry. In: Walter C, Carson M (eds) Plantation forest biotechnology for the 21st century. Research Signpost, Kerala, India, pp 275–301
Svobodová H, Albrechtová J, Kumstyřová L, Lipavská H, Vágner M, Vondraková Z (1999) Somatic embryogenesis in Norway spruce: anatomical study of embryo development and influence of polyethylene glycol on maturation process. Plant Physiol Biochem 37:209–221. doi:10.1016/S0981-9428(99)80036-9
Timmis R (1998) Bioprocessing for tree production in the forest industry: conifer somatic embryogenesis. Biotechnol Prog 14:156–166. doi:10.1021/bp970143y
Van Winkle SC, Johnson S, Pullman GS (2003) The impact of gelrite and activated carbon on the elemental composition of two conifer embryogenic tissue initiation media. Plant Cell Rep 21:1175–1182. doi:10.1007/s00299-003-0637-2
Von Aderkas P, Bonga JM (2000) Influencing micropropagation and somatic embryogenesis in mature trees by manipulation of phase change, stress and culture environment. Tree Physiol 20:921–928
Von Aderkas P, Label P, Lelu MA (2002) Charcoal affects early development and hormonal concentrations of somatic embryos of hybrid larch. Tree Physiol 22:431–434
Vooková B, Kormut'ák A (2001) Effect of sucrose concentration, charcoal, and indole-3 butyric acid on germination of Abies numidica somatic embryos. Biol Plant 44:181–184. doi:10.1023/A:1010278704613
Vooková B, Kormut'ák A (2002) Some features of somatic embryo maturation of Algerian fir. In Vitro Cell Dev Biol Plant 38:549–551. doi:10.1079/IVP2002330
Vooková B, Kormut'ák A (2003) Plantlet regeneration in Abies cilicica Carr. and Abies cilicica × Abies nordmanniana hybrid via somatic embryogenesis. Turk J Bot 27:71–76
Vooková B, Kormut'ák A (2006) Comparison of induction frequency, maturation capacity and germination of Abies numidica during secondary somatic embryogenesis. Biol Plant 50:785–788. doi:10.1007/s10535-006-0132-z
Acknowledgments
The Finnish Forest Research Institute (Metla), Punkaharju Research Unit (Finland) is acknowledged for providing the working facilities and the ability to conduct the experiments. We especially thank Ms. Aila Viinanen, Mr Jouko Lehto (from Metla) and Ms. Danica Trangošová (from the National Forest Centre, Forest Research Institute, Slovakia) for their excellent technical assistance. The research was funded by Centre for International Mobility. JK is grateful to CIMO for a Finnish Government scholarship and COST 834 for providing her with STSM.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krajňáková, J., Häggman, H. & Gömöry, D. Effect of sucrose concentration, polyethylene glycol and activated charcoal on maturation and regeneration of Abies cephalonica somatic embryos. Plant Cell Tiss Organ Cult 96, 251–262 (2009). https://doi.org/10.1007/s11240-008-9482-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-008-9482-x