Abstract
Introduction
Various risk stratification methods exist for patients with pulmonary embolism (PE). We used the simplified Pulmonary Embolism Severity Index (sPESI) as a risk-stratification method to understand the Veterans Health Administration (VHA) PE population.
Materials and methods
Adult patients with ≥ 1 inpatient PE diagnosis (index date = discharge date) from October 2011–June 2015 as well as continuous enrollment for ≥ 12 months pre- and 3 months post-index date were included. We defined a sPESI score of 0 as low-risk (LRPE) and all others as high-risk (HRPE). Hospital-acquired complications (HACs) during the index hospitalization, 90-day follow-up PE-related outcomes, and health care utilization and costs were compared between HRPE and LRPE patients.
Results
Of 6746 PE patients, 95.4% were men, 67.7% were white, and 22.0% were African American; LRPE occurred in 28.4% and HRPE in 71.6%. Relative to HRPE patients, LRPE patients had lower Charlson Comorbidity Index scores (1.0 vs. 3.4, p < 0.0001) and other baseline comorbidities, fewer HACs (11.4% vs. 20.0%, p < 0.0001), less bacterial pneumonia (10.6% vs. 22.3%, p < 0.0001), and shorter average inpatient lengths of stay (8.8 vs. 11.2 days, p < 0.0001) during the index hospitalization. During follow-up, LRPE patients had fewer PE-related outcomes of recurrent venous thromboembolism (4.4% vs. 6.0%, p = 0.0077), major bleeding (1.2% vs. 1.9%, p = 0.0382), and death (3.7% vs. 16.2%, p < 0.0001). LRPE patients had fewer inpatient but higher outpatient visits per patient, and lower total health care costs ($12,021 vs. $16,911, p < 0.0001) than HRPE patients.
Conclusions
Using the sPESI score identifies a PE cohort with a lower clinical and economic burden.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Highlights
-
sPESI score identifies a PE cohort with lower clinical and economic burden.
-
LRPE patients were less likely to have HACs and other PE-related outcomes than HRPE patients.
-
LRPE patients also had shorter inpatient length of stay and lower health care costs.
-
Prognostic risk stratification is fundamental in choosing appropriate PE treatment.
Introduction
Pulmonary embolism (PE) is a major health concern causing hospitalization in the United States, with 176,000 admissions annually [1, 2]. In the span of 20 years, the incidence of PE increased by 81%, from 62.1 to 112.3 cases per 100,000 people [3]. PE is a potentially fatal disease with a 90-day mortality rate of up to 37.2% and ~ 100,000 annuals deaths in the United States [4, 5].
The economic burden of PE is substantial, with estimated annual cumulative costs ranging from $8.5 to $19.8 billion in the United States [6]. Additionally, the annual cost per patient has been estimated at $13,300 to $31,000 in PE patients [7]. However, risk stratification remains a challenge in clinical practice. The American college of chest physicians recommends that low-risk PE (LRPE) patients can benefit from abbreviated hospital stays or outpatient therapy which could substantially reduce the clinical and economic disease burden [8, 9]. Several risk-stratification methods have been developed to identify LRPE patients, including the Geneva score, the Pulmonary Embolism Severity Index (PESI) score, the simplified PESI (sPESI) score, etc [10].
The European Society of Cardiology (ESC) advocates for the risk stratification of PE patients and the consideration of an outpatient management option for LRPE patients. However, physicians in the United States have not widely adopted an outpatient or observation management strategy [11, 12]. Also, the new recommendations of the ESC suggest using the sPESI score as the first step for prognostic assessment of acute hemodynamic stability of PE [13]. Therefore, we compared the PE-related outcomes, health care resource utilization (HRU), and costs in LRPE vs high-risk PE (HRPE) identified using the sPESI score.
Materials and methods
Data source
This was a longitudinal, retrospective cohort study that used data from the Veterans Health Administration (VHA) during October 1, 2010 to September 30, 2015 (study period). The VHA is the largest integrated health care system in the United States, providing care at 1245 health care facilities—including 170 US Department of Veterans Affairs medical centers and 1065 outpatient clinics—and serving > 9 million enrolled veterans across the country [14].
Institutional Review Board approval was not required because this study did not use individually identifiable data from the VHA.
Study population
Patients were included in the study if they had ≥ 1 inpatient diagnosis for PE (ICD-9-CM codes 415.1, 415.11, and 415.19) during the identification period (October 1, 2011–June 30, 2015), with the hospital discharge date designated as the index date. Patients were required to be aged ≥ 18 years, have a prescription claim for an anticoagulant [unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), warfarin, or novel oral anticoagulants (NOACs)] during the index hospitalization, and be continuously enrolled in their health plan with medical and pharmacy benefits for ≥ 12 months prior to the index date (baseline period) until 3 months post-index date or death (follow-up period), whichever occurred first (Appendix in Supplementary material). Patients administered subcutaneous heparin during the hospital stay were not included, since many patients are given subcutaneous heparin as a prophylaxis for PE. Also, patients with a PE claim or any anticoagulant claim prior to the index hospitalization were excluded.
Eligible PE patients were stratified based on the presence of sPESI parameters during the index hospitalization into LRPE and HRPE cohorts. The sPESI is an extensively validated prognostic tool, encompassing 6 items—including age ≥ 80 years, history of cancer/chronic cardiopulmonary diseases, heart rate ≥ 110/min, systolic blood pressure < 100 mmHg, and arterial oxygen saturation < 90%—each burdening 1 point when present [15, 16]. Patients scoring 0 points are considered at low risk and all others are considered at high risk.
Baseline measures
Patient demographics including age, gender, race, and body mass index during the baseline period were assessed. In addition, clinical characteristics including Charlson Comorbidity Index (CCI) score, past medical history, and the administration of various diagnostic tests were recorded. We assessed the association of hospital-acquired complications (HACs) and clinical markers with the risk level of PE patients during the index hospitalization (Appendix in Supplementary material).
Outcome measures
PE-related clinical outcomes [recurrent venous thromboembolism (VTE), major bleeding, and death] and diagnostic tests, including computed tomography angiography (CTA), echocardiogram, lung ventilation/perfusion scan, and venous Doppler ultrasound, during the 90-day post-discharge period, were evaluated. Recurrent VTE was defined as having a hospitalization claim for deep vein thrombosis (DVT) or PE between 8 and 90 days after the index date. Major bleeding was defined using a previously validated algorithm developed by Cunningham et al. [17] (Appendix in Supplementary material). Additionally, PE-related clinical outcomes were reported during the 30-day post-discharge period. Health care resource utilization (HRU) including the percentage of patients with any (i.e., not disease-specific) inpatient hospitalization, and outpatient visit, as well as the mean number of visits per patient and associated health care costs during the 90-day follow-up period were reported.
Statistical analysis
Descriptive statistics were provided for all study variables, including baseline demographics, clinical characteristics, and outcome variables among the LRPE and HRPE cohorts. Statistical tests of significance (Chi square for categorical variables and Wilcoxon-rank sum test for continuous variables) were conducted to assess differences between the cohorts. All analyses were conducted using Statistical Analysis Software (SAS)® (Version 9.3, SAS Institute, Cary, North Carolina, 2012).
Results
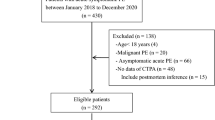
The study included 6746 PE patients, of which 1918 (28.4%) were stratified as LRPE and 4828 (71.6%) as HRPE (Fig. 1).
Baseline characteristics
The demographic and clinical characteristics during the baseline period were summarized in Table 1.
During the index hospitalization, LRPE patients had a shorter average inpatient length of stay (LOS) (8.8 vs. 11.2, p < 0.0001) and fewer HACs (11.4% vs. 20.0%, p < 0.0001) and were also less likely to have clinical marker testing (Table 1).
90-day follow-up outcomes
During the 90-day follow-up period, LRPE patients had fewer PE-related outcomes, including recurrent VTE (4.4% vs. 6.0%, p = 0.0077), major bleeding (1.2% vs. 1.9%, p = 0.0382), or death (3.7% vs. 16.2%, p < 0.0001). Additionally, the clinical outcomes during the 30-day follow-up period were reported (Table 2).
90-day follow-up HRU and costs
As compared to HRPE patients, LRPE patients had a fewer number of hospital re-admissions (0.2 vs. 0.3, p < 0.0001) with a shorter average re-hospitalization LOS (1.9 vs. 2.5 days, p < 0.0001). However, LRPE patients had higher outpatient visits per patient (19.7 vs. 18.4, p = 0.0002) (Fig. 2). Also, LRPE patients were more likely to have a venous Doppler ultrasound (8.5% vs. 4.9%, p < 0.0001) during the 90-day follow-up than HRPE patients (data not shown). LRPE patients incurred lower inpatient ($4503 vs. $7332, p < 0.0001), pharmacy ($1294 vs. $2502, p = 0.0013), and total costs ($12,021 vs. $16,911, p < 0.0001) than HRPE patients (Fig. 2).
Discussion
The results of our study showed that in a real-world clinical setting, sPESI identifies a subset of PE patients at a lower risk for adverse clinical outcomes, including mortality. These patients might benefit from early discharge or outpatient therapy, thus reducing their economic burden, especially considering the extended length of stay of LRPE in this VA cohort (mean 8.8 days).
Although a few studies have evaluated the value of sPESI in identifying those PE patients at an early risk of adverse clinical events, our study is unique in evaluating the 90-day clinical events [16, 18]. The results of previous studies showed that about 26.1–30.7% of PE patients were stratified as low-risk using the sPESI score [18,19,20]. Although right ventricular dysfunction (RVD) was considered as a significant predictor of early prognosis in LRPE patients, we observed that very few patients (~ 5) had evidence of RVD during the index hospital stay [21]. The results of our study were consistent with these studies, as 28.4% of the PE patients were classified as low-risk. Additionally, the results of our study showed that LRPE patients had ~ 2 days shorter LOS than the HRPE patients. However, in a study conducted by Shafiq et al. using the PESI criteria, no significant differences were observed in the hospital LOS between the LRPE and HRPE patients [22]. The study also observed that only 9% of the LRPE patients were discharged early (≤ 3 days), albeit the American College of Chest Physicians guidelines recommending early discharge of LRPE patients [22]. Our study also showed that LRPE patients were less likely to have HACs, which could possibly be related to the shorter hospital LOS among these patients. However, as pointed out in earlier studies, we could not conclude if HACs cause a longer LOS or if a longer LOS is caused by the HACs [23, 24]. Therefore, further research is warranted to evaluate the hospital LOS among PE patients stratified using the sPESI criteria.
The results of our study showed that the mortality rate in LRPE patients was 4 times lower than the HRPE patients, while several previous studies observed a 10 times lower mortality rate in the sPESI LRPE patients [16, 18, 25, 26]. A possible explanation for the larger difference in the magnitude of the mortality risk is that these previous studies evaluated the early mortality risk (30-day mortality) while our study assessed the 90-day mortality in PE patients. Similarly, the rate of major bleeding was lower in the LRPE patients, which is similar to the findings from Masotti et al. [16], who observed an increase in the bleeding rate with an increase in the sPESI score. Additionally, the combined rate of non-fatal major bleeding and recurrent VTE was about 5% in our study, which is consistent with a study by Ozsu et al. [18].
There is no evidence in the literature to date that evaluates the HRU and the associated costs among PE patients stratified by using any of the risk-stratification tools. The results of our study showed that LRPE patients had fewer hospital re-admissions well as lower health care costs. Surprisingly, LRPE patients had higher outpatient utilization, which could be related to the higher proportion of LRPE patients with venous Doppler ultrasound imaging during the follow-up period. This may be due to the possibility that clinicians performed more tests to confirm that LRPE patients were at low-risk for the complications and could be safely managed as outpatients or due to a higher death rate in HRPE patients, thus less opportunity for visits and extra testing. However, previous studies validating the sPESI score suggested that PE patients with a sPESI score of 0 do not require further prognostic imaging procedures or laboratory biomarker testing to define the low risk [8, 16]. Therefore, the burden of diagnostic tests can be reduced in the LRPE patients. Several previous studies suggested that reducing the hospital LOS, which is an important cost driver among PE patients, would substantially reduce the costs [18, 27, 28]. Also, in a previous study using the In-hospital mortality for pulmonary using claims data (IMPACT) criteria for the risk stratification of PE patients, the LRPE patients discharged early (≤ 3 days) had lower health care costs than those who stayed longer in the hospital [29]. Previous studies have suggested that PE outpatient management has cost savings in the range of $500 to $2500 per patient [7, 9]. Our study showed that LRPE patients incurred $4890 lower health care costs than the HRPE patients. However, further research is warranted to support the findings of our study in using sPESI for the outpatient management of the LRPE patients.
The findings from our study should be viewed in the context of some study limitations. First, the study relied on retrospective claims data. While claims data are extremely valuable for the efficient and effective examination of health care outcomes, treatment patterns, and costs, they are collected for payment and not research. To ensure exclusion of any rule-out PE diagnosis, PE patients were required to have an anticoagulant claim during their hospital stay. The presence of a claim for a filled prescription does not indicate the medication was consumed or taken as prescribed. Thus, the true number of medications prescribed may not be accurately recorded. Third, certain clinical and disease-specific parameters are not readily available in claims data, which could influence study outcomes. Additionally, the risk stratification in our study did not consider right ventricular dysfunction or elevated cardiac biomarkers which are considered as two of the important prognostic values in PE, and further analysis using these measures is warranted. Also, our study only quantified the overall clinical burden in the index hospitalization, and further research aiming to understand the early versus late complications after admission would be more beneficial in clarifying that the early discharge would minimize the risk of these HACs. The current study also represented only US data from a specific subpopulation (VHA veterans) who were mostly elderly men. Therefore, the general applicability of our findings to young male patients or females requires further study.
Conclusions
In summary, the results of this study showed that LRPE patients stratified using the sPESI score were unlikely to have complications or adverse clinical outcomes; hence, they had a lower clinical and economic burden than HRPE patients. Therefore, prognostic risk stratification may be considered a fundamental tool in choosing appropriate treatment in PE patients, and may substantially reduce the economic burden in LRPE patients.
Abbreviations
- CCI:
-
Charlson Comorbidity Index
- CTA:
-
Computed tomography angiography
- DVT:
-
Deep vein thrombosis
- ECHO:
-
Echocardiogram
- ESC:
-
European Society of Cardiology
- HAC:
-
Hospital-acquired complication
- HRPE:
-
High-risk pulmonary embolism
- HRU:
-
Health care resource utilization
- ICD-9-CM:
-
International Classification of Diseases, 9th Revision, Clinical Modification
- IMPACT:
-
In-hospital mortality for pulmonary embolism using claims data
- LMWH:
-
Low-molecular-weight heparin
- LOS:
-
Length of stay
- LRPE:
-
Low-risk pulmonary embolism
- LV:
-
Left ventricular
- NOAC:
-
Novel oral anticoagulant
- PE:
-
Pulmonary embolism
- SAS:
-
Statistical analysis software
- SD:
-
Standard deviation
- sPESI:
-
Simplified Pulmonary Embolism Severity Index
- STD:
-
Standardized difference
- UFH:
-
Unfractionated heparin
- VHA:
-
Veterans Health Administration
- VQ:
-
Lung ventilation/perfusion
- VTE:
-
Venous thromboembolism
References
Weeda ER, Wells PS, Peacock WF et al (2017) Hospital length-of-stay and costs among pulmonary embolism patients treated with rivaroxaban versus parenteral bridging to warfarin. Intern Emerg Med 12(3):311–318. https://doi.org/10.1007/s11739-016-1552-1
Rali P, Gandhi V, Malik K (2016) Pulmonary embolism. Crit Care Nurs Q 39(2):131–138. https://doi.org/10.1097/CNQ.0000000000000106
Wiener RS, Schwartz LM, Woloshin S (2011) Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med 171(9):831–837. https://doi.org/10.1001/archinternmed.2011.178
Heit JA, Spencer FA, White RH (2016) The epidemiology of venous thromboembolism. J Thromb Thormbolysis 41(1):3–14. https://doi.org/10.1007/s11239-015-1311-6
Bĕlohlávek J, Dytrych V, Linhart A (2013) Pulmonary embolism, part I: epidemiology, risk factors and risk stratification, pathophysiology, clinical presentation, diagnosis and nonthrombotic pulmonary embolism. Exp Clin Cardiol 18(2):129–138
Dasta JF, Pilon D, Mody SH et al (2015) Daily hospitalization costs in patients with deep vein thrombosis or pulmonary embolism treated with anticoagulant therapy. Thromb Res 135(2):303–310. https://doi.org/10.1016/j.thromres.2014.11.024
LaMori JC, Shoheiber O, Mody SH, Bookhart BK (2015) Inpatient resource use and cost burden of deep vein thrombosis and pulmonary embolism in the United States. Clin Ther 37(1):62–70. https://doi.org/10.1016/j.clinthera.2014.10.024
Jiménez D, Lobo JL, Barrios D, Prandoni P, Yusen RD (2016) Risk stratification of patients with acute symptomatic pulmonary embolism. Intern Emerg Med 11(1):11–18. https://doi.org/10.1007/s11739-015-1388-0
Kearon C, Akl EA, Ornelas J et al (2016) Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 149(2):315–352. https://doi.org/10.1016/j.chest.2015.11.026
Stamm JA, Long JL, Kirchner HL, Keshava K, Wood KE (2014) Risk stratification in acute pulmonary embolism: frequency and impact on treatment decisions and outcomes. South Med J 107(2):72–78. https://doi.org/10.1097/SMJ.0000000000000053
Aujesky D, Mazzolai L, Hugli O, Perrier A (2009) Outpatient treatment of pulmonary embolism. Swiss Med Wkly 139(47–48):685–690. https://doi.org/10.4414/smw.2009.12661
Hellenkamp K, Kaeberich A, Schwung J, Konstantinides S, Lankeit M (2015) Risk stratification of normotensive pulmonary embolism based on the sPESI—does it work for all patients? Int J Cardiol 197:162–163. https://doi.org/10.1016/j.ijcard.2015.06.065
Konstantinides S, Torbicki A, Agnelli G, Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) et al (2014) 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 35(43):3033–3069. https://doi.org/10.1093/eurheartj/ehu283
Veterans Health Administration. About VHA. https://www.va.gov/health/aboutVHA.asp. Accessed 6 July 2016
Fermann GJ, Erkens PM, Prins MH, Wells PS, Pap ÁF, Lensing AW (2015) Treatment of pulmonary embolism with rivaroxaban: outcomes by simplified pulmonary embolism severity index score from a post hoc analysis of the EINSTEIN PE study. Acad Emerg Med 22(3):299–307. https://doi.org/10.1111/acem.12615
Masotti L, Panigada G, Landini G et al (2016) Simplified PESI score and sex difference in prognosis of acute pulmonary embolism: a brief report from a real life study. J Thromb Thrombolysis 41(4):606–612. https://doi.org/10.1007/s11239-015-1260-0
Cunningham A, Stein CM, Chung CP, Daugherty JR, Smalley WE, Ray WA (2011) An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacoepidemiol Drug Saf 20(6):560–566. https://doi.org/10.1002/pds.2109
Ozsu S, Ozlu T, Şentürk A et al (2014) Combination and comparison of two models in prognosis of pulmonary embolism: results from Turkey pulmonary embolism group (TUPEG) study. Thromb Res 133(6):1006–1010. https://doi.org/10.1016/j.thromres.2014.02.032
Dentali F, Riva N, Turato S et al (2013) Pulmonary embolism severity index accurately predicts long-term mortality rate in patients hospitalized for acute pulmonary embolism. J Thromb Haemost 11(12):2103–2110. https://doi.org/10.1111/jth.12420
Jiménez D, Aujesky D, Moores L et al (2010) Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 170(15):1383–1389. https://doi.org/10.1001/archinternmed.2010.199
Barco S, Mahmoudpour SH, Planquette B, Sanchez O, Konstantinides SV, Meyer G (2018) Prognostic value of right ventricular dysfunction or elevated cardiac biomarkers in patients with low-risk pulmonary embolism: a systematic review and meta-analysis. Eur Heart J. https://doi.org/10.1093/eurheartj/ehy873.
Shafiq A, Lodhi H, Ahmed Z, Bajwa A (2015) Is the pulmonary embolism severity index being routinely used in clinical practice? Thrombosis. https://doi.org/10.1155/2015/175357. 2015.
Sorbello D, Dewey HM, Churilov L et al (2009) Very early mobilisation and complications in the first 3 months after stroke: further results from phase II of a very early rehabilitation trial (AVERT). Cerebrovasc Dis 28(4):378–383. https://doi.org/10.1159/000230712
Ingeman A, Andersen G, Hundborg HH, Svendsen ML, Johnsen SP (2011) In-hospital medical complications, length of stay, and mortality among stroke unit patients. Stroke 42(11):3214–3218. https://doi.org/10.1161/STROKEAHA.110.610881
Masotti L, Panigada G, Landini G et al (2015) Comparison and combination of a hemodynamics/biomarkers-based model with simplified PESI score for prognostic stratification of acute pulmonary embolism: findings from a real world study. Int J Res Med Sci 3(11):3230–3237. https://doi.org/10.18203/2320-6012.ijrms20151168
Elias A, Mallett S, Daoud-Elias M, Poggi JN, Clarke M (2016) Prognostic models in acute pulmonary embolism: a systematic review and meta-analysis. BMJ Open 6(4):e010324. https://doi.org/10.1136/bmjopen-2015-010324
Piran S, Le Gal G, Wells PS et al (2013) Outpatient treatment of symptomatic pulmonary embolism: a systematic review and meta-analysis. Thromb Res 132(5):515–519. https://doi.org/10.1016/j.thromres.2013.08.012
Berghaus TM, Thilo C, Bluethgen A, von Scheidt W, Schwaiblmair M (2010) Effectiveness of thrombolysis in patients with intermediate-risk pulmonary embolism: influence on length of hospital stay. Adv Ther 27(9):648–654. https://doi.org/10.1007/s12325-010-0058-x
Coleman CI, Kohn CG, Crivera C, Schein JR, Peacock WF (2015) Validation of the multivariable in-hospital mortality for pulmonary embolism using claims data (IMPACT) prediction rule within an all-payer inpatient administrative claims database. BMJ Open 5(10):e009251. https://doi.org/10.1136/bmjopen-2015-009251
Funding
This study was funded by Janssen Scientific Affairs, LLC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
WFP has received grants from Abbott, Alere, Banyan, Cardiorentis, Janssen, Portola, Pfizer, Roche, and ZS Pharma; is a consultant to Alere, Beckman, Boehringer-Ingelheim, Cardiorentis, Instrument Labs, Janssen, Phillips, Portola, Prevencio, Singulex, The Medicine’s Company, and ZS Pharma; and also has ownership interests at the Comprehensive Research Associate LLC, Emergencies in Medicine LLC. CIC has received grant funding and consulting fees from Janssen Scientific Affairs, LLC, Raritan, NJ and Bayer Pharma AG, Berlin, Germany. PW receives speaker fees from Bayer Healthcare and Daiichi Sankyo, writing committee fees from Itreas, and grant support fees from Pfizer/BMS. GJF has received research support from Novartis, Siemens, Pfizer, Portola, and PCORI; has advised Janssen Scientific Affairs, LLC; and receives speaker fees from Janssen. CC and JS and are employees of Janssen Scientific Affairs. LW and OB are employees of STATinMED Research, which is a paid consultant to Janssen Scientific Affairs.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wells, P., Peacock, W.F., Fermann, G.J. et al. The value of sPESI for risk stratification in patients with pulmonary embolism. J Thromb Thrombolysis 48, 149–157 (2019). https://doi.org/10.1007/s11239-019-01814-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-019-01814-z