Abstract
We sought to compare length-of-stay (LOS), total hospital costs, and readmissions among pulmonary embolism (PE) patients treated with rivaroxaban versus parenterally bridged warfarin. We identified adult PE (primary diagnostic code = 415.1x) patients in the Premier Database (11/2012-9/2015), and included those with ≥1 PE diagnostic test on days 0–2. Rivaroxaban users (allowing ≤2 days of prior parenteral therapy) were 1:1 propensity score matched to patients parenterally bridged to warfarin. LOS, total costs, and readmission for venous thromboembolism (VTE) or major bleeding within the same or subsequent 2 months were compared between cohorts. Separate analyses were performed in low-risk PE patients. Rivaroxaban use was associated with a 1.4-day [95 % confidence interval (CI) −1.47 to −1.28] shorter LOS, and $2322 (95 % CI −$2499 to −$2146) reduction in costs compared to parenterally bridged warfarin (p < 0.001 for both). There was no difference in readmission for VTE (1.5 versus 1.7 %) or major bleeding (0.3 versus 0.2 %) between the rivaroxaban and parenterally bridged warfarin cohorts (p ≥ 0.27 for both). Results were similar in low-risk patients (0.2–1.0 day and $251–$1751 reductions in LOS and costs, respectively, p ≤ 0.01 for all). In patients with PE, rivaroxaban was associated with reduced LOS and costs, without increased risk of readmission versus parenterally bridged warfarin. Similar results were observed in low-risk PE patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute pulmonary embolism (PE) is a common condition causing hospitalization in the United States (US), with an incidence of 176,000 admissions annually and a case fatality rate of 3.1 % [1]. Direct hospital treatment costs alone for PE in the US are estimated to approach $1.9 billion per year [1].
Traditional long-term oral anticoagulation therapy for patients presenting with acute symptomatic PE in the US has consisted of warfarin [2]. However, since warfarin requires 5+ days to achieve therapeutic anticoagulation, ‘bridging’ therapy (i.e., transition from an immediate acting agent, usually unfractionated or low-molecular-weight heparin, to warfarin) is required. During this time period, patients receive both agents, and, depending on the immediate acting therapy used, may remain in the hospital for anticoagulation monitoring or until outpatient international normalized ratio (INR) measurements can be coordinated [3].
Rivaroxaban is a US Food and Drug Administration approved direct oral factor Xa inhibitor to treat PE and reduce the risk of recurrent venous thromboembolism (VTE). It does not require bridging therapy or coagulation monitoring. In the EINSTEIN PE multicenter, randomized controlled trial (RCT), rivaroxaban was shown to be noninferior to parenteral bridging to a vitamin K antagonist (VKA) in preventing recurrent VTE with no difference in clinically relevant bleeding [4]. Additional analysis of EINSTEIN PE subjects also demonstrated a reduced index hospital length-of-stay (LOS) with rivaroxaban use [5].
To preserve internal validity, RCTs apply strict management protocols and rarely enroll the full spectrum of patients with a condition. Medications are often used in populations not evaluated by RCTs in clinical practice, which may affect real-world outcomes. Consequently, the assessment of outcomes outside of RCTs is needed to confirm results in routine clinical practice.
In this study, we sought to compare index hospital LOS, costs, and readmission among patients treated with rivaroxaban versus parenteral bridging to warfarin in a real-world setting.
Methods
This retrospective claims study used Premier Perspective Comparative Hospital Database data from November 2012–September 2015. The Premier database captures 20 % of all acute care hospital discharges in the US. This database provides detailed data regarding hospital encounters, including a date-stamped listing of all charged items at the individual patient level (medications, laboratory, and diagnostic tests, therapeutic services), as well as, discharge status and subsequent hospital encounters to the same institution over time. Data in Premier are de-identified and fully compliant with all HIPAA privacy and security requirements to protect participant anonymity and confidentiality. As the study used only de-identified patient records, it was granted an exemption from institutional review board oversight.
We included adult patients (≥18 years of age) if they had all of the following: (1) a hospital encounter for PE (including those discharged directly from emergency department [ED], coded as an observation stay or treated as an inpatient); (2) an International Classification of Diseases, 9th-edition, Clinical Modification (ICD-9-CM) diagnosis code for PE (ICD-9-CM: 415.1x) in the primary position; (3) ≥1 claim for a diagnostic test for PE [computed tomography (CT), ventilation–perfusion scan, or pulmonary angiography] on days 0–2; and (4) treatment with either rivaroxaban or parenteral anticoagulation (unfractionated heparin, low-molecular-weight heparin, or fondaparinux) followed by warfarin. Consistent with the EINSTEIN PE trial [4], rivaroxaban patients were allowed to receive ≤2 days of prior parenteral therapy and still be included. Patients transferred from another healthcare facility were excluded from this analysis. While no formal sample size calculation was performed, we made an a priori decision to include all identified patients meeting the aforementioned selection criteria. Given the magnitude of the LOS benefit observed with rivaroxaban in the EINSTEIN PE trial [5], we had high confidence that a suitable sample size would be reached in our study.
The primary endpoint for this study was LOS for the index PE hospital encounter. Secondary endpoints included total hospital costs (consisting of all costs associated with the index hospital stay, including but not limited to room and board, diagnostic, and laboratory tests as well as medications and other treatment modalities) and readmission rates for VTE (ICD-9-CM: 451.1, 451.2, 453.4, 453.8, 453.9, and 415.1x) or major bleeding per the Cunningham algorithm [6]. This algorithm uses ICD-9-CM diagnostic and procedure codes to systematically identify hospitalizations related to major bleeding episodes. The algorithm identifies these bleeding-related hospitalizations via the primary discharge diagnosis, and had a positive predictive value >88 % in its validation study. Eligible bleeding events may occur at various sites (e.g., intracranial, gastrointestinal, and genitourinary), but are excluded if they are trauma related. In Premier, specific dates of patient discharge and readmission are not provided (therefore, the total number of days to readmission cannot be calculated). However, sufficient data to determine if a patient is readmitted to the same hospital within the same month or any subsequent month are available. For this study, we assessed the readmission rate during the same or the 2 months subsequent to the index PE (i.e., no more than 3 months after the index event).
We also performed additional subgroup analyses on two cohorts of patients deemed to be at low risk for early post-PE mortality: (1) those with an estimated in-hospital mortality risk ≤1.5 % according to the validated In-Hospital Mortality for Pulmonary Embolism using Claims Data (IMPACT) prediction rule [absolute risk = 1/(1 + exp(−x)), where x = −5.833 + (0.026 × age) + (0.402 × myocardial infarction) + (0.368 × chronic lung disease) + (0.464 × stroke) + (0.638 × prior major bleeding) + (0.298 × atrial fibrillation) + (1.061 × cognitive impairment) + (0.554 × heart failure) + (0.364 × renal failure) + (0.484 × liver disease) + (0.523 × coagulopathy) + (1.068 × cancer)] [7, 8] and (2) PE patients who were considered low risk as evidenced by ED discharge or the decision to utilize observation status [9].
As this was an observational study, the investigators had no control over which patients received rivaroxaban, and which received parenteral bridging to warfarin. As a result, differences in important observed covariates were expected, which could lead to a biased estimate of treatment effect. To reduce bias due to confounding factors, rivaroxaban users were 1:1 propensity score matched to patients receiving parenteral bridging to warfarin using a greedy matching algorithm with calipers set at 1/2 the standard deviation of the propensity score [10]. Propensity scores were calculated using a multivariable logistic regression model incorporating age, gender, race, marital status, primary payer, attending physician specialty, year of index encounter, geographic region, hospital characteristics, Agency for Healthcare Research and Quality (AHRQ)-29 comorbidity status [11], and other comorbidities not included in AHRQ-29 (atrial fibrillation, myocardial infarction, stroke, prior major bleeding, and cognitive dysfunction). Propensity-score matching using these same covariates was also performed separately for the analyses of the two low-risk PE cohorts.
Separate statistical analyses were performed on the overall population and the two low-risk PE cohorts. Means ± standard deviations (SDs) were used to describe continuous variables, while percentages were used to summarize dichotomous/categorical variables. The adequacy of the matching procedures was assessed by comparing differences in baseline characteristics between the anticoagulation cohorts by calculating standardized differences (with difference <10 % considered well balanced) [10]. Any baseline characteristic exhibiting a standardized difference >10 % was further adjusted for in subsequent regression analysis. Differences in LOS and total costs were compared between anticoagulation groups using a generalized linear regression model with a gamma-distributed error and log-link. In-hospital mortality, need for thrombolysis >2 days after presentation and the proportion of patients readmitted during the same or subsequent 2 months following the index PE were compared between cohorts via logistic regression. SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) and IBM SPSS version 22.0 (IBM Corp., Armonk, NY, USA) were used to perform database management and statistical analysis. A p value <0.05 considered statistically significant in all cases.
Results
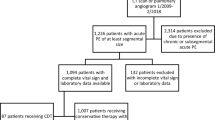
After the requirements for a primary ICD-9-CM code for PE, diagnostic confirmation and initiation of PE treatment with rivaroxaban or parenteral bridging to warfarin between days 0–2 were applied, 8824 patients were included in the overall PE analysis cohort. Of these, 4412 rivaroxaban patients were propensity score matched to 4412 patients receiving parenteral bridging to warfarin. Following restriction to patients deemed to be at low risk of early PE mortality using the IMPACT risk stratification rule, 2012 rivaroxaban patients were matched to 2012 patients receiving parenteral bridging to warfarin. When low risk was inferred by ED discharge or observation status, 585 rivaroxaban patients were matched to 585 patients receiving parenteral bridging to warfarin. Patient and hospital characteristics for the overall and low-risk cohorts stratified by anticoagulation management strategy can be found in Table 1 and eTables 1 and 2. Upon comparing the rivaroxaban group to matched parenteral bridging to warfarin patients, all baseline characteristics had a standardized difference <10 % in the overall population as well as the low risk by IMPACT cohort. After matching of rivaroxaban and parenterally bridged warfarin patients in the ED discharge or observation status cohort analysis, several characteristics (as reported in eTable 2) displayed standardized differences >10 % (requiring their inclusion into subsequent multivariable regression analyses). In the full PE cohort analysis, mean ± SD LOS for all patients was 3.9 ± 2.8 and costs were $5887 ± 5846. Rivaroxaban was associated with a 1.4-day shorter LOS and lower treatment costs (−$2322) versus parenteral bridging to warfarin (p < 0.001 for both) (Table 2). We observed a low incidence of in-hospital mortality (0.4 %) and thrombolysis >2 days after presentation (0.3 %), although rates were higher in patients receiving parenteral bridging to warfarin (p < 0.001 for both). There were no differences in readmission for VTE or major bleeding in the same or subsequent 2 months between anticoagulation cohorts (p ≥ 0.27 for both).
Mean ± SD hospital LOS and treatment costs for the low-risk cohort identified by IMPACT were 3.3 ± 2.3 days and $4758 ± 4235. Rivaroxaban again was associated with a shorter LOS (−1.0 days) and lower treatment costs (−$1751) compared to parenteral bridging to warfarin (p < 0.001 for both) (Table 3). Higher in-hospital mortality (0 versus 0.4 %) and thrombolysis >2 days after presentation (0 versus 0.5 %) were observed in patients receiving parenteral bridging to warfarin (p ≤ 0.003), but readmission for VTE or major bleeding did not differ between anticoagulant strategies (p ≥ 0.66 for both).
In the analysis restricted to inclusion of only patients discharged from the ED or managed in observation, the mean ± SD hospital LOS and treatment costs were 2.0 ± 0.8 and $2528 ± 1715. We observed a shorter LOS (−0.2 days), fewer encounters lasting >2 midnights (15.4 versus 25.1 %) and lower treatment costs (−$251) with rivaroxaban versus parenteral bridging to warfarin (p ≤ 0.01 for all) (Table 4). No patient experienced in-hospital mortality or required thrombolysis within 2 days of presentation. VTE and major bleeding readmission do not differ between anticoagulation cohorts (p ≥ 0.43 for both).
Discussion
In this large, administrative claims database study of acute PE patients, rivaroxaban was associated with both decreased LOS and lower treatment costs compared to parenteral bridging to warfarin. These reductions were observed in the overall PE cohort, as well as, in analyses restricted to low-risk patients. Furthermore, in the overall cohort, rivaroxaban was associated with a lower hospital mortality and lower rates of thrombolysis, although this was not duplicated in the ED discharge and observation status cohort, where we would expect extremely low rates of these events. Finally, regardless of patients estimated risk of early PE mortality, rivaroxaban use was not associated with differences in the short-term readmission rates for VTE or major bleeding.
Our results are consistent with RCT data from EINSTEIN PE and two smaller real-world studies [5, 12–14]. In an analysis of the intention-to-treat population of EINSTEIN PE (n = 4832), van Bellen and colleagues found patients taking rivaroxaban had a shorter LOS versus those receiving parenteral bridging to a VKA (6.6 versus 7.5 days, p < 0.0001) [5]. To eliminate any potential impact of geographic treatment trends on LOS results, Bookhart and colleagues [12] performed a post hoc analysis of EINSTEIN PE data restricted to US and Canada study sites. Of the 486 patients enrolled in this analysis, 321 were hospitalized for their index PE, with rivaroxaban associated with a lower mean LOS of nearly 2 days (p = 0.0002) versus parenteral bridging to warfarin.
Roberts and colleagues [13] retrospectively evaluated LOS with rivaroxaban compared to parenteral bridging to warfarin in 158 PE patients treated at several centers within a Texas hospital network. The study required patients to have a primary diagnosis of PE at discharge and excluded patients with abnormal renal function, receiving prior anticoagulation or having other indications for an anticoagulant (e.g., atrial fibrillation). A shorter median LOS was observed in patients receiving rivaroxaban versus parenteral bridging to warfarin (1.8 versus 4.5 days, p < 0.001). Moreover, the 2.7-day LOS reduction with rivaroxaban was observed despite 44 % of warfarin-treated PE patients having a subtherapeutic INR (<2.0) value at the time of hospital discharge.
A second real-world retrospective study performed at a single center in the Northeastern US compared LOS and total hospital treatment costs between rivaroxaban and parenteral bridging to warfarin among low-risk PE patients identified using the simplified Pulmonary Embolism Severity Index (sPESI) (n = 115), Hestia (n = 87), or IMPACT (n = 108) risk stratification rules [14]. Consecutive low-risk PE patients with a primary diagnosis of PE objectively confirmed by CT were included. The authors report that in low-risk patients, mean LOS was shorter in patients receiving rivaroxaban versus parenteral bridging to warfarin (∆ = −3.7, −2.1 and −4.3 days in low-risk patients by sPESI, Hestia, and IMPACT, respectively, p ≤ 0.001 for all). This study also demonstrated that total hospital costs were decreased with rivaroxaban compared to parenterally bridged warfarin by $3835–$7094 (p ≤ 0.001 for all); a finding consistent with this current administrative claims-based analysis.
Several limitations to our analysis are worthy of discussion. First, errors or omissions in coding may exist in administrative claims databases (i.e., readmissions not occurring at the same Premier institution would be missed). Second, Premier is a subset of US hospital encounters rather than a random sample; thus, our population may not be fully representative of the US population as a whole. Furthermore, these data from US claims may not be generalizable to other counties, although shorter hospital stays would likely be associated with lower costs in any country. Fourth, we could not determine the exact types of hospital costs impacted by the different anticoagulant treatment regimens evaluated in our study. Next, Premier does not contain data on INR and we could not determine if (time to) achievement of a therapeutic INR impacted discharge timing among patients receiving warfarin. Similarly, Premier does not contain vital signs; therefore, we could not assess hemodynamic stability or identify low-risk patients using common PE risk stratification rules (e.g., PESI, sPESI, and Hestia) [15]. Nonetheless, we did use two methods of identifying low-risk patients that are supported by the previous literature [8, 9]. Finally, despite propensity-score matching and regression analysis, residual confounding cannot be ruled out.
Conclusion
Compared with parenteral bridging to warfarin, rivaroxaban was associated with shorter index PE LOS and lower total hospital treatment costs, without an increased risk of early readmission for VTE or major bleeding. While somewhat attenuated, similar cost and LOS findings were also observed in lower risk PE populations.
References
HCUPnet, Healthcare Cost and Utilization Project (2013) Agency for Healthcare Research and Quality. http://hcupnet.ahrq.gov/. Accessed 28 June 2016
Kearon C, Akl EA, Ornelas J et al (2016) Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 149:315–352
Coleman CI, Peacock WF, Weeda ER, Ashton V, Fermann GJ (2016) Outcomes related to variation in hospital pulmonary embolus observation stay utilization. Hosp Pract 27:1–5
EINSTEIN–PE Investigators, Büller HR, Prins MH et al (2012) Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 366:1287–1297
van Bellen B, Bamber L, Correa de Carvalho F, Prins M, Wang M, Lensing AW (2014) Reduction in the length of stay with rivaroxaban as a single-drug regimen for the treatment of deep vein thrombosis and pulmonary embolism. Curr Med Res Opin 30:829–837
Cunningham A, Stein CM, Chung CP, Daugherty JR, Smalley WE, Ray WA (2011) An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacoepidemiol Drug Saf 20:560–566
Coleman CI, Kohn CG, Bunz TJ (2015) Derivation and validation of the In-hospital Mortality for Pulmonary embolism using Claims daTa (IMPACT) prediction rule. Curr Med Res Opin 31:1461–1468
Weeda ER, Kohn CG, Fermann GJ et al (2016) External validation of prognostic rules for early post-pulmonary embolism mortality: assessment of a claims-based and three clinical-based approaches. Thromb J 14:7
Piran S, Le gal G, Wells PS et al (2013) Outpatient treatment of symptomatic pulmonary embolism: a systematic review and meta-analysis. Thromb Res 132:515–519
Austin PC (2011) An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res 46:399–424
Elixhauser A, Steiner C, Harris DR, Coffey RM (1998) Comorbidity measures for use with administrative data. Med Care 36:8–27
Bookhart BK, Haskell L, Bamber L, Wang M, Schein J, Mody SH (2014) Length of stay and economic consequences with rivaroxaban vs enoxaparin/vitamin K antagonist in patients with DVT and PE: findings from the North American EINSTEIN clinical trial program. J Med Econ 17:691–695
Roberts KM, Knight TB, Padilla-tolentino E, Murthy M, Peterson EJ (2015) Length of stay comparison between rivaroxaban and warfarin in the treatment of pulmonary embolism: results from a real-world observational cohort study. Thrombosis 2015:414–523
Weeda ER, Kohn CG, Peacock WF et al (2016) Rivaroxaban versus heparin bridging to warfarin in low-risk patients with pulmonary embolism. Pharmacotherapy. doi:10.1002/phar.1828 (Epub ahead of print)
Kohn CG, Mearns ES, Parker MW, Hernandez AV, Coleman CI (2015) Prognostic accuracy of clinical prediction rules for early post-pulmonary embolism all-cause mortality: a bivariate meta-analysis. Chest 147:1043–1062
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This analysis was sponsored by Janssen Scientific Affairs, LLC, Raritan, NJ, USA.
Conflict of interest
Dr. Coleman has received Grant funding and consultancy fees from Janssen Pharmaceuticals; Bayer Pharma AG and Boehringer-Ingelheim Pharmaceuticals, Inc. Mrs. Ashton and Drs. Crivera, Schein, and Wildgoose are employees of Janssen Scientific Affairs LLC. Dr. Peacock has received Grant funding and consultancy fees from Janssen Pharmaceuticals and Portola. Dr. Fermann has received Grant funding from Pfizer and is on the advisory board and speaker’s bureau for Janssen Pharmaceuticals. Dr. Baugh is a Roche Diagnostics Advisory Board member and received consulting fees from Janssen Pharmaceuticals. He also reports research support from Janssen Pharmaceuticals and Boehringer Ingelheim. Dr. Wells has received Grant funding from Bristol Myers Squib and Pfizer, is on the advisory board and has received speaker’s fees from Bayer Healthcare, has received consultancy fees from Janssen Pharmaceuticals, and served on a writing committee with Itreas. Dr. Weeda has no conflict of interest germane to this manuscript to report. No non-financial conflicts of interest exist for any of the authors.
Statements on human and animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required. It should also be noted that all data were de-identified; thus, our study was exempted from institutional review board oversight.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Weeda, E.R., Wells, P.S., Peacock, W.F. et al. Hospital length-of-stay and costs among pulmonary embolism patients treated with rivaroxaban versus parenteral bridging to warfarin. Intern Emerg Med 12, 311–318 (2017). https://doi.org/10.1007/s11739-016-1552-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-016-1552-1