Abstract
Agrobacterium rhizogenes-mediated transformation has become a powerful tool for studying gene function and root biology due to its quick and simple methodology. This transformation method is particularly suitable for those plants, including legumes, whose transformation using Agrobacterium tumefaciens has been challenging. Although there are some reports on A. rhizogenes-mediated transformation of legumes to produce ‘composite’ plants, conditions influencing A. rhizogenes-mediated transformation of soybean [Glycine max (L.) Merr.] have not been yet fully investigated. To better understand A. rhizogenes-mediated root transformation in soybean, we have evaluated the impact of genotype, plant age for infection, bacterial inoculating concentration, inoculation temperature, and other factors on transformation of soybean. The results have shown that there are significant differences among soybean genotypes in their susceptibility to A. rhizogenes. Soybean cv. Zigongdongdou is the most susceptible to A. rhizogenes strain K599 among 10 genotypes tested. The effects of seedling age have been evaluated, and 1-day-old plantlets are found to be optimal for hairy root induction. There are no significant differences in hairy root induction for bacterial suspension from OD600 = 0.2 to OD600 = 1.2. Under 16 h photoperiod, hairy roots can be induced both at 23°C/20°C and 28°C/25°C, but not at 33°C/30°C as day/night temperature regimes. Using this transformation protocol, almost 100% of the composite plants formed hairy roots within 2 weeks, and based on GUS histochemical analysis, 94.2% transformation frequency is obtained. Transgene integration has been also confirmed by Southern blot analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Genetic transformation is of great importance for studying gene function and molecular farming. Although numerous methods have been developed for introducing genes into plants, the transformation efficiency for some recalcitrant plants such as soybean [Glycine max (L.) Merr.] still remains low. Since the first successful transformation of soybean was reported (Hinchee et al. 1988), two major methods have been used in soybean transformation: one is particle bombardment of embryogenic tissue and another is Agrobacterium tumerfaciens-mediated transformation. Both methods have limitations: the former is highly genotype-dependent, requires a prolonged tissue culture period and tends to produce multiple insertion events, while the latter is labor intensive and requires special trained personnel to undertake the work. As a result, in spite of previous improvements (Olhoft et al. 2003; Paz et al. 2004; Zeng et al. 2004), soybean transformation is still challenging for most of laboratories. It is estimated that only if one performer can produce at least 300 transgenic lines every year, can the needs of existing soybean genomics initiatives be met (Olhoft et al. 2003). Obviously, few laboratories can hit this target to date.
In some species, a rapid and simple method of hairy root transformation with Agrobacterium rhizogenes has been developed. Such a system can be used to produce secondary metabolites (Lodhi et al. 1996; Fujimoto et al. 2000; Sevon et al. 2002; Moyano et al. 2003), characterize promoters (Xiao et al. 2005), analyze gene function in connection with root development (Ivashuta et al. 2005), and regenerate whole plant (Cho and Wildholm 2002; Kuntal et al. 2006). In recent years, a new transformation method has been developed which represented a significant advancement due to its less time consuming to generate transgenic plant tissue. This method produces ‘composite’ plant consisting of a wild-type non-transgenic shoot and transgenic hairy roots (Hansen et al. 1999). As a potential substitution of A. tumerfaciens transformation, the system has now been successfully used in legumes mainly to identify genes related to nodulation and mycorrhizal symbiosis (Ane et al. 2004; Bersoult et al. 2005; Frendo et al. 2005; Kaló et al. 2005; Limpens et al. 2005; Vieweg et al. 2005). Soybean transformation using A. rhizogenes has also been reported elsewhere (Cheon et al. 1993; Lee et al. 1993; Taylor et al. 2006; Hayashi et al. 2008). To date the hairy root transformation can be implemented through ‘ex vitro’ which is less costly than either in vitro or the infection (Collier et al. 2005; Kereszt et al. 2007).
Similar to A. tumefaciens, A. rhizogenes transfers its endogenous T-DNA from the extrachromosomal replicon, called the root-inducing (Ri) plasmid, into the plant genomic DNA. As most previous studies reported on A. tumefaciens, factors such as plant genotypes, bacterial density, inoculation temperature and the age of plants are known to affect T-DNA transfer. However, there is very limited information on evaluation of composite plant production by A. rhizogenes. Therefore, this study was undertaken to assess the effect of plant genotypes, bacterial inoculating concentrations, different inoculation temperatures and seedling ages on soybean hairy roots induction.
Materials and methods
Agrobacterium rhizogenes strain and plasmid
The vector pCAMBIAl305.1113 or pGFPGUSPlus (Vickers et al. 2007; EF546437) (a kind gift from Dr. Peter Gresshoff, University of Queensland, Australia) was introduced into A. rhizogenes strain K599 (NCPPB2659) by electrotransformation (Gene Pulser Xcell, Bio-Rad). The pCAMBIAl305.1113 was created by replacing the GUS fragment of pGFPGUSPlus with a GmNMH7 gene coding region (AY310303). The transformed A. rhizogenes were streaked onto solid LB media containing 50 mg l−1 Kanamycin. Then single isolated colonies were incubated overnight at 28°C in 40 ml liquid LB media culture with shaking at 180 rpm. In the next morning, the cultures were resuspended in sterile distilled water for infection.
Plant material
Ten different soybean genotypes including Beifeng 11, Heihe 27, Jindou 19, Suinong 14, Yudou 25, Yuechun 04–5, Zhongdou 19, Zhonghuang 40, Zhongpin 661, and Zigongdongdou were tested to evaluate the effect of plant genotypes on the A. rhizogenes-mediated transformation. Soybean seeds were surface-sterilized for 16 h using chlorine gas produced by mixing 3.5 ml of 12 N HCl and 100 ml commercial bleach in a tightly sealed desiccator. The sterilized seeds were germinated in flowerpots covered with transparent plastic film as a wrap to keep high humidity and then kept in greenhouse. For each genotype, 30 uniform seedlings were selected for inoculation.
Induction of hairy roots
To assess the effect of seedling age on the DAI, 0–5 day(s) old seedlings (Fig. 1a showed the 1-day-old seedlings), about 20 seedlings for each age, were injected with A. rhizogenes by stabbing at the cotyledonary node twice with a syringe needle (Fig. 1b). To prevent the influences of seed quality and seeding depth, the uniform seedlings were selected for inoculation. The seedlings which just emerged out of vermiculite surface and the cotyledons were still yellow (about 3 days after sowing) were defined as 0-day-old seedlings.
Soybean hairy root transformation. a 1-day seedlings used for infection; b Inoculation of A. rhizogenes around the cotyledonary node area using a syringe needle; c Composite plant 10 days after inoculation, and then the primary roots were removed; d Transformed root grown in greenhouse for one month
To evaluate the effect of bacterial density on root transformation, four different A. rhizogenes concentrations (OD600 = 0.2, 0.6, 1.0, or 1.3) were compared. The freshly harvested bacterial culture as described above was then resuspended in distilled water before the infection. Thirty plants were inoculated for each treatment and DAI was recorded.
To analyze the effect of culture temperatures on transformation, three different day/night temperature regimes (23°C/20°C, 28°C/25°C, or 33°C/30°C) were applied to growth chambers immediately after soybean plantlets, 35 plants for each treatment, were inoculated with A. rhizogenes.
To establish an efficient, simple and credible protocol for A. rhizogenes-mediated transformation system, we used Zigongdongdou as material and combined the optimal parameters of the various conditions as determined in the above single-factor experiments. About 30 composite plants transformed with K599 harboring vector pGFPGUSPlus were analyzed with GUS histochemical analysis when hairy roots were about 2–3 cm long, and the number of GUS+ plants was recorded in each of the three replications. To further confirm the transformation, 50 composite plants, induced by K599 harboring vector pCAMBIAl305.1113, with removal of primary roots by cutting the hypocotyl approximately 1 cm under the wounded site where the hairy roots were formed (Fig. 1c), were transplanted in new flowerpots and were kept in greenhouse. One mouth later, some roots were further analyzed by PCR and Southern blot.
Assay for β-glucuronidase activity
The hairy roots (about 1–2 cm in length) were assayed for GUS gene expression by histochemical staining using a modified method of Jefferson et al. (1987). Both wild-type (negative control) and putatively transformed roots were incubated at 37°C in the dark in an X-Gluc solution. The X-Gluc solution contained 50 mM Na3PO4 (pH 7.0), 10 mM Na2EDTA, 0.1% (v/v) Triton X-100, 0.1 M K3[Fe(CN)6], 0.1 M K4[Fe(CN)6], 0.5 g l−1 5-bromo-4-chloro-3-indolyl-β-d-glucuronide (X-Gluc) and 20% methanol. The roots were subsequently washed in an ethanol gradient at room temperature (30 min in 70% ethanol, 30 min in 40% ethanol, and then 30 min in 20% ethanol). After rehydration, the roots were kept in water and then mounted on a slide for microscopic observations (OLYMPUS BX51).
PCR and Southern blot analysis
Genomic DNA was extracted from putatively transformed and untransformed hairy roots tissues using a CTAB method as described by Murray and Thompson (1980). Genomic DNA was extracted from putatively transformed and untransformed hairy roots tissues. PCR analysis was performed with a pair of GFP gene specific primers: primer 1: 5′-CTTCTCGTTGGGGTCTTT-3′ and primer 2: 5′-ACAAGTTCAGCGTGTCCG-3′. The PCR amplification was performed under the conditions of pre-denaturing at 94°C for 5 min, denaturing at 94°C for 30 s, annealing at 57°C for 50 s, and prolonging at 72°C 50 s for 30 cycles. The gel was stained in SYBR Green I Nucleic Acid Gel Stains, and visualized under UV illumination.
About 30 μg of genomic DNA was completely digested with EcoR I (a single restriction site in the plasmid), separated on a 0.7% agarose gel and transferred to Hybond N membrane (Amersham Biosciences, UK). The membrane was hybridized with α-32P-labeled 915 bp fragment of the GmNMH7 coding region at 65°C for 16 h according to the instruction of Prime-a-Gene Labeling System (Promega), then washed in solutions of SDS detergent and SSC to remove excess probe, and then exposed to X-ray film at −80°C and subsequently developed.
Statistical analysis
Data were analyzed with SPSS (Version 11.5) using LSD Multiple Comparison Test and means were separated at α = 0.05 or 0.01 level. Transformation frequency is calculated by the number of composite plants divided by the total number of inoculated seedlings.
Results and discussion
Genotypic effects on DAI, the number of hairy roots and the transformation frequency
Ten soybean genotypes were transformed with A. rhizogenes strain K599 harboring the vector pGFPGUSPlus. Significant differences were found among genotypes in DAI, the number of hairy roots and the transformation frequency. The average DAI were less than 8 in Zigongdongdou, 11 in Heihe 27 and between 8 and 11 in remaining genotypes. The numbers of hairy roots were significantly different among different genotypes (P < 0.05) with Yuechun 04–5 being about six times more than that of Yudou 25 (Table 1). Although not all hairy roots were transgenic, a plant is considered to be transgenic as long as it is composite, i.e., consisting of transgenic hairy roots as detected by GUS gene expression. The genotype Zigongdongdou displayed the highest transformation frequency at over 93% contrasting to Zhongdou 19 at only 30% (Table 2). The transformation frequency of each of three other genotypes, Yuechun 04–5, Suinong 14 and Zhongpin 661, was over 80%. The average number of transgenic hairy roots was 1.9 for each composite plant (except Zhongdou 19) in the current experiment (data not shown).
In the soybean hairy root transformation system, it is desirable for a soybean genotype to have short DAI, a large number of hairy roots and a high transformation frequency. However, the present study illustrated no single genotype posses all these desirable characters. For example, Yuechun 04–5 produced the most number of hairy roots among 10 genotypes evaluated but its DAI was longer than that of Zhongpin 661 and Zigongdongdou (Table 1); Zhongpin 661 had a relatively shorter DAI but produced a much less number of hairy roots than many other genotypes and displayed a lower transformation frequency (only 80%). Of all 10 genotypes, Zigongdongdou and Yuechun 04–5 were the top two candidates as hairy root transformation materials when the DAI, the number of hairy roots and the transformation frequency were overall considered. Therefore, Zigongdongdou was used in our subsequent experiments.
Previously, the susceptibility of soybean genotypes to A. rhizogenes infection was reported (Savka et al. 1990; Cho et al. 2000). However, these studies concentrated on the genotype capacity to produce hairy roots using the cotyledons as the explant with no comparison of susceptibility among different genotypes. In this study, we assayed the genotypic effects on DAI, the capacity of hairy root production and the transformation frequency and identified two genotypes that are superior for transformation by A. rhizogenes strain K599. We emphasize that a short DAI is important because of space and time saving.
Effect of seedling age on DAI
The experiment was conducted to evaluate the infection age of seedlings. Twenty seedlings were used for each treatment. The hairy roots were induced at all six different ages of seedlings (0, 1, 2, 3, 4, 5 days old), but the average DAI of 0-day-old and 1-day-old seedlings was much shorter than the others (Table 3). When infections were conducted with the seedlings which were above 1-day-old, the average DAI was above eight and there were no significant difference among ages. This result indicated that hairy roots can be induced on a wide range of ages of seedlings, and it is better to use younger seedlings than the older ones for rapid transformation.
Effect of A. rhizogenes concentration for infection on DAI
Four different inoculating concentrations (optical density: OD600 = 0.2, 0.6, 1.0, or 1.3) of A. rhizogenes were used to determine the optimal concentration for transformation. The average DAI were approximate 9 days, but there was no significant difference among the four concentrations on DAI (data not shown). This result was different from the previous reports using A. tumefaciens-mediated transformation in which the optimal inoculation concentration was OD600 = 0.7–0.8 (Paz et al. 2005) but concurs with the results of Kereszt et al. (2007). And the similar results were observed with the concentrated paste. It is likely that strain K599 was so effective that even a low bacterial inoculum was sufficient enough to induce hairy roots on cotyledons of Zigongdongdou. On the other hand, a high bacterial concentration was not detrimental to the cells or tissues of composite soybean plants. Therefore, the effect of A. rhizogenes concentration for infection on composite soybean plants was little. We subsequently used the overnight low concentration (OD600 ≈ 0.3) of A. rhizogenes for inoculation in the following experiments.
Effect of temperatures on DAI
Three different day/night temperature regimes (23°C/20°C, 28°C/25°C or 33°C/30°C) were examined in this study. There was barely hairy root production at continuous high temperatures (33°C/30°C). At 28°C/25°C, the average DAI were 6.4, and the hairy roots emerged continuously and grew well in all soybean plantlets. At 23°C/20°C, the average DAI were 8.3 (Table 4). At 33°C/30°C, only three plantlets each produced one hairy root 6.0 days after inoculation and died 3 days latter. Apparently, 28°C/25°C was optimal for A. rhizogenes-mediated hairy root induction in soybean, and a higher temperature (33°C/30°C) was harmful for hairy root induction. That fact that hairy roots were induced at both 28°C/25°C and 23°C/20°C, suggests that the hairy roots can be induced at least within the temperature range from 20°C to 28°C.
Inoculation temperature has been considered as a factor affecting Agrobacterium to transfer the T-DNA to plant cells (Fullner and Nester 1996; Banta et al. 1998; Bash and Matthysse 2002). Baron et al. (2001) demonstrated that incubation of explants with Agrobacterium at 28°C but not at 26°C strongly inhibited extracellular assembly of the major T-pilus component VirB2 as well as of pilus-associated protein VirB5. Fullner and Nester (1996) showed that 19°C was the optimal temperature for transfer. Salas et al. (2001) indicated that 19°C may be the best temperature for the Agrobacterium transfer machinery, and co-culture at 25°C appears to be beneficial for plant cell susceptibility to infection and for stable T-DNA insertion into the plant chromosomes. Under our experimental conditions, 28°C/25°C was found to be optimal for A. rhizogenes to induce hairy roots. It is possible that the hairy roots grow faster at 28°C/25°C than at 23°C/20°C. The lack of hairy roots at 33°C/30°C is also consistent with previous observations on plant tumor induction that A. tumefaciens does not form tumors at 31.5°C (Lin and Kado 1977).
Other results in our laboratory showed that the inhibition by high temperature on the hairy roots induction was greatly alleviated when the plantlets were first inoculated at 25°C for one day and then moved to 33°C/30°C (data not shown), suggesting that a short time co-culture of A. rhizogenes with plant cells at appropriate temperature was inductive to hairy root growth. This treatment analogy can be applied to the ‘composite’ plants which, after inoculated with Agrobacterium, can be moved to the outside-lab conditions for large-scale propagation.
Other transformation-related factors including humidity, light, and the infection site were also investigated in the present study. No hairy roots were induced when soybean seedlings were cultured in a pot without covering by plastic bag after inoculation, indicating that high humidity is essential for the hairy root induction. Previous reports also illustrated that light could affect the A. tumefaciens transformation during the co-cultivation (Zambre et al. 2003). Our results showed that there was no remarkable difference between strong and weak illuminations in hairy root induction (data not shown). The results also indicated that, besides the cotyledonary node, many other organs and parts of the plant, such as hypocotyl, stem and cotyledon, can also be used as sites of infection to induce hairy roots.
A rapid and highly efficient soybean composite plant production
By combining the optimal conditions determined above, we established a rapid and efficient A. rhizogenes-mediated root transformation system of soybean as highlighted in Fig. 1. The protocol can be best illustrated by soybean cv Zigongdongdou as follows: 1-day-old seedlings (about 4 days after sowing) are inoculated at the cotyledonary node with A. rhizogenes strain K599 (containing a binary vector such as pGFPGUSPlus or pCAMBIAl305.1113) (Fig. 2) suspension (OD600 ≈ 0.3) and the inoculated plants are kept in 12 h light/12 h dark at 28°C/25°C in a growth chambers for about 6 days until hairy roots appear. Then the plantlets can be transplanted into new flowerpots and kept in the greenhouse for further analysis. At day 11 after inoculation, the number of hairy roots per plant was 10.8, the frequency of composite plants showing expression of GUS (Fig. 3) was 94.2% (Table 5). The number of transgenic hairy roots for each GUS-positive composite plant ranged from 1 to 5.
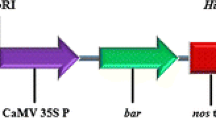
a Binary vector T-DNA region of vector pCAMBIAl305.1113 used for soybean transformation. GmNMH7 is the probe for Southern blotting DNA analysis. b Binary vector T-DNA region with GUS of vector pGFPGUSPlus used for plant transformation. LB, T-DNA left border; HPTII, hygromycin phosphotransferases, hygromycin resistance gene; 2 × 35S, CaMV35S eukaryotic promoter with duplicated enhancer region; NOS, nos terminator; GFP, green fluorescent protein, S565T variant; 35S, CaMV35S promoter; GUS, GUSPlus-His6, beta-glucuronidase; RBR, T-DNA right border repeat. GmNMH7, Glycine max NMH7, NMH7: Nodule MADS-box Homologue 7
For preliminary screening of composite plants, we did PCR analysis of GFP gene from hairy root tissues of 18 composite plants transformed with vector pCAMBIAl305.1113 a month after inoculation. The results showed that hairy roots from all PCR-positive plants carried the GFP gene (Fig. 4). For Southern blot analysis, soybean genomic DNA from hairy roots of composite plants was digested with EcoR I and the stable integration of the transgene into the hairy root genome was confirmed using an α-32P-labeled GmNMH7 probe (Fig. 5). No hybridization signal was detected in wild-type negative control hairy roots induced by A. rhizogenes strain K599 without vector pCAMBIAl305.1113.
Southern blot analysis of transgenic soybean hairy roots. Genomic soybean genomic DNA (approximately 30 μg) was digested with EcoR I, which recognizes a single site within pCAMBIAl305.1113, and hybridized with the GmNMH7 probe. 1–2. pCAMBIAl305.1113 plasmid DNA digested with EcoR I; 3. Marker probed with GeneRuler DNA Ladder Mix; 4–6. DNA from transgenic hairy roots transformed with pCAMBIAl305.1113; 7. DNA from wild-type hairy roots induced by K599 (containing no vector) as negative control
In summary, previous studies were conducted to investigate the conditions affecting A. rhizogenes-mediated transformation in some plant species, but no assessments have been done in soybean. Here, we assessed the conditions affecting the hairy root transformation process based on the previously published results (Kereszt et al. 2007). Two soybean genotypes, Zigongdongdou and Yuechun 04–5, were found to be superior for genetic transformation mediated by A. rhizogenes K599. Other factors that influence the hairy root induction and the transformation efficiency were also studied. The protocol described in this study is rapid, simple and little environment-dependent and therefore, can be used in the outdoor conditions and for large-scale experiments of gene functional characterization. And using this protocol, salt tolerance genes were analyzed well in our lab (data not shown).
Abbreviations
- DAI:
-
Days of hairy root emergence after bacterial infection
References
Ane JM, Kiss GB, Riely BK et al (2004) Medicago truncatula DMI1 required for bacterial and fungal symbioses in legumes. Science 303:1364–1367. doi:10.1126/science.1092986
Banta LM, Bohne J, Lovejoy SD et al (1998) Stability of the Agrobacterium tumefaciens VirB10 protein is modulated by growth temperature and periplasmic osmoadaption. J Bacteriol 180:6597–6606
Baron C, Domke N, Beinhofer M et al (2001) Elevated temperature differentially affects virulence, VirB protein accumulation, and T-pilus formation in different Agrobacterium tumefaciens and Agrobacterium vitis strains. J Bacteriol 183:6852–6861. doi:10.1128/JB.183.23.6852-6861.2001
Bash R, Matthysse AG (2002) Attachment to roots and virulence of a chvB mutant of Agrobacterium tumefaciens are temperature sensitive. Mol Plant Microbe Interact 15:160–163. doi:10.1094/MPMI.2002.15.2.160
Bersoult A, Camut S, Perhald A et al (2005) Expression of the Medicago truncatula DMI2 gene suggests roles of the symbiotic nodulation receptor kinase in nodules and during early nodule development. Mol Plant Microbe Interact 18:869–876. doi:10.1094/MPMI-18-0869
Cheon CI, Lee N, Sissique A et al (1993) Roles of plant homologues of Rab1p and Rab7p in the bio-genesis of the peribacteroid membrane, a subcellular compartment formed de novo during root nodule symbiosis. EMBO J 12:4125–4135
Cho HJ, Wildholm JM (2002) Improved shoot regeneration protocol for hairy roots of legume Astragalus sincus. Plant Cell Tissue Organ Cult 69:259–269. doi:10.1023/A:1015624316573
Cho HJ, Farrand SK, Noel GR et al (2000) High-efficiency induction of soybean hairy roots and propagation of the soybean cyst nematode. Planta 210:195–204. doi:10.1007/PL00008126
Collier R, Fuchs B, Walter N et al (2005) Ex vitro composite plants: an inexpensive, rapid method for root biology. Plant J 43:449–457. doi:10.1111/j.1365-313X.2005.02454.x
Frendo P, Harrison J, Norman C et al (2005) Glutathione and homoglutathione play a critical role in the nodulation process of Medicago truncatula. Mol Plant Microbe Interact 18:254–259. doi:10.1094/MPMI-18-0254
Fujimoto Y, Ohyama K, Nomura K et al (2000) Biosynthesis of sterols and ecdysteroids in Ajuga hairy roots. Lipids 35:279–288. doi:10.1007/s11745-000-0524-z
Fullner KJ, Nester EW (1996) Temperature affects the T-DNA transfer machinery of Agrobacterium tumefaciens. J Bacteriol 178:1498–1504
Hansen AC, Busk H, Marcker A et al (1999) VsENBP1 regulates the expression of the early nodulin PsENOD12B. Plant Mol Biol 40:495–506. doi:10.1023/A:1006238303309
Hayashi S, Gresshoff PM, Kinkema M (2008) Molecular analysis of lipoxygenases associated with nodule development in soybean. Mol Plant Microbe Interact 21:843–853. doi:10.1094/MPMI-21-6-0843
Hinchee M, Connorward DV et al (1988) Production of transgenic soybean plants using Agrobacterium-mediated DNA transfer. Biotechnology (N Y) 6:915–922. doi:10.1038/nbt0888-915
Ivashuta S, Liu J, Liu J et al (2005) RNA interference identifies a calcium-dependent protein kinase involved in Medicago truncatula root development. Plant Cell 17:2911–2921. doi:10.1105/tpc.105.035394
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS-fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Kaló P, Gleason C, Edwards A et al (2005) Nodulation signaling in legumes requires NSP2, a member of the GRAS family of transcriptional regulators. Science 308:1786–1789. doi:10.1126/science.1110951
Kereszt A, Li D, Indrasumunar A et al (2007) Agrobacterium rhizogenes-mediated transformation of soybean to study root biology. Nat Protoc 2:948–952. doi:10.1038/nprot.2007.141
Kuntal KN, Narayan B, Chaudhuri D et al (2006) Spontaneous plant regeneration in transformed roots and calli from Tylophora indica: changes in morphological phenotype and tylophorine accumulation associated with transformation by Agrobacterium rhizogenes. Plant Cell Rep 25:1059–1066. doi:10.1007/s00299-006-0164-z
Lee NG, Stein B, Suzuki H et al (1993) Expression of antisense nodulin-35 RNA in Vigna aconitifolia transgenic root nodules retards peroxisome development and affects nitrogen availability to the plant. Plant J 3:599–606. doi:10.1046/j.1365-313X.1993.03040599.x
Limpens E, Mirabella R, Fedorova E et al (2005) Formation of organelle-like N2-fixing symbiosomes in legume root nodules is controlled by DMI2. Proc Natl Acad Sci USA 102:10375–10380. doi:10.1073/pnas.0504284102
Lin BC, Kado CI (1977) Studies on Agrobacterium tumefaciens. VIII. Avirulence induced by temperature and ethidium bromide. Can J Microbiol 23:1554–1561
Lodhi AH, Bongaerts RJM, Verpoorte R et al (1996) Expression of bacterial isochorismate synthase in transgenic root cultures of Rubia peregrina. Plant Cell Rep 15:54–57. doi:10.1007/BF01275449
Moyano E, Jouhikainen K, Tammela P (2003) Effect of pmt gene overexpression on tropane alkaloid production in transformed root cultures of Datura metel and Hyoxcyamus muticus. J Exp Bot 54:203–211. doi:10.1093/jxb/54.381.203
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4326. doi:10.1093/nar/8.19.4321
Olhoft PM, Flagel LE, Donovan CM et al (2003) Efficient soybean transformation using hygromycin B selection in the cotyledonary-node method. Planta 216:723–735
Paz M, Shou H, Guo Z et al (2004) Assessment of conditions affecting Agrobacterium-mediated soybean transformation using the cotyledonary node explants. Euphytica 136:167–179. doi:10.1023/B:EUPH.0000030670.36730.a4
Paz M, Carlos Martinez C, Kalvig AB et al (2005) Improved cotyledonary node method using an alternative explant derived from mature seed for efficient Agrobacterium-mediated soybean transformation. Plant Cell Rep 25:248–256. doi:10.1007/s00299-005-0113-2
Salas MG, Park SH, Srivatanakul M et al (2001) Temperature influence on stable T-DNA integration in plant cells. Plant Cell Rep 20:701–705. doi:10.1007/s002990100374
Savka MA, Ravillion B, Noel GR et al (1990) Induction of hairy roots on cultivated soybean genotypes and their use to propagate the soybean cyst nematode. Phytopathology 80:503–508. doi:10.1094/Phyto-80-503
Sevon N, Oksman C, Kirsi M (2002) Agrobacterium rhizogenes-mediated transformation: root cultures as a source of alkaloids. Planta Med 68:859–868. doi:10.1055/s-2002-34924
Taylor CG, Fuchs B, Collier R et al (2006) Generation of composite plants using Agrobacterium rhizogenes. Methods Mol Biol 343:155–167
Vickers CE, Schenk PM, Li D et al (2007) pGFPGUSPlus, a new binary vector for gene expression studies and optimising transformation systems in plants. Biotechnol Lett 29:1793–1796. doi:10.1007/s10529-007-9467-6
Vieweg MF, Hohnjec N, Küster H (2005) Two genes encoding different truncated hemoglobins are regulated during root nodule and arbuscular mycorrhiza symbioses of Medicago truncatula. Planta 220:757–766. doi:10.1007/s00425-004-1397-0
Xiao K, Zhang C, Harrison M et al (2005) Isolation and characterization of a novel plant promoter that directs strong constitutive expression of transgenes in plants. Mol Breed 15:221–231. doi:10.1007/s11032-004-5679-9
Zambre M, Terryn N, De Clercq J et al (2003) Light strongly promotes gene transfer from Agrobacterium tumefaciens to plant cells. Planta 216:580–586
Zeng P, Vadnais DA, Zhang Z et al (2004) Refined glufosinate selection in Agrobacterium-mediated transformation of soybean Glycine max (L.) Merrill. Plant Cell Rep 22:478–482. doi:10.1007/s00299-003-0712-8
Acknowledgments
The authors would like to thank: Professor Peter Gresshoff and Ms. Dongxue Li at University of Queensland, Australia, for providing A. rhizogenes strain K599 and vector pCAMBIAl305.1; Professor Sandui Guo for providing growth chambers and Yan Luo, Liang Li, Yang Yu, Jialin Wu, Xiaomin Ge for plant care; Dr. Zhanyuan J. Zhang for a critical review of this manuscript. This project was supported by the Special Fund for Routine Research of Central Public Welfare-oriented Institutions (072060302-18, 08206302-10), Natural Science Foundation of China (30771358) and National High Technology Research and Development Program of China (2007AA10Z133).
Author information
Authors and Affiliations
Corresponding author
Additional information
D. Cao and W. Hou contributed equally to this work.
Rights and permissions
About this article
Cite this article
Cao, D., Hou, W., Song, S. et al. Assessment of conditions affecting Agrobacterium rhizogenes-mediated transformation of soybean. Plant Cell Tiss Organ Cult 96, 45–52 (2009). https://doi.org/10.1007/s11240-008-9458-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-008-9458-x