Abstract
Patients with acute symptomatic pulmonary embolism (PE) who present with arterial hypotension or shock have a high risk of death (high-risk PE), and treatment guidelines recommend strong consideration of thrombolysis in this setting. For normotensive patients diagnosed with PE, risk stratification should aim to differentiate the group of patients deemed as having a low risk for early complications (all-cause mortality, recurrent venous thromboembolism, and major bleeding) (low-risk PE) from the group of patients at higher risk for PE-related complications (intermediate-high risk PE), so low-risk patients could undergo consideration of early outpatient treatment of PE and intermediate-high risk patients would undergo close observation and consideration of thrombolysis. Clinicians should also use risk stratification and eligibility criteria to identify a third group of patients that should not undergo escalated or home therapy (intermediate-low risk PE). Such patients should initiate standard therapy of PE while in the hospital. Clinical models [e.g., Pulmonary Embolism Severity Index (PESI), simplified PESI (sPESI)] may accurately identify those at low risk of dying shortly after the diagnosis of PE. For identification of intermediate-high risk patients with acute PE, studies have validated predictive models that use a combination of clinical, laboratory and imaging variables.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pulmonary embolism (PE) remains one of the leading causes of cardiovascular morbidity and mortality. However, patients with PE have a heterogeneous presentation and prognosis. While treated PE has a short-term mortality of 2 % in normotensive patients who do not have evidence of right ventricular (RV) dysfunction, the mortality rate rises up to 30 % in patients with shock, and up to 65 % in patients with cardiac arrest at presentation [1].
The key to an effective treatment of PE in the acute phase lies in the assessment of the patient’s prognosis. High-risk PE is characterized by the presence of PE-associated arterial hypotension or shock, and has a short-term mortality of at least 15 % [2]. For patients with high-risk PE, guidelines generally recommend consideration of treatment with thrombolytic agents [3–5]. For normotensive patients diagnosed with PE, risk stratification should aim to differentiate the group of patients deemed as having a low risk for early complications (low-risk PE) who might benefit from an abbreviated hospital stay or outpatient therapy, from the group of patients with preserved systemic arterial pressure deemed as having a high risk for a complicated course (intermediate-high risk PE) who might benefit from an escalation of therapy. Clinicians should also use risk stratification and eligibility criteria to identify a third group of patients who should not undergo escalated or home therapy (intermediate-low risk PE). Risk stratification should also take into consideration the risk of bleeding from anticoagulant or thrombolytic therapy, the risk of early venous thromboembolism recurrence, and their consequences.
The outcomes assessed by prognostic tools for patients with acute PE should have a relationship to the therapeutic options. All-cause mortality, recurrent venous thromboembolism (VTE) and potential complications of anticoagulant therapy (i.e., major bleeding) serve as appropriate outcomes when aiming to identify a low-risk group of patients who could safely undergo an abbreviated hospital stay or initial PE therapy at home. On the other hand, PE-specific mortality, non-fatal PE-related complications, and potential complications of anticoagulant or thrombolytic therapy function as appropriate outcomes when aiming to identify intermediate-high risk patients who might benefit from intensive monitoring or more aggressive PE-specific therapy. Thus, clinicians might consider using separate prognostic approaches for identification of low-risk and intermediate-high risk patients [6].
Identification of patients with PE at low risk for early adverse outcomes
We assume the following definition of low-risk PE: normotensive patients diagnosed with acute symptomatic PE and a low risk for early complications (all-cause mortality, recurrent VTE, and major bleeding). We suggest that more than a negligible risk (i.e., <1 %) of early complications within the first week of diagnosis would not be acceptable for early hospital discharge or outpatient therapy to many clinicians and patients.
Clinically based risk stratification models may be preferred to biomarkers [i.e., troponins, brain natriuretic peptides (BNP)] and imaging testing [i.e., echocardiography, spiral computed tomographic (CT) angiography] to accurately identify patients at low risk of complications short after the diagnosis of PE. The strengths of standardized clinical scores lie in the following facts: (1) they are based on clearly defined, routinely available “simple” clinical parameters on admission; (2) they take into account both the clinical severity of acute PE and the burden of concomitant illness; and (3) they do not require the routine use of costly or potentially time-consuming laboratory tests or echocardiographic procedures. However, use of a clinically based risk stratification model does not mean that biomarkers and imaging studies cannot provide additional useful information.
Understanding the causes of death after acute PE helps with the assessment of the strengths of the clinical scores for identification of low-risk PE patients. Sanchez et al. assessed the cause of death during the first 30 days after the diagnosis of acute symptomatic PE in a consecutive series of patients, and the prognostic characteristics of a clinical score based on baseline clinical findings and comorbidity [i.e., simplified Pulmonary Embolism Severity Index (sPESI)] and cardiac troponin I (cTnI) obtained at the time of PE diagnosis [7]. Within the first 30 days after the diagnosis of acute symptomatic PE, death due to PE and death due to other causes occurs in a similar proportion of patients. As cTnI only predicted PE-associated mortality, low-risk sPESI has a higher negative predictive value for all-cause mortality compared with cTnI. Patients with a low-risk sPESI are unlikely to have an adverse early outcome, and they do not require additional imaging procedures or laboratory biomarker testing to improve prediction of their low risk for adverse outcomes.
One study compared the usefulness of the PESI and troponin testing for the identification of low-risk patients with acute symptomatic PE [8]. Compared with cTnl testing, PESI classification more accurately identified patients with PE who had a low risk of all-cause death within 30 days of presentation. The addition of cTnI to the PESI model didn't improve the negative predictive value or the negative likelihood ratio compared with either test alone.
Lankeit et al. compared a strategy combining imaging and laboratory biomarkers [i.e., European Society of Cardiology (ESC) model] with a simplified clinical score (i.e., sPESI) for risk stratification of normotensive patients with acute symptomatic PE [9]. Although the sPESI classified fewer patients as low risk [31 % (165 of 526), 95 % confidence interval (CI), 27–35 %] compared with the ESC model [39 % (207 of 526), 95 % CI, 35–44 %; P < 0.01], low-risk patients based on the sPESI had no 30-day mortality compared with 3.4 % [95 % CI, 0.9–5.8 %] in low-risk patients by the ESC model.
These studies and others support the notion that compared with imaging and laboratory biomarkers (or their combinations), standardized clinical scores more accurately identify patients who are at low risk of fatal and non-fatal adverse medical outcomes in the acute phase after PE diagnosis [10–12].
Clinical prediction rules used to identify patients with PE at low risk for early adverse outcomes
Aujesky et al. derived a clinical prediction rule (i.e., PESI) that includes 11 routinely available clinical parameters at the time of presentation [13] (Table 1). For each patient, the model assigns weighted points for each applicable characteristic, and calculates a total point score by summing these points and the patient’s age in years. Total points assignment corresponds with five severity classes (I through V) of increasing risk of mortality within 30 days of hospitalization. Patients in classes I and II are categorized as low risk, while patients in classes III, IV and V are categorized as high risk. Multiple retrospective and prospective studies have validated the prognostic accuracy of the PESI [14–16]. Furthermore, a trial that randomized patients with acute PE and a low risk of complications (according to the PESI) to receive low-molecular-weight heparin entirely out of the hospital (discharged within 24 h) vs. at least partly in hospital further validated the PESI [17]. This study suggests that treating appropriately selected patients with acute PE at home does not increase recurrent VTE, bleeding, or mortality.
Investigators derived and externally validated a simplified version of the PESI [18]. The simplified PESI (sPESI) includes the variables of age (>80 years vs. other), history of cancer (yes/no), history of chronic cardiopulmonary disease (yes/no), heart rate (>110 beats/min vs. other), systolic blood pressure (<100 mmHg vs. other), and oxyhemoglobin saturation (<90 % vs. other). The sPESI categorizes patients with none of the variables present as low risk, and those with any variable present as high risk (Table 2). In an external validation cohort of 7106 patients included in the RIETE registry, the 36.1 % (2569/7106) of patients classified by the sPESI as having a low risk of death had a 30-day all-cause mortality of 1.1 % (28 of 2569 patients; 95 % CI, 0.7–1.5 %), while the high-risk group had a 30-day all-cause mortality of 8.9 % (95 % CI, 8.1–9.8 %).
The Hestia criteria comprise a set of clinical parameters that can easily be obtained at the bedside (Table 3). In a single-arm management trial that used these criteria to select candidates for home treatment, the rate of recurrent VTE was 2.0 % (95 % CI, 0.8–4.3 %) in patients with acute PE who were discharged within 24 h [19]. A validation study of the Hestia criteria has not yet been published.
A recent systematic review and metaanalysis assessed the prognostic accuracy of different clinical prediction rules to identify PE patients at low risk for early mortality, and thus, suitable for outpatient treatment or early hospital discharge [20]. This study found three prediction rules [i.e., PESI, sPESI, Global Registry of Acute Coronary Events (GRACE)] with sensitivities above 90 % [18, 21, 22]. Since the GRACE score was tested in only one study with a small number of patients, we suggest that clinicians use the PESI and the sPESI for identification of low-risk normotensive patients with acute symptomatic PE.
Combination of clinical prediction rules with imaging of the right ventricle or lower limb ultrasound testing to identify patients with PE at low risk for early adverse outcomes
The PROgnosTic valuE of Computed Tomography scan in haemodynamically stable patients with acute symptomatic PE (PROTECT) study prospectively assessed the prognostic significance of multidetector CT pulmonary angiography findings and other prognostic tools in 848 normotensive patients with acute symptomatic PE [23]. Of the 37 % (313/848) who had a low-risk sPESI score, 5 (1.6 %; 95 % CI, 0.5–3.7 %) experienced a complicated course that included 1 (0.3 %, 95 % CI, 0–1.8 %) death. In this low-risk sPESI subgroup, 87 % (273/313) did not show and 13 % (40/313) did show RV dysfunction on echocardiography. Three (1.1 %; 95 % CI, 0.2–3.2 %) of the 273 sPESI low-risk patients without RV dysfunction had a complicated course, and none died. In the low-risk sPESI subgroup, 143 patients (46 %) had concomitant deep vein thrombosis (DVT) assessed by complete lower limb ultrasound testing (CCUS). One (0.6 %; 95 % CI, 0–3.2 %) of the 170 sPESI low-risk patients without concomitant DVT had a complicated course, and the patient survived. These results suggest that incorporation of echocardiographic RV dysfunction or concomitant DVT diagnosed by lower limb ultrasound testing into the sPESI does not significantly improve prognostication for the low-risk sPESI subgroup.
Identification of patients with PE at intermediate-high risk for early adverse outcomes
Since identification of normotensive patients at higher risk for complications associated with PE would facilitate selection of patients for escalation of PE therapy (e.g., transfer to the intensive care unit, thrombolysis), we suggest the following definition of intermediate-high risk PE: confirmed PE, normal blood pressure, and a risk of PE-related complications similar to patients with PE and cardiovascular instability [24]. We suggest that a significant risk of early PE-related complications would usually encourage intensive monitoring or even possibly encourage thrombolysis [2]. A combination of clinical variables, blood tests, and imaging studies may assist with classification of patients into intermediate-high risk categories.
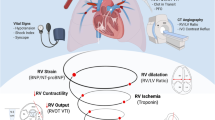
Assessment of RV dysfunction
Echocardiographic criteria used to risk stratify patients with PE include RV dilatation, an increased RV/left ventricle (LV) diameter ratio, hypokinesis of the free RV wall, increased velocity of the jet of tricuspid regurgitation, decreased tricuspid annulus plane systolic excursion (TAPSE), or combinations of the above. These findings have been identified as independent predictors of an adverse outcome in patients with acute symptomatic PE, but are heterogeneous, and have proven difficult to standardize [25–27]. Moreover, echocardiographic RV dysfunction has a weak association with short-term PE-related complications. Sanchez et al. performed a meta-analysis and calculate an odds ratio (OR) for short-term mortality for RV dysfunction on echocardiography of 2.5 (95 % CI, 1.2–5.5) [28]. More recently, studies have validated the use of CT angiography for assessing RV dilatation (end-diastolic diameter, compared with that of the left ventricle) [29, 30]. A recent systematic review shows that CT-assessed RV dysfunction has an association with an increased risk of mortality in normotensive patients with PE (OR 1.8; 95 % CI, 1.3–2.6), but the relatively small likelihood ratios and the small increase in the ability to classify risk with this approach suggest that the usefulness of basing therapeutic decision-making solely on CT results does not appear warranted [31]. Taken together, these findings suggest that RV dysfunction (assessed either by echocardiography or CT angiography) in itself should not significantly drive the decision to give thrombolytic therapy to normotensive patients with acute PE.
Assessment of myocardial injury
Studies of patients with acute PE have demonstrated an association between myocardial injury [assessed by elevated serum levels of troponin or heart-type fatty acid-binding protein (HFABP)] and short-term adverse in-hospital outcome [32–34]. Jimenez et al. performed a meta-analysis of studies in normotensive patients with acute PE to assess the prognostic value of elevated troponin levels for all-cause mortality [35]. Elevated troponin levels were associated with a high mortality (OR 4.3; 95 % CI, 2.1–8.5). However, troponin by itself does not appear to clinically significantly change the pre-test to post-test probabilities, and the usefulness of basing therapeutic decision-making solely on troponin levels does not appear warranted.
Thrombus burden
In a prospective single center cohort study of outpatients diagnosed with a first episode of acute symptomatic PE, investigators assessed the prognostic significance of concomitant DVT during the 3 months of follow-up after PE diagnosis [36]. In this study, DVT assessment by CCUS has a positive predictive value for 90-day PE-related mortality of 6.6 % (95 % CI, 4.1–9.2 %). For patients with a DVT on CCUS, the positive predictive value of high-risk PESI score (classes III–V) increases to 18.1 % (95 % CI, 13.1–26.1 %).
The degree of thrombus load and central thrombus location on CT angiography are not predictive of all-cause mortality, although both are associated with adverse clinical outcome [37]. This observation can be explained from a pathophysiologic perspective, where the severity of a PE event depends not only on the size and distribution of thrombi, but also on the patient’s underlying cardiopulmonary status.
Combination of prognostic tests
Single markers of RV dysfunction and myocardial injury have an insufficient positive predictive value for PE-specific complications to drive decision-making toward aggressive (e.g., thrombolytic) therapy [38]. Observational studies have suggested an incremental prognostic value of the association of markers of RV dysfunction and injury over either alone [39, 40], or the combination of imaging testing and biomarkers to clinical scores [41].
The PREP score includes the variables of cancer, underlying cardiac or respiratory disease, cardiogenic shock, altered mental status, BNP and right to left ventricle diameter ratio [42]. In the derivation study, 247 of 570 patients (43 %) were classified in the highest risk category, and have a risk of PE-related adverse event at 30 days of 22.9 % [41].
The PROTECT study of 848 normotensive patients with acute PE derived and validated a multimarker prognostication that consisted of sPESI, BNP, cTnI, and CCUS imaging for concomitant DVT for normotensive patients diagnosed with acute symptomatic PE in an Emergency Department (available at http://www.PEprognosis.com) [23]. The combination of results for all PROTECT prognostic tests has a positive predictive value for the prediction of a complicated course (defined as death from any cause, haemodynamic collapse, or adjudicated recurrent PE) of 25.8 % in the derivation cohort and 21.2 % in the validation cohort.
A patient-level metaanalysis involving 2874 normotensive patients presenting to the hospital with acute PE found that significant predictors of PE-related complications include tachycardia, mild hypotension, cardiac dysfunction, and myocardial injury [43]. The Bova et al. model identifies three stages (I, II, and III) with 30-day PE-related complication rates of 4.2, 10.8, and 29.2 %, respectively. A recent study validated the Bova score for accurately assessing risk for PE-related complications that occur within 30 days of PE diagnosis [44].
In summary, observational studies suggest that the combination of clinical variables (i.e., tachycardia and mild hypotension), myocardial injury, and RV dysfunction, particularly in those with concomitant DVT, identifies the more severe intermediate-risk patients with acute PE (i.e., “intermediate-high risk” according to European Society of Cardiology guideline [4]) who might benefit from intensive monitoring and recanalization therapy if haemodynamic decompensation appears [45].
Conclusions
Risk evaluation and prognostic stratification should drive the management of patients with acute PE (Fig. 1).
Stepwise approach to risk stratification of normotensive pulmonary embolism. 1Markers of myocardial injury include cardiac troponins I and T, or hear-type fatty acid binding protein (H-FABP). 2Assessed by echocardiography, computed tomographic angiogram, brain natriuretic peptide (BNP) or N-terminal pro-brain natriuretic peptide (NT-proBNP). 3For older patients, clinicians might consider half-dose thrombolytic therapy or catheter-directed thrombolysis. †Stable patients in the PESI class I–II or with sPESI of 0, and elevated cardiac biomarkers or signs of right ventricle dysfunction on imaging tests, are classified into the intermediate-low risk category. This might apply to situations in which imaging or biomarker results become available before calculation of the clinical severity index. These patients have a good prognosis when admitted to the hospital and adequately treated with standard anticoagulation. Stable patients in the PESI class III–V or with sPESI > 0, with negative test results, are classified into the intermediate-low risk category. These patients have a good prognosis when admitted to the hospital and adequately treated with standard anticoagulation. *Patients with concomitant deep vein thrombosis are at increased risk of PE-related complications. PESI Pulmonary Embolism Severity Index, sPESI simplified Pulmonary Embolism Severity Index, PE pulmonary embolism, UFH unfractionated heparin
References
White RH (2003) The epidemiology of venous thromboembolism. Circulation 107:I4–I8
Kasper W, Konstantinides S, Geibel A, Olschewski M, Heinrich F, Grosser KD, Rauber K, Iversen S, Redecker M, Kienast J (1997) Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol 30:1165–1171
Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, Nelson ME, Wells PS, Gould MK, Dentali F, Crowther M, Kahn K (2012) Antithrombotic therapy for VTE disease antithrombotic therapy and prevention of thrombosis, 9th ed: American College of chest physicians. Evidence-based clinical practice guidelines. Chest 141:419S–494S
Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M, Force Task, Members. Authors (2014) ESC guidelines on the diagnosis and management of acute pulmonary embolism: the task force for the diagnosis and management of acute pulmonary embolism of the European society of cardiology (ESC) endorsed by the European respiratory society (ERS). Eur Heart J 2014(35):3033–3073
Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, Jenkins JS, Kline JA, Michaels AD, Thistlethwaite P, Vedantham S, White RJ, Zierler BK, American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology (2011) Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis and chronic thromboembolic pulmonary hypertension. Circulation 123:1788–1830
Jiménez D, Aujesky D, Yusen RD (2010) Risk stratification of normotensive patients with acute pulmonary embolism. Br J Haematol 151:415–424
Sánchez D, De Miguel J, Sam A, Wagner C, Zamarro C, Nieto R, García L, Aujesky D, Yusen RD, Jiménez D (2011) The effects of cause of death classification on prognostic assessment of patients with pulmonary embolism. J Thromb Haemost 9:2201–2207
Moores L, Aujesky D, Jiménez D, Díaz G, Gómez V, Martí D, Briongos S, Yusen R (2010) Pulmonary Embolism Severity Index and troponin testing for the selection of low-risk patients with acute symptomatic pulmonary embolism. J Thromb Haemost 8:517–522
Lankeit M, Gómez V, Wagner C, Aujesky D, Recio M, Briongos S, Moores LK, Yusen RD, Konstantinides S, Jiménez D, Instituto Ramón y Cajal de Investigación Sanitaria Pulmonary Embolism Study Group (2012) A strategy combining imaging and laboratory biomarkers in comparison with a simplified clinical score for risk stratification of patients with acute pulmonary embolism. Chest 141:916–922
Jiménez D, Díaz G, Molina J, Martí D, Del Rey J, García-Rull S, Escobar C, Vidal R, Sueiro A, Yusen RD (2008) Troponin I and risk stratification of patients with acute nonmassive pulmonary embolism. Eur Respir J 31:847–853
Vanni S, Nazerian P, Pepe G, Baioni M, Risso M, Grifoni G, Viviani G, Grifoni S (2011) Comparison of two prognostic models for acute pulmonary embolism: clinical vs. right ventricular dysfunction-guided approach. J Thromb Haemost 9:1916–1923
Jiménez D, Lobo JL, Monreal M, Moores L, Oribe M, Barrón M, Otero R, Nauffal D, Rabuñal R, Valle R, Navarro C, Rodriguez-Matute C, Alvarez C, Conget F, Uresandi F, Aujesky DA, Yusen RD, PROTECT investigators (2014) Prognostic significance of multidetector CT in normotensive patients with pulmonary embolism: results of the protect study. Thorax 69:109–115
Aujesky D, Obrosky DS, Stone RA, Auble TE, Perrier A, Cornuz J, Roy PM, Fine MJ (2005) Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 172:1041–1046
Aujesky D, Roy PM, Le Manach CP, Verschuren F, Meyer G, Obrosky DS, Stone RA, Cornuz J, Fine MJ (2006) Validation of a model to predict adverse outcomes in patients with pulmonary embolism. Eur Heart J 27:476–481
Jiménez D, Yusen RD, Otero R, Uresandi F, Nauffal D, Laserna E, Conget F, Oribe M, Cabezudo MA, Díaz G (2007) Prognostic models for selecting patients with acute pulmonary embolism for initial outpatient therapy. Chest 132:24–30
Donzé J, Le Gal G, Fine MJ, Roy PM, Sanchez O, Verschuren F, Cornuz J, Meyer G, Perrier A, Righini M, Aujesky D (2008) Prospective validation of the pulmonary embolism severity index. A clinical prognostic model for pulmonary embolism. Thromb Haemost 100:943–948
Aujesky D, Roy PM, Verschuren F, Righini M, Osterwalder J, Egloff M, Renaud B, Verhamme P, Stone RA, Legall C, Sanchez O, Pugh NA, N’gako A, Cornuz J, Hugli O, Beer HJ, Perrier A, Fine MJ, Yealy DM (2011) Outpatient versus inpatient treatment for patients with acute pulmonary embolism: an international, open-label, randomised, non-inferiority trial. Lancet 378:41–48
Jiménez D, Aujesky D, Moores L, Gómez V, Lobo JL, Uresandi F, Otero R, Monreal M, Muriel A, Yusen RD, RIETE Investigators (2010) Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 170:1383–1389
Zondag W, Mos IC, Creemers-Schild D, Hoogerbrugge AD, Dekkers OM, Dolsma J, Eijsvogel M, Faber LM, Hofstee HM, Hovens MM, Jonkers GJ, van Kralingen KW, Kruip MJ, Vlasveld T, de Vreede MJ, Huisman MV; Hestia Study Investigators (2011) Outpatient treatment in patients with acute pulmonary embolism: the Hestia study. J Thromb Haemost 9:1500–1507
Kohn CG, Meanrs EL, Parker MW, Hernandez AW, Coleman CI (2014) Prognostic accuracy of clinical prediction rules for early post-pulmonary embolism all-cause mortality: a bivariate meta-analysis. Chest. doi:10.1378/chest.14-1888
Paiva LV, Providencia RC, Barra SN, Faustino AC, Botelho AM, Marques AL (2013) Cardiovascular risk assessment of pulmonary embolism with the GRACE risk score. Am J Cardiol 111:425–431
Aujesky D, Obrosky DS, Stone RA, Auble TE, Perrier A, Cornuz J, Roy PM, Fine MJ (2006) A prediction rule to identify low-risk patients with pulmonary embolism. Arch Intern Med 166:169–175
Jimenez D, Kopecna D, Tapson V, Briese B, Schreiber D, Lobo JL, Monreal M, Aujesky D, Sanchez O, Meyer G, Konstantinides S, Yusen RD, On Behalf Of The Protect Investigators (2014) Derivation and validation of multimarker prognostication for normotensive patients with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med 189:718–726
Wood KE (2002) Major pulmonary embolism: review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest 121:877–905
Ribeiro A, Lindmarker P, Juhlin-Dannfelt A, Johnsson H, Jorfeldt L (1997) Echocardiography Doppler in pulmonary embolism: right ventricular dysfunction as a predictor of mortality rate. Am Heart J 134:479–487
Grifoni S, Olivotto I, Cecchini P, Pieralli F, Camaiti A, Santoro G, Conti A, Agnelli G, Berni G (2000) Short term clinical outcome of patients with pulmonary embolism, normal blood pressure and echocardiographic right ventricular dysfunction. Circulation 101:2817–2822
Lobo JL, Holley A, Tapson V, Moores L, Oribe M, Barron M, Otero R, Nauffal D, Valle R, Monreal M, Yusen RD (2014) Jimenez D; PROTECT and RIETE investigators. Prognostic significance of tricuspid annular displacement in normotensive patients with acute symptomatic pulmonary embolism. J Thromb Haemost 12:1020–1027
Sanchez O, Trinquart L, Colombet I, Durieux P, Huisman MV, Chatellier G, Meyer G (2008) Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J 29:1569–1577
Becattini C, Agnelli G, Vedovati MC, Pruszczyk P, Casazza F, Grifoni S, Salvi A, Bianchi M, Douma R, Konstantinides S, Lankeit M, Duranti M (2011) Multidetector computed tomography for acute pulmonary embolism: diagnosis and risk stratification in a single test. Eur Heart J 32:1657–1663
Schoepf UJ, Kucher N, Kipfmueller F, Quiroz R, Costello P, Goldhaber SZ (2004) Right ventricular enlargement on chest computed tomography: a predictor of early death in acute pulmonary embolism. Circulation 110:3276–3280
Trujillo J, den Exter P, Gómez V, Castillo H, Moreno C, van der Hulle T, Huisman M, Monreal M, Yusen RD, Jimenez D (2013) Computed tomography-assessed right ventricular dysfunction and risk stratification of patients with acute non-massive pulmonary embolism: systematic review and meta-analysis. J Thrombosis Haemost 11:1823–1832
Janata K, Holzer M, Laggner AN, Müllner M (2003) Cardiac troponin T in the severity assessment of patients with pulmonary embolism: cohort study. BMJ 326:312–313
Lankeit M, Friesen D, Aschoff J, Dellas C, Hasenfuß G, Katus H, Konstantinides S, Giannitsis E (2010) Highly sensitive troponin T assay in normotensive patients with acute pulmonary embolism. Eur Heart J 31:1836–1844
Dellas C, Puls M, Lankeit M, Schafer K, Cuny M, Berner M, Hasenfuss G, Konstantinides S (2010) Elevated heart-type fatty acid-binding protein levels on admission predict an adverse outcome in normotensive patients with acute pulmonary embolism. J Am Coll Cardiol 55:2150–2157
Jiménez D, Uresandi F, Otero R, Lobo JL, Monreal M, Martí D, Zamora J, Muriel A, Aujesky D, Yusen RD (2009) Troponin-based risk stratification of patients with nonmassive pulmonary embolism: systematic review and metaanalysis. Chest 136:974–982
Jiménez D, Aujesky D, Díaz G, Monreal M, Otero R, Martí D, Marín E, Aracil E, Sueiro A, Yusen RD, RIETE Investigators (2010) Prognostic significance of deep vein thrombosis in patients presenting with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med 181:983–991
Meinel FG, Nance JW, Schoepf UJ, Hoffmann VS, Thierfelder KM, Costello P, Goldhaber SZ, Bamberg F (2015) Predictive value of computed tomography in acute pulmonary embolism: systematic review and meta-analysis. Am J Med 128:747–759
Jiménez D, Aujesky D, Moores L, Gómez V, Martí D, Briongos S, Monreal M, Barrios V, Konstantinides S, Yusen RD (2011) Combinations of prognostic tools for identification of high-risk normotensive patients with acute symptomatic pulmonary embolism. Thorax 66:75–81
Binder L, Pieske B, Olschewski M, Geibel A, Klostermann B, Reiner C, Konstantinides S (2005) N-terminal pro-brain natriuretic peptide or troponin testing followed by echocardiography for risk stratification of acute pulmonary embolism. Circulation 112:1573–1579
Scridon T, Scridon C, Skali H, Alvarez A, Goldhaber SZ, Solomon SD (2005) Prognostic significance of troponin elevation and right ventricular enlargement in acute pulmonary embolism. Am J Cardiol 96:303–305
Sanchez O, Trinquart L, Planquette B, Couturaud F, Verschuren F, Caille V, Meneveau N, Pacouret G, Roy PM, Righini M, Perrier A, Bertoletti L, Parent F, Lorut C, Meyer G (2013) Echocardiography and pulmonary embolism severity index have independent prognostic roles in pulmonary embolism. Eur Respir J 42:681–688
Sanchez O, Trinquart L, Caille V, Couturaud F, Pacouret G, Meneveau N, Verschuren F, Roy PM, Parent F, Righini M, Perrier A, Lorut C, Tardy B, Benoit MO, Chatellier G, Meyer G (2010) Prognostic factors for pulmonary embolism: the PREP study, a prospective multicenter cohort study. Am J Respir Crit Care Med 181:168–173
Bova C, Sanchez O, Prandoni P, Lankeit M, Konstantinides S, Vanni S, Jimenez D (2014) Identification of intermediate-risk patients with acute symptomatic pulmonary embolism: analysis of individual participants’ data from six studies. Eur Respir J 44:694–703
Fernandez C, Bova C, Sanchez O, Prandoni P, Lankeit M, Konstantinides S, Vanni S, Fernandez-Golfin C, Yusen RD, Jimenez D (2015) Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest. doi:10.1378/chest.14-2551
Meyer G, Vicaut E, Danays T, Agnelli G, Becattini C, Beyer-Westendorf J, Bluhmki E, Bouvaist H, Brenner B, Couturaud F, Dellas C, Empen K, Franca A, Galiè N, Geibel A, Goldhaber SZ, Jimenez D, Kozak M, Kupatt C, Kucher N, Lang IM, Lankeit M, Meneveau N, Pacouret G, Palazzini M, Petris A, Pruszczyk P, Rugolotto M, Salvi A, Schellong S, Sebbane M, Sobkowicz B, Stefanovic BS, Thiele H, Torbicki A, Verschuren F, Konstantinides SV, PEITHO Investigators (2014) Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 370:1402–1411
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
None.
Rights and permissions
About this article
Cite this article
Jiménez, D., Lobo, J.L., Barrios, D. et al. Risk stratification of patients with acute symptomatic pulmonary embolism. Intern Emerg Med 11, 11–18 (2016). https://doi.org/10.1007/s11739-015-1388-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-015-1388-0