Abstract
Fluted pumpkin (Telfairia occidentalis Hook. f.) is traditionally propagated by seeds, which have low viability after pod harvest, low percentage germination, and poor root development and often germinate during storage. The competition between use of seeds for consumption and propagation causes scarcity of propagules, necessitating development of more efficient propagation systems. Efficient protocols were developed for the induction of somatic embryos (SEs) and conversion into plantlets using cotyledons from mature zygotic embryos. This study evaluated the effects of 2,4-dichlorophenoxyacetic acid (2,4-D) and kinetin (25 combinations) on the induction of SEs, and of indole-3-acetic acid (IAA), 2,4-D, and kinetin (7 combinations) on conversion of SEs into plantlets. Significantly more SEs (381.7; p < 0.01) were obtained on medium with 0.5 mg l−1 2,4-D and 0.1 mg l−1 kinetin than from the other 24 treatments after 4 wk of cotyledon culture. All SEs were obtained through an intermediary callus. For the production of SE-derived plantlets, treatments with 0.01 mg l−1 IAA and 0.02 mg l−1 kinetin resulted in significantly more shoots and roots than other treatments (p < 0.001 for each) and gave 100% conversion of SEs to plantlets. The mean numbers of roots and shoots on this treatment were 3.7 and 1.3, respectively, and the mean shoot length was 2.2 cm. The plantlets had broad leaves and good vigor, similar to the parent cultivar. Nearly all plantlets (98–100%) survived acclimatization. The production of SEs from cotyledons and the high rate of conversion into quality plants will allow development of a mass production system for Telfairia planting material to meet the increasing demand for this crop.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The fluted pumpkin (Telfairia occidentalis Hook. f.) is an indigenous, multi-purpose leafy vegetable consumed by millions of people in Nigeria and other parts of Africa as food and used in ethno-medicine (Ajayi et al. 2006a; Kayode and Kayode 2011). The plant is traditionally propagated by mature seeds, and average leaf production is 16.5 mt ha−1 (Odiaka et al. 2007). The pod ranges from 20 to 50 cm in length and from 10 to 20 cm in diameter (Balogun et al. 2002; Chukwudi and Agbo 2014a). The number of seeds within a pod varies depending on the cultivar. The larger the pod size, the larger the seeds produced (Chukwudi and Agbo 2014b).
Telfairia seeds are recalcitrant being limited by low seed viability after pod harvest, low percentage germination, and poor rooting (Balogun et al. 2002, 2007; Akinyemi and Esuola 2012). In part, this is because the seeds are sensitive to desiccation (Ajayi et al. 2006b): The critical moisture content required for the seeds to remain viable is 40% (Nkang et al. 2000). Storage under moist conditions was suggested as a means to prolong the viability of Telfairia seeds (Akoroda 1986). Frozen or low-temperature storage conditions are not possible because the seeds are sensitive to chilling (Ajayi et al. 2006b). Consequently, the common storage practice among local growers is to leave the seeds in the pod after harvest in order to prevent excessive desiccation prior to sowing (Fig. 1A ). A major limitation of this practice is that the mature seeds germinate within the pod (Fig. 1B ). This viviparous nature of the seeds limits long-term storage. Pod rot resulting from the activity of microorganisms also reduces the viability of the seeds (Nwufo and Emebiri 1990).
The relatively long interval between seed sowing and pod maturation in the field further limits the future supply of planting material (Alegbejo 2012). Ehiagbonare (2008) reported that the development of normal seedlings from germinated seeds of Telfairia can also be a problem. Modupeola et al. (2014) investigated the effects of seed size and position within the pod on seedling development. They found that large seeds (21–25 g) and seeds positioned at the top of the pod (i.e., closest to the stem) had significantly improved seedling development compared with other seeds in the pod.
In vitro culture techniques have been used to grow, multiply, and conserve vegetative parts of many plants (Engelmann 2011) including Telfairia occidentalis. A number of studies evaluated the effects of various plant growth regulators (PGRs) on several explants including embryonic axes (Ajayi et al. 2006a; Akinyemi and Esuola 2012), meristems and shoot tips (Adesoye et al. 2012), nodal cuttings (Sanusi et al. 2008), and stems and leaves (Sakpere et al. 2014). Ajayi et al. (2006a) cultured embryo axes and shoot tips of Telfairia occidentalis and obtained the greatest growth response (80%) from excised shoot tips planted on Murashige and Skoog (MS) medium (Murashige and Skoog 1962) with 0.1 mg l−1 1-naphthaleneacetic acid (NAA) and 2.0 mg l−1 kinetin. This study did not provide data on plantlet production. Adesoye et al. (2012) compared the effects of PGRs on growth of shoot tips and meristem cultures of Telfairia occidentalis and found that 2.0 mg l−1 6-benzylaminopurine (BAP) and 2.0 mg l−1 indole-3-acetic acid (IAA) were the best for shoot tip culture, while 2.0 mg l−1 BAP was most effective for meristem culture. The authors concluded that shoot tip culture was better than meristem culture for shoot regeneration, but provided no information on root production. Sanusi et al. (2008) cultured nodal segments derived from seedlings of Telfairia occidentalis and found that indole-3-butyric acid (IBA; 0.05 mg l−1) and BAP (0.01 mg l−1) gave the best result for both rooting and shooting, but the experiment was not replicated. Sakpere et al. (2014) examined the effects of PGRs on the leaf, stem, and nodal segments of Telfairia occidentalis and determined that a combination of kinetin (3.3 mg l−1) and 2,4-dichlorophenoxyacetic acid (2,4-D; 5 mg l−1) significantly increased the percentage of callus induction on the nodal segments compared to a combination of BAP (0.25 mg l−1) and NAA (0.25 or 0.5 mg l−1). The authors concluded that 2,4-D was better for callus induction than NAA. It appears that no previous in vitro report has addressed a complete production system.
Somatic embryogenesis has not been studied for the purpose of vegetative propagation and conservation of Telfairia occidentalis. An efficient protocol for somatic embryogenesis of Telfairia occidentalis could enable its rapid clonal propagation, widen the genetic base, and create a platform for mass production and conservation. The present study focused on the development of a somatic embryo (SE)-based production system for planting materials of Telfairia occidentalis by using cotyledons to create an efficient protocol for SE induction and conversion into plants. Studies were also carried out to determine the best practices for plant acclimatization and survival.
Materials and Methods
Source of plant material.
Mature pods of Telfairia occidentalis cv. NHTOCS-13, harvested within 1 mo, were obtained from the field genebank collection (accession, NHTO-2) of the National Horticultural Research Institute (NIHORT) in Ibadan, Nigeria.
Explants disinfection.
Mature seeds (Fig. 1C ) were isolated from pods of Telfairia occidentalis and surface disinfected with 70% ethanol for 5 min followed by bleach (Clorox® regular bleach [5.25% sodium hypochlorite]) at 35% (v/v) for 20 min and 17.5% (v/v) for an additional 10 min. The seeds were then thoroughly rinsed thrice with sterile distilled water (~300 ml).
Explant culture.
Cotyledons were excised from zygotic embryos (Fig. 1D ) of sterilized seeds and cultured in Petri dishes on MS medium (Sigma Aldrich® #M5519, St. Louis, MO; 4.43 g l−1) with myo-inositol (100 mg l−1), kinetin (0.02 mg l−1), indole-3-acetic acid (0.01 mg l−1), and 2 g l−1 gellan gum (PhytagelTM, Sigma Aldrich® #P8169) at pH 5.7. Medium was autoclaved at 15 psi and 121°C for 15 min and dispensed (20 ml per Petri dish). The cultures were maintained at 25 ± 1°C under a 16-h photoperiod at 25 μmol m−2 s−1 provided by cool-white fluorescent tubes (Eastar lighting, Zhejiang, China). Light green cotyledons from 3-wk-old in vitro cultures were used for the induction of SEs. All chemicals used in this study were sourced from Sigma Aldrich® Co.
Induction of somatic embryos.
Light green cotyledon segments (~0.5-cm length) were excised and cultured in Petri dishes on MS medium with 24 PGR combinations (20 ml per dish); medium with no PGRs (T25) served as the control (Table 1). Other medium components included 4.43 g l−1 MS, 100 mg l−1 myo-inositol, 30 g l−1 sucrose, and 2 g l−1 Phytagel™, and the pH of each medium was adjusted to 5.7. The PGR concentrations were chosen based on relevant literature (see “Introduction” section). The experimental design was completely randomized with three replicates. A total of 375 seeds were extracted from seven pods. For each treatment, ten excised cotyledons were cultured (one cotyledon per 5-cm sterile plastic Petri dish) for a total of 750 excised cotyledons on the 25 treatment combinations. Data were collected at 4 wk on the percentage of cotyledons per treatment that produced embryogenic callus and the number of SEs per treatment. Callus was examined and embryo counts done using an XDY-1 Fluorescence Inverted Microscope (Vanco Industries Co. Ltd., Ningbo, China), and photographs were taken with the camera on an OLYMPUS IX51 inverted microscope (Olympus America Inc., Melville, NY). All SEs were transferred to PGR-free MS medium with 3% sucrose for 7 d to mature before transferring to conversion medium.
Somatic embryo conversion into plants.
The culture medium for converting SEs into plantlets consisted of either 2,4-D or IAA (0 and 0.01 mg l−1) in combination with kinetin (0, 0.02, and 0.10 mg l−1); the control (M7) contained no PGRs (Table 2). All treatments contained MS (4.43 g l−1), sucrose (30 g l−1), myo-inositol (100 mg l−1), and Phytagel (2 g l−1), and the pH of each medium was adjusted to 5.7. The experimental design was completely randomized with three replicates. Twenty SEs were randomly selected and cultured per treatment, per replicate (n = 60). Data were collected at 4 wk after culture and included the number of roots, number of shoots and shoot length per treatment, the percentage of SEs per treatment that converted to plantlets, and the number of SEs that produced callus during the conversion stage.
Establishment of plants in the screenhouse.
Plantlets obtained from the conversion experiment were washed in running tap water to remove the culture medium. The plantlets were transplanted into sterile topsoil in small (4 × 4 cm) polyethylene bags and watered twice daily (morning and evening). The plantlets were initially transferred to a shaded area of the screenhouse for 5 d and then transplanted into larger plastic containers (15 cm diameter × 20 cm height) and transferred to an area with more exposure to sunshine. Two planting substrates were tested for the establishment of plantlets: sawdust (pulverized wood) and topsoil mixed with straw of dried Tridax procumbens L. (soil mixture, ratio 1:1 [w/v]). The chemical profile of Tridax procumbens was previously reported (Ikewuchi et al. 2009). Each planting substrate had ten plants per replicate (one plant per pot). The plants on sawdust were watered once daily (morning) while the plants on topsoil mixed with straw of dried Tridax procumbens L. were watered twice daily (morning and evening) with a plastic watering can and about a liter of water per pot, per watering regime. The experiment was completely randomized with three replicates (n = 30). Plant survival data were collected at 4 wk after transplanting.
Statistical analysis.
The data were subjected to analysis of variance (ANOVA) using the Genstat 2010 statistical package. Differences among treatment means were considered significant at p ≤ 0.05. Duncan’s multiple range test was used for means separation.
Results
Effects of 2,4-D and kinetin on the induction of somatic embryos.
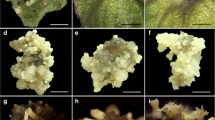
The 2,4-D and kinetin had significant (p < 0.01) effects on the production of embryogenic callus and SEs of Telfairia (Table 1 and Fig. 2A–E ). Treatments T13 and T17 produced embryogenic callus on all cotyledon segments (100%), which was significantly (p < 0.01) better than any of the other treatments. The control (T25) and T21–T24 (kinetin only) did not produce any callus. Other treatments (T1–T4, T6, T11, T18) produced only non-embryogenic callus. The most SEs (381.7) were obtained on T17, which was significantly more than on any other treatment. Among the treatments that produced SEs, the fewest were produced on T5 (67.3, Table 1).
Indirect somatic embryogenesis: (A) callus formed on a cotyledon segment (~0.5-cm segment length) after 4 wk of initial culture, (B) proliferated embryogenic callus, (C) somatic embryos formed after 4 wk of initial culture, (D) 4-wk-old somatic embryo of Telfairia occidentalis (2-mm length), (E) 5-wk old somatic embryo of T. occidentalis undergoing growth changes.
Effects of plant growth regulators on the conversion of somatic embryos into plantlets.
The conversion of SEs into plantlets was achieved using IAA and kinetin. Among the six PGR combinations and non-PGR control, only M5 (0.01 mg l−1 IAA and 0.02 mg l−1 kinetin) produced plantlets (Fig. 3). M5 produced conversion of all SEs (100%) to plantlets (data not shown). The mean number of shoots (NS) was 1.3 (p < 0.001) and shoot length (SL) was 2.2 cm. The highest number of roots (NR) was observed on M5 (3.7), which was significantly higher (p < 0.001) than the other treatments. The SEs produced roots within the first 3 d of culture while it took about 2 wk for the shoots to emerge. A few callus formation (CF) occurred on M1, M4, and M5. All plants produced through SEs or somatic embryogenesis (Fig. 4A, B ) appeared morphologically similar to the parent cultivar (Fig. 4C ), with broad leaves and good vigor, and had a relatively uniform growth pattern.
Mean number of roots (NR), number of shoots (NS), shoot length (SL; measured in centimeters), and number of explants with callus formation (CF) on treatments M1–M7 (Table 2). Error bars indicate standard errors.
(A) In vitro-derived plantlets of Telfairia occidentalis (4 wk old) ready for transplanting into the screenhouse. (B) In vitro-derived plantlet of T. occidentalis (4 wk old) with broad leaves and good vigor. (C) Mature seed-grown T. occidentalis cultivar during late-season growth in the field. (D, E) Somatic embryo–derived plantlets of T. occidentalis (photographed 3 wk after transplanting) established in the screenhouse on two planting substrates: soil mixed with straw (D) and sawdust (E).
Effects of two planting substrates on plant growth.
There was no significant difference between the survival of plantlets on the sawdust and soil mixture although they had different water retention capabilities. The sawdust retained more water and remained too moist if watered twice a day. Survival in pots with sawdust was 98% while survival in the soil mixture was 100% (data not shown). Plants established in the screenhouse (Fig. 4D–E ) were morphologically similar to the parent cultivar (Fig. 4C ), with broad leaves and good vigor.
Discussion
This study demonstrates an efficient protocol for the induction of SEs of Telfairia occidentalis using cotyledons from mature zygotic embryos and the conversion of SEs into plantlets. The SE method is a suitable system for the mass production of quality planting materials and could be an alternative strategy for the in vitro germplasm storage of Telfairia occidentalis. An SE-based production system may improve the yield of leaves per hectare because the plant materials are expected to be of higher quality. SEs are also useful materials for genetic transformation for future improvement in yield and nutrients of this crop. In addition, the availability of an SE protocol for Telfairia occidentalis makes possible the production of synthetic seeds (encapsulated SEs), which have many biotechnological applications (Redenbaugh 1990).
Other tissue culture protocols have been reported as alternative methods for the propagation of Telfairia (Ajayi et al. 2006a; Sanusi et al. 2008; Adesoye et al. 2012; Akinyemi and Esuola 2012; Sakpere et al. 2014). This is the first study addressing a complete production system, from somatic embryogenesis to screenhouse-established plants, that is directly applicable or transferable to the commercial production of Telfairia.
This study demonstrated that the induction of SEs in Telfairia was significantly increased by 2,4-D (0.5 mg l−1) and kinetin (0.1 mg l−1). The cytokinin alone was not effective for induction of embryogenic callus and SEs. Both of these PGRs are commonly used for the induction of callus and were previously tested for callus induction in Telfairia (Sakpere et al. 2014). This is the first report in Telfairia that shows the effects of these PGRs on SE induction and conversion into plantlets. Among the treatments tested, the highest number of SEs (381.7) was observed on medium containing 2,4-D (0.5 mg l−1) and kinetin (0.1 mg l−1). This is similar to the work of Tiwari et al. (1998), who produced SEs of Brahmi [Bacopa monnieri L. (Wettst.)] using similar amounts of the same PGRs: 2,4-D (0.2 mg l−1) and kinetin (0.1 or 0.5 mg l−1). Al-Sabah et al. (2012) obtained embryogenic callus of Amla (Emblica officinalis Gaertn.) on MS medium with 2,4-D (5 mg l−1) and kinetin (1 mg l−1) and proembryos on MS medium with 2,4-D (1 mg l−1) and kinetin (0.1 mg l−1).
Somatic embryogenesis of Telfairia involves an intermediary embryogenic callus phase (Fig. 2A, B ). A high percentage of explants with embryogenic callus was observed. The SEs had well-organized structures (Fig. 2C–E ). In the absence of a well-organized structure, many SEs are unable to form or convert to plants (Nickle and Yeung 1993).
Only treatment M5 (0.01 mg l−1 IAA and 0.02 mg l−1 kinetin) promoted the conversion of SEs into plantlets; NR was 3.7, NS was 1.3, and SL was 2.2 cm (Fig. 3). Olowe et al. (2014) obtained 1.6 shoots and SL of 1.46 cm from shoot tip cultures of field-grown Telfairia occidentalis planted on 1/2-strength N6 medium (Chu 1981) with 0.05 mg l−1 BAP, but unlike in the present study, the authors observed no root formation in their optimal treatment. Adesoye et al. (2012) obtained only one shoot from each shoot tip culture on 1/2-strength N6 medium with 2.0 mg l−1 BAP and 2.0 mg l−1 IAA.
A few treatments in the present study (M1, M4, and M5) promoted callus formation (Fig. 3), which is not uncommon during organogenesis in Telfairia occidentalis. Olowe et al. (2014) observed callus formation on all combinations of BAP and 2,4-D as well as on almost all combinations of BAP and IAA tested with shoot tips derived from field-grown Telfairia occidentalis, whereas BAP alone had no effect on callus production. Balogun et al. (2007) also reported callus formation during the in vitro regeneration of Telfairia occidentalis stem segments cultured on medium with 3.0 mg l−1 BAP and 1.5 mg l−1 NAA. Sakpere et al. (2014) developed a good callus production system for Telfairia occidentalis using field-grown plants. They obtained about 80% callus induction from stem segments cultured on medium with 0.25 mg l−1 BA and 0.25 mg l−1 NAA; the highest cumulative percentage of callus induction (35%) was on MS medium with 2,4-D (5 mg l−1) and kinetin (0.1 mg l−1). In the present study, 100% callus induction was obtained with a combination of 2,4-D and kinetin (T13 and T17, Table 1). The difference between these results may be due in part to explant type or the concentration of 2,4-D used. However, the results of both studies agreed that kinetin alone was not adequate for callus induction. Callus production could be of advantage in producing important variants (Fras and Maluszynska 2004), particularly for a crop plant such as Telfairia that has narrow genetic diversity.
Plantlets transplanted into the screenhouse survived at high percentages (98–100%) in both treatments. Given the high survival of plants on both soil mixture and sawdust, either one could be used in the propagation of Telfairia. All plants appeared healthy with good vigor (Fig. 4D, E ). Telfairia is a crop with enormous potential for food, medicine, and income generation for millions of people in Africa (Okoli and Mgbeogu 1983; Alegbejo 2012). The traditional propagation of this crop by seed limits conservation efforts due to the seed’s inherent physiological problems (short-duration viability and vivipary) and to diseases transmitted through seeds such as Telfairia mosaic virus (TeMV) (Anno-Nyako 1988). TeMV causes severe damage to the plant including reductions in chlorophyll and nitrogen content, resulting in poor growth (Mofunanya et al. 2014). The results of the present research offer a biotechnology-based alternative to seed propagation. The production of SEs from virus-free plants could greatly improve planting stock. The efficient SE protocol developed in this study could be a catalyst toward the large-scale production of this crop. Currently, 80% of Telfairia production and marketing is undertaken by rural women and girls in Africa (Odiaka et al. 2007). This study, by providing a method to generate quality planting materials, may increase income and improve the livelihood of Telfairia growers.
From this study, the following steps are recommended for propagating Telfairia through somatic embryogenesis: (1) initiation of bacteria- and virus-free explants in vitro, (2) induction of embryogenic cells/SEs using 2,4-D (0.5 mg l−1) and kinetin (0.1 mg l−1), (3) maturation of SEs on PGR-free medium, (4) conversion of SEs to plants using medium with IAA (0.01 mg l−1) and kinetin (0.02 mg l−1), and (5) establishment of plantlets in top soil mixed with straw as this produced a higher survival percentage than sawdust.
References
Adesoye AI, Okooboh GO, Akande SR, Balogun MO, Odu BO (2012) Effect of phytohormones and genotype on meristem and shoot tip culture of Telfairia occidentalis Hook. f. J Appl Biosci 49:3415–3424
Ajayi S, Berjak P, Kioko J (2006a) Observations on in vitro behaviour of the zygotic axes of fluted pumpkin. Afr J Biotechnol 5:1397–1404
Ajayi SA, Berjak P, Kioko JI, Dulloo ME, Voduohe RS (2006b) Responses of fluted pumpkin (Telfairia occidentalis Hook. f.; Cucurbitaceae) seeds to desiccation, chilling and hydrated storage. S Afr J Bot 72:544–550
Akinyemi SOS, Esuola CO (2012) Rapid in vitro germination of zygotic embryos of fluted pumpkin (Telfairia occidentalis Hook. f.). J Hortic Sci Biotechnol 87:413–418
Akoroda MO (1986) Seed desiccation and recalcitrance in Telfairia occidentalis. Seed Sci Technol 14:327–332
Alegbejo JO (2012) Production, marketing, nutritional value and uses of fluted pumpkin (Telfairia occidentalis Hook. f.) in Africa. J Biol Sci Bioconservation 4:20–27
Al-Sabah L, Sudhersan C, Jibi-Manuel S (2012) Somatic embryogenesis and plantlet regeneration in Amla. Am Eurasian J Sustain Agric 6:417–421
Anno-Nyako FO (1988) Seed transmission of Telfairia mosaic virus in fluted pumpkin (Telfairia occidentalis Hook. f.) in Nigeria. J Phytopathol 121:85–87
Balogun MO, Ajibade SR, Ogunbodede BA (2002) Micropropagation of fluted pumpkin by enhanced axillary shoot formation. Niger J Hortic Sci 6:85–88
Balogun MO, Akande SR, Ogunbodede BA (2007) Effects of plant growth regulators on callus, shoot and root formation in fluted pumpkin (Telfairia occidentalis). Afr J Biotechnol 6:355–358
Chu CC (1981) The N6 medium and its application to anther culture of cereal crops. In: Plant tissue culture: Proceedings of the Peking Symposium. Pitman, Boston, pp 43–50
Chukwudi UP, Agbo CU (2014a) Leaf and fruit yield performance of Telfairia occidentalis Hook. f. (fluted pumpkin) as influenced by fruit size. Notulae Sci Biologicae 6:509–514
Chukwudi UP, Agbo CU (2014b) Influence of fruit characteristics on seeds and seedling emergence of fluted pumpkin (Telfairia occidentalis Hook. f.). J Anim Plant Sci 24:600–605
Ehiagbonare JE (2008) Conservation studies on Telfairia occidentalis Hook. f.A. indigenous plant used for ethnomedicinal treatment of anemia in Nigeria. Afr J Agric Res 3:074–077
Engelmann F (2011) Use of biotechnologies for the conservation of plant biodiversity. In Vitro Cell Dev Biol Plant 47:5–16
Fras A, Maluszynska J (2004) The correlation between the chromosome variation in callus and genotype of explants of Arabidopsis thaliana. Genetica 121:145–154
Ikewuchi CJ, Ikewuchi CC, Igboh MN (2009) Chemical profile of Tridax procumbens Linn. Pak J Nutr 8:548–550
Kayode AAA, Kayode OT (2011) Some medicinal values of Telfairia occidentalis: a review. Am J Biochem Mol Biol 1:30–38
Modupeola TO, Olaniyi JO, Abdul-Rafiu AM, Akinyode ET, Taylor OO, Bidmos FA, Oyewusi AD (2014) Effect of seed size and position in pod on the early seedling growth of fluted pumpkin (Telfairia occidentalis) in southwestern Nigeria. Res J Seed Sci 7:26–30
Mofunanya AAJ, Owolabi AT, Nkang A (2014) Effects of Telfairia mosaic virus (TeMV) on the chlorophyll content and photosynthetic capabilities of two ecotypes of Telfairia occidentalis Hook. f. (fluted pumpkin). Int J Plant Pathol 5:54–62
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco cultures. Physiol Plant 15:473–497
Nickle TC, Yeung EC (1993) Failure to establish a functional shoot meristem may be a cause of conversion failure in somatic embryos of Daucus carota (Apiaceae). Am J Bot 80:1284–1291
Nkang A, Omokoro D, Egbe A (2000) Effects of desiccation on the lipid peroxidation and activities of peroxide and polyphenoloxidase in seeds of Telfairia occidentalis. Seed Sci Technol 28:1–9
Nwufo MI, Emebiri LC (1990) Pod rots of fluted pumpkin (Telfairia occidentalis Hook. f.) in Imo State, Nigeria. Int Biodeterior 26:63–68
Odiaka NI, Akoroda M, Odiaka E (2007) Telfairia production: consideration for alleviating rural poverty among Nigerian women. J Appl Hortic 9:136–139
Okoli BA, Mgbeogu CM (1983) Fluted pumpkin, Telfairia occidentalis: West African vegetable crop. Econ Bot 37:145–149
Olowe O, Adesoye A, Ojobo O, Amusa O, Liamngee S (2014) Effects of sterilization and phytohormones on shoot tip culture of Telfairia occidentalis. J Nat Sci Res 4:53–58
Redenbaugh K (1990) Application of artificial seed to tropical crops. HortSci 25:251–255
Sakpere AMA, Ajayi SA, Adelusi AA (2014) Effect of growth regulators and explant types on callus induction in Telfairia occidentalis Hook. f. Afr J Biotechnol 13:2015–2021
Sanusi IS, Odofin WT, Aladele SE, Olayode MN, Gamra EO, Fajimi O (2008) In vitro culture of Telfairia occidentalis under different cytokinins and auxin combination. Afr J Biotechnol 7:2407–2408
Tiwari V, Singh BD, Tiwari NK (1998) Shoot regeneration and somatic embryogenesis from different explants of Brahmi [Bacopa monniera (L.) Wettst.]. Plant Cell Rep 17:538–543
Acknowledgments
This work was made possible with funds provided by the Alliance for a Green Revolution in Africa (AGRA), Nairobi, Kenya; the National Biotechnology Development Agency (NABDA), Abuja, Nigeria; and the University of Ibadan, Ibadan, Nigeria, for the upgrade of the Biotechnology Laboratory in the Department of Agronomy. The authors would like to thank Drs. S.O.S. Akinyemi and A.O. Faneye for their advice and help during the course of this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Krystyna Klimaszewska
Rights and permissions
About this article
Cite this article
Awosika, D.O., Uchendu, E.E., Balogun, M.O. et al. A somatic embryogenesis–based system for the production of fluted pumpkin (Telfairia occidentalis Hook. f.) planting materials. In Vitro Cell.Dev.Biol.-Plant 51, 612–618 (2015). https://doi.org/10.1007/s11627-015-9728-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-015-9728-3