Abstract
To identify patient-related risk factors for venous thrombosis in patients with central venous catheters (CVC) or peripherally inserted central catheters (PICC). We performed a systematic review of the literature assessing patient-related risk factors for thrombosis related to CVC or PICC. The databases PubMed, Ovid and the Cochrane library were searched for observational studies pertaining to patient-related risk factors for CVC and PICC-related thrombosis. The initial search through PubMed, Ovid and the Cochrane library yielded 516 results. After 71 duplicates were removed, 445 articles were assessed for eligibility based on title and abstract. Four hundred and eleven articles were then excluded and 33 full text articles were manually assessed for eligibility. Eight articles were eliminated as they did not contain content relevant to the review. Twenty-five studies were then selected to assess 20 risk factors. There were no consistent significant associations for catheter-related thrombosis across the twenty-five studies. Multiple studies identified age, malignancy, diabetes, obesity, chemotherapy, thrombophilia and a history of thrombosis as significant risk factors for catheter-related thrombosis. Inconsistent findings among studies make it difficult to establish which patient-related risk factors are associated with catheter-related thrombosis. Future studies could include larger sample sizes and more cases of catheter-related thrombosis to produce more significant results. Identification of patient-related risk factors could lead to early recognition of upper limb deep vein thrombosis in patients with catheters, thereby preventing complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Long term indwelling venous catheters are frequently used to secure vascular access for delivery of intravenous medications and fluids [1]. Central venous catheters (CVCs) and peripherally inserted central catheters (PICCs) can significantly improve the quality of patient’s lives, preventing repeated venipuncture and the subsequent associated pain [2, 3]. Due to increased accessibility and cost effectiveness of indwelling venous catheters, their use has considerably increased in recent times [4].
Catheter-related thrombosis (CRT) is an important complication associated with the use of CVCs and PICCs [1]. Thrombosis is believed to occur due to the friction contact between the inner lining of the vein and the catheter, along with reduced blood flow. The development of a thrombus poses significant health risks. Thrombosis can result in obliteration of the veins of the upper limb, catheter occlusion and subsequent loss of vascular access [5]. The most concerning complication is a pulmonary embolism where the majority of associated deaths occur within hours [6].
Up to 66 % of cases of CRT are asymptomatic [7]. The first signs of thrombosis may be catheter occlusion or, more worryingly, symptomatic pulmonary embolism [8]. There is, therefore, a clinical imperative to identify patients who are more likely to develop CRT. Closer monitoring of patients assessed as high risk for developing CRT may prevent thrombotic complications. There have been no previous systematic reviews focusing on the clinical characteristics of patients who develop CRT. The aim of this systematic review is to explore the patient-related risk factors associated with the development of a CRT.
Methods
Literature search and article selection
A systematic literature search was conducted to determine the patient-related risk factors associated with CRT. Original published studies on patient-related risk factors for CRT were identified through a search on PubMed, the Cochrane Library and OVID. The search terms used were (‘upper limb deep vein thrombosis’ or ‘deep vein thrombosis’) and (‘risk factor’) and (‘PICC’ or ‘CVC’ or ‘catheter’). The reference lists of all identified articles were manually reviewed to identify further studies potentially suitable for the review. All articles were manually assessed for suitability for this review.
Studies were selected for this review based on predetermined selection criteria. Observational studies (cohort, case–control and cross sectional studies) on patient-related risk factors for CRT were identified for possible inclusion. Inclusion criteria comprised studies with greater than 10 participants, articles involving humans and studies published in the English language. Abstracts, review articles, letters, expert opinions, studies involving catheters that were not CVCs or PICCs and studies that did not include patient-related risk factors were excluded. Based on these criteria, two independent reviewers (AL, CH) selected articles suitable for inclusion for analysis.
Data extraction
The author developed a standardised data extraction protocol for the purposes of a systematic review. Data extracted included the patient population, number of participants, type of study, type of catheter, study period, methodology, statistical tests used, risk factors assessed and results. Potential risk factors were further explored in this review if they were investigated in two or more studies. Data was extracted from the articles, text, tables and figures from the selected studies. Due to the wide heterogeneity of the studies and a lack of a comparison group for most studies, a meta-analysis was not practicable.
Quality appraisal
The quality assessment of each of the included cohort studies is displayed in Table 1. Quality appraisal was based on the National Heart, Lung and Blood Institute checklist for observational cohort and case–control studies [9]. Each criterion was given equal weighting. A score of 13–14 was good, 9–12 fair and studies scoring below 9 were deemed to be of poor quality. Case–control studies were assessed according to a quality assessment for case–control studies from the National, Heart, Lung and Blood Institute [10]. Each criterion was given equal weighting. A score of 11–12 was good, 9–10 was fair and below 9 was poor. Quality assessment of each included case–control study is presented in Table 2.
Results
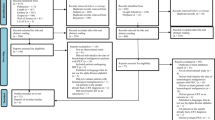
The literature search using the method below through OVID, MEDLINE and the Cochrane library yielded 533 results. After 51 duplicates were removed 482 articles were assessed for eligibility based on article title and abstract. Subsequently 449 records were eliminated and 33 full text articles were assessed for eligibility. Eight articles were excluded as they did not concern patient-related risk factors for CRT. Twenty-one cohort studies and four case–control studies were then selected for this review. A flowchart of the identification and appraisal of the studies is displayed in Fig. 1. The characteristics of the studies are presented in Table 3. In all, 25 studies comprising 14,107 patients were assessed for patient-related risk factors for CRT.
Patient Demographics
Twenty studies investigated age as a risk factor for CRT, with 17 of the studies finding no association [2, 5, 11–25]. Three prospective cohort studies identified a significant association between age and catheter related thrombosis [26–28]. Shi et al. found age >60 years to be strongly associated with thrombosis in patients undergoing chemotherapy via PICCs (OR 10.15, 95 % CI 8.14–14.52) [26]. Similarly, Timsit et al. reported age >64 years to be an individual risk factor for thrombosis related to CVCs (RR 2.44 95 % CI 2.05–3.19) [27]. Gentile et al. indicated an increased risk of CVC-related thrombosis in patients over 30 years of age (RR 2.3 95 % CI 1.2–4.4) [28].
No statistically significant association was found for gender as a risk factor in 17 studies [2, 11–14, 16–27]. Two studies demonstrated a significant association between male gender and thrombosis on univariate χ2 analysis, however significance was not retained on multivariate analysis [26, 29].
Increased body mass index (BMI) was considered in eight studies as a risk factor [2, 18, 19, 21, 24, 26, 29, 30], with two studies reporting a significant association between obesity (BMI >25 kg/m2) and PICC-related thrombosis [2, 26]. In a prospective cohort study Shi et al. found patients with a BMI >25 kg/m2 were more likely to develop PICC-related thrombosis (OR 51.65 95 % CI 30.72–65.05) [26]. Another prospective cohort study found BMI >25 kg/m2 to be independently associated with CRT [2].
Medical comorbidities and active therapies
Malignancy was investigated in eight studies with no effect detected in six studies [14, 20, 27, 29–31]. Two retrospective cohort studies reported a positive association between malignancy and catheter related thrombosis on multivariate analysis(OR 4.1 95 % CI 1.9–8.9 P < 0.001) (OR 1.953 95 % CI 1.014–3.761 P 0.05) [5, 22].
Seven studies investigated whether anticoagulation was negatively associated with CRT [11, 12, 15, 20, 24, 29, 32]. With only one study reporting a significant association. A retrospective case–control analysis reported a positive association between the use of anticoagulation and PICC-related thrombosis [32]. Anticoagulation was reported to have no effect in six studies [11, 12, 15, 20, 24, 29].
Seven studies assessed the relationship between thrombocytopenia and CRT [11, 15, 18, 20, 21, 25, 27, 32].. Only one study found a significant association with CVC related thrombosis on univariate analysis (OR 75.5 95 % CI 6–645) [18]. Six studies recorded no association [11, 15, 20, 21, 25, 27]. Thrombophilia was assessed in three studies with a divided response [24, 29, 31]. De Cicco et al. investigated anti-thrombin III deficiency as a risk factor in a prospective cohort study revealing a significant association [31]. In a prospective cohort study Rooden et al. reported a significant association for thrombophilia in general (RR 2 95 % CI 0.6–2.4) [24], whereas Wilson et al. reported no association between thrombophilia and thrombosis in patients with CVCs [29]. Eight studies investigated whether a personal history of thrombosis would increase the risk of CRT [11, 14, 20, 23, 24, 29, 30, 32]. Three studies detected a positive correlation between having a personal history of thrombosis and CRT. In a retrospective cohort study, Lobo et al. identified a personal history of thrombosis as a significant risk factor for CRT (OR 10.36 95 % CI 4.81–22.34) [14]. Similarly another retrospective cohort study reported a significant association on multivariate analysis (OR 6.659 95 % CI 2.381–18.622) [29]. Furthermore a prospective cohort study found a positive association (RR 2 95 % CI 1.3–3) [24]. The other five studies reported no significant association [11, 20, 23, 30, 32].
Diabetes was assessed in eight studies with inconsistent results [2, 17, 19–22, 26, 32]. Three of the studies reported a significant association with diabetes [2, 19, 22] with two of the studies retaining significance on multivariate analysis [2, 19]. In a prospective single arm cohort study, Yi et al. investigated diabetes as a risk factor in patients with peripherally inserted central lines. Each participant was screened every 3 days with colour duplex ultrasound for thrombosis. The study found a significant association with diabetes on multivariate analysis (OR 1.12 95 % CI 0.89–4.57) [2]. Aw et al. retrospectively analyzed charts of chemotherapy patients with PICCs. Within this population, diabetes was considered a significant risk factor for thrombosis (OR 3.18 95 % CI 1.06–9.53) [19]. The other five studies did not indicate any association [2, 17, 19–22, 26, 32].
Five studies examined hypertension as a risk factor for CRT [19–22, 26]. Two studies reported hypertension to be independently associated with CRT [20, 26]. In contrast, three studies reported hypertension to have no effect on the development of CRT [19, 21, 22]. Two studies investigated hypercholesterolaemia with mixed findings [21, 22]. Cheng et al. observed a significant association between hypercholesterolaemia and CRT (OR 1.463 95 % CI 1.067–2.007) [21], whereas Liem et al. reported no effect [22].
Recent surgery as a potential risk factor for CRT was investigated by seven studies [2, 5, 22, 24, 27, 29, 33]. Only one study found a positive association with surgery. Wilson et al. reported a significant association with recent surgery (OR 3.01 95 % CI 1.50–6.06) [29]. Six studies detected no association with CRT [2, 5, 22, 24, 27, 33].
Eleven studies explored chemotherapy as a possible risk factor for CRT providing a divided response [2, 5, 11, 13, 15, 17, 22, 24, 30–32]. Three studies reported chemotherapy as a significant risk factor for CRT with an OR ranging from 3.19 to 4.109 [2, 5, 22]. Similarly Liem et al. reported a significant association [22]. Six other studies reported no association between chemotherapy and CRT [11, 13, 15, 17, 24, 30–32]. Hormone replacement therapy was assessed as a risk factor in four studies [18, 20, 22, 24]. Only one study found a positive correlation on univariate analysis (P < 0.001) [22]. Three studies reported no association with CRT [18, 20, 24]. Seven studies investigated the role of smoking in CRT [2, 19–22, 26, 29]. Two studies found smoking to have a positive association with CRT [22, 26]. The other studies reported no effect [2, 19–21, 29].
Metastasis, trauma, haemoglobin, renal insufficiency and ethnicity were found to have no effect [2, 14, 15, 19–22, 24, 26, 29–31].
Discussion
Twenty-five studies were investigated for 20 patient-related risk factors for CRT. No risk factors were consistently identified with a significant association with CRT. Age, malignancy, diabetes, obesity, chemotherapy, thrombophilia and history of thrombosis were identified in multiple studies as significant risk factors. However due to a lack of consensus across all of the studies, a definitive conclusion in regards to the clinical characteristics of patients that predispose to CRT was unable to be established.
There was a lack of consistency among the results for each of the investigated risk factors for this review. This could be attributed to a variety of reasons. Firstly there was a wide variation in the screening methods used for thrombus detection. Some studies screened only symptomatic patients while others screened all participants. There was also a variation in the mode of investigation to determine an upper limb deep vein thrombosis. While most studies utilised ultrasound, De Cicco et al. used venography to detect a thrombus [31]. Secondly, the risk factors included in this review were sourced from different methods. The majority of the studies were retrospective and derived the patient-related risk factors from chart reviews or electronic chart records. However, in the prospective setting there was a variation in the collection of the patient-related risk factors. Yi et al. collected the information on risk factors from interviews with the patients while Van Rooden et al. utilized blood samples to determine the presence of a thrombophilia [2, 24]. Additionally, the majority of studies did not include the definitions of the risk factor assessed. Therefore there were inconsistencies present across all of the studies regarding the variation in methodology and definitions of risk factors.
Despite the wide heterogeneity across studies, some risk factors showed significant associations with CRT across multiple studies. Chemotherapy, a personal history of thrombosis and diabetes were recognised as significant risk factors in three studies each [2, 5, 14, 19, 22, 24, 29]. The identification of a personal history of thrombosis as a risk factor is in concordance with results from a recent meta-analysis [34]. Chemotherapy and diabetes have not been extensively investigated as risk factors for CRT thus have not been established in any previous systematic reviews. Chemotherapy and diabetes predispose to thrombus formation through venous injury [2, 35].
Surprisingly the use of prophylactic warfarin as an anti-coagulant showed a positive association with thrombus formation in one study. It was postulated that this likely occurred as the hospital in which the study was performed placed all hospitalized patients with PICCs on low dose warfarin for DVT prophylaxis [32].
There are several potential benefits to identifying patient-related risk factors for catheter related thrombosis. Firstly, by recognizing the clinical characteristics of patients more prone to developing CRT, these patients can be more closely monitored for upper limb deep vein thrombosis. This may involve routine screening with ultrasound, as the majority of cases of CRT are asymptomatic [7]. If a thrombus is then identified, the patients can receive treatment to prevent any thrombotic complications.
There were several limitations to performing this systematic review. Firstly there was wide heterogeneity between the studies with different population groups investigated. Some studies only included cancer patients with indwelling venous catheters. One study investigated patients who had undergone recent head injury [28]. Another study assessed CRT in patients in the neurological intensive care unit [29]. Secondly, the methods used for screening of thrombosis varied between studies. Some studies screened all study participants for thrombosis while other studies investigated only symptomatic patients. Thus the incidence of upper limb deep vein thrombosis varied widely between studies. Studies where all participants were screened for thrombosis produced an incidence rate of up to 90 % as seen in the study by De Cicco et al. [31]. The incidence rate was considerably lower in studies where only symptomatic patients were screened with an incidence ranging from 1.5 to 10 % [11, 15]. Therefore, some studies had a small number of participants with CRT which would heavily influence the risk factors associated with catheter-related thrombosis. The major sources of bias within the studies in this review were from a lack of sample size calculation and lack of blinding of the assessors. These were the main contributing factors to the ‘fair’ grade given by the quality assessment tool for the majority of the studies. Furthermore, some studies were underpowered; therefore the results are unlikely to be statistically significant. Additionally, this review only included observational studies which have a higher risk of bias than randomised control trials as potential confounders are unable to be controlled for.
There were several weaknesses with this review method. Firstly, this review is subject to publication bias as all of the articles selected were published studies. The review also included articles published in English thus making the risk factors more difficult to generalise for other population groups. Additionally some potential risk factors were widely investigated in the published studies while others received little attention, producing a risk of bias. Some clinical characteristics of patients previously established as risk factors for CRT were not significantly associated with CRT in the studies included in this review. For example no studies in this review found metastasis to be associated with CRT [15, 19, 31]. This conflicts with findings from Verso et al. who conducted a randomised control trial concluding that distant metastases increased the risk of thrombosis in patients with CVCs [36]. As this review included only observational studies, this study was not included in the analysis.
Despite these limitations, this review is the first to synthesise a wide range of potential patient-related risk factors for CRT. A previous meta-analysis on risk factors for CRT utilized higher quality studies but concentrated mainly on catheter-related factors for thrombosis. The only patient-related factor investigated was a past history of thrombosis which was found to be significant on multivariate logistic regression analysis [34].
Conclusion
In conclusion, several studies have identified increased age, malignancy, diabetes, thrombophilia, chemotherapy, obesity and a history of thrombosis as significant risk factors for CRT. In terms of the implication for clinical practice, practitioners should be aware of these risk factors when they decide that a CVC or a peripherally inserted central line is required for patient care. Such patients may therefore require routine ultrasounds for catheter-related thrombosis to allow earlier detection of a thrombus. Future studies would benefit from having more robust study designs to decrease the amount of bias. Secondly, future research would benefit from larger sample sizes and screening all of the patients within the study as the majority of cases of upper limb deep vein thrombosis are asymptomatic.
References
Lee JA, Zierler BK, Zierler RE (2012) The risk factors and clinical outcomes of upper extremity deep vein thrombosis. Vasc Endovasc Surg 46(2):139–144. doi:10.1177/1538574411432145
Yi XL, Chen J, Li J et al (2014) Risk factors associated with PICC-related upper extremity venous thrombosis in cancer patients. J Clin Nurs 23(5–6):837–843. doi:10.1111/jocn.12227
Linenberger ML (2006) Catheter-related thrombosis: risks, diagnosis, and management. J Natl Compr Cancer Netw 4(9):889–901. http://www.jnccn.org/content/4/9/889.full.pdf+html. Accessed 7 May 2014
Periard D, Monney P, Waeber G et al (2008) Randomized controlled trial of peripherally inserted central catheters vs. peripheral catheters for middle duration in-hospital intravenous therapy. J Thromb Haemost 6(8):1281–1288. doi:10.1111/j.1538-7836.2008.03053.x
Chopra V, Ratz D, Kuhn L, Lopus T, Lee A, Krein S (2014) Peripherally inserted central catheter-related deep vein thrombosis: contemporary patterns and predictors. J Thromb Haemost 12(6):847–854. doi:10.1111/jth.12549
Ozsu S, Oztuna F, Bulbul Y et al (2011) The role of risk factors in delayed diagnosis of pulmonary embolism. Am J Emerg Med 29(1):26–32. doi:10.1016/j.ajem.2009.07.005
Grant JD, Stevens SM, Woller SC et al (2012) Diagnosis and management of upper extremity deep-vein thrombosis in adults. Thromb Haemost 108(6):1097–1108. doi:10.1160/th12-05-0352
Tesselaar ME, Ouwerkerk J, Nooy MA, Rosendaal FR, Osanto S (2004) Risk factors for catheter-related thrombosis in cancer patients. Eur J Cancer 40(15):2253–2259. doi:10.1016/j.ejca.2004.06.023
National Heart, Blood and Lung Institute. Quality assessment tool for observational cohort and cross-sectional studies. http://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort.htm.Mar2014. Accessed 22 Aug 2014
National Heart, Blood and Lung Institute. Quality Assessment of Case-Control Studies. http://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/case-control.htm.Mar2014. Accessed 22 Aug 2014
Cortelezzi A, Moia M, Falanga A et al (2005) Incidence of thrombotic complications in patients with haematological malignancies with central venous catheters: a prospective multicentre study. Br J Haematol 129(6):811–817. doi:10.1111/j.1365-2141.2005.05529.x
Evans RS, Sharp JH, Linford LH et al (2013) Reduction of peripherally inserted central catheter-associated DVT. Chest 143(3):627–633. doi:10.1378/chest.12-0923
De Cicco M, Matovic M, Balestreri L, et al. (1997) Central venous thrombosis: an early and frequent complication in cancer patients bearing long-term silastic catheter. A prospective study. Thromb Res 86(2):101–113. http://ac.els-cdn.com.elibrary.jcu.edu.au/S0049384897000546/1-s2.0-S0049384897000546-main.pdf?_tid=ed29fa9e-2f7b-11e4-ae0e-00000aab0f6c&acdnat=1409317183_b33f54faccbb0519ba5517140d9b9c5e. Accessed 7 May 2014
Lobo BL, Vaidean G, Broyles J, Reaves AB, Shorr RI (2009) Risk of venous thromboembolism in hospitalized patients with peripherally inserted central catheters. J Hosp Med 4(7):417–422. doi:10.1002/jhm.442
Ahn DH, Illum HB, Wang DH, Sharma A, Dowell JE (2013) Upper extremity venous thrombosis in patients with cancer with peripherally inserted central venous catheters: a retrospective analysis of risk factors. J Oncol Pract 9(1):e8–e12. doi:10.1200/jop.2012.000595
Richters A, Van Vliet M, Peer PGM, et al. (2014) Incidence of and risk factors for persistent gram-positive bacteraemia and catheter-related thrombosis in haematopoietic stem cell transplantation. Bone Marrow Transpl. 49(2):264–269. http://www.scopus.com/inward/record.url?eid=2-s2.0-84893770878&partnerID=40&md5=e2e62c45510eac547a8cd8ca83db4852. Accessed 20 Aug 2014
Baxi SM, Shuman EK, Scipione CA et al (2013) Impact of postplacement adjustment of peripherally inserted central catheters on the risk of bloodstream infection and venous thrombus formation. Infect Control Hosp Epidemiol 34(8):785–792. doi:10.1086/671266
Del Principe MI, Buccisano F, Maurillo L et al (2013) Infections increase the risk of central venous catheter-related thrombosis in adult acute myeloid leukemia. Thromb Res 132(5):511–514. doi:10.1016/j.thromres.2013.08.007
Aw A, Carrier M, Koczerginski J, McDiarmid S, Tay J (2012) Incidence and predictive factors of symptomatic thrombosis related to peripherally inserted central catheters in chemotherapy patients. Thromb Res 130(3):323–326. doi:10.1016/j.thromres.2012.02.048
Maneval RE, Clemence BJ (2014) Risk factors associated with catheter-related upper extremity deep vein thrombosis in patients with peripherally inserted central venous catheters: a prospective observational cohort study: part 2. J Infus Nurs 37(4):260–268. doi:10.1097/nan.0000000000000042
Cheng Y, Cui T, Fu P, Liu F, Zhou L. (2013) Dyslipidemia is associated with tunneled-cuffed catheter-related central venous thrombosis in hemodialysis patients: a retrospective, multicenter study. Artif Organs 37(8):E155–E161. doi. http://www.scopus.com/inward/record.url?eid=2-s2.0-84881616341&partnerID=40&md5=e7acdbc3e6544af4a063b6cdeba0fd10. Accessed 7 May 2014
Liem TK, Yanit KE, Moseley SE et al (2012) Peripherally inserted central catheter usage patterns and associated symptomatic upper extremity venous thrombosis. J Vasc Surg 55(3):761–767. doi:10.1016/j.jvs.2011.10.005
Lee AY, Levine MN, Butler G et al (2006) Incidence, risk factors, and outcomes of catheter-related thrombosis in adult patients with cancer. J Clin Oncol 24(9):1404–1408. doi:10.1200/jco.2005.03.5600
Van Rooden CJ, Rosendaal FR, Meinders AE, Van Oostayen JA, Van Der Meer FJ, Huisman MV (2004) The contribution of factor V Leiden and prothrombin G20210A mutation to the risk of central venous catheter-related thrombosis. Haematologica. 89(2):201–206. http://www.jnccn.org/content/4/9/889.full.pdf+html. Accessed 7 May 2014
Joks M, Czyz A, Poplawski D, Komarnicki M (2014) Incidence and risk factors for central venous catheter-related thrombosis in hematological patients. Med Oncol 31(1):772. doi:10.1007/s12032-013-0772-8
Shi Y, Wen L, Zhou Y, Tao S (2014) Thrombotic risk factors in patients undergoing chemotherapy via peripherally inserted central catheter. J Int Med Res 42(3):863–869. doi:10.1177/0300060514527061
Timsit JF, Farkas JC, Boyer JM, et al. (1998) Central vein catheter-related thrombosis in intensive care patients: incidence, risks factors, and relationship with catheter-related sepsis. Chest. 114(1):207–213. http://journal.publications.chestnet.org/data/Journals/CHEST/21768/207.pdf. Accessed Aug 20 2014
Gentile A, Petit L, Masson F et al (2013) Subclavian central venous catheter-related thrombosis in trauma patients: incidence, risk factors and influence of polyurethane type. Crit Care 17(3):R103. doi:10.1186/cc12748
Wilson TJ, Brown DL, Meurer WJ, Stetler WR Jr, Wilkinson DA, Fletcher JJ (2012) Risk factors associated with peripherally inserted central venous catheter-related large vein thrombosis in neurological intensive care patients. Intensive Care Med 38(2):272–278. doi:10.1007/s00134-011-2418-7
Moran J, Colbert CY, Song J, et al. (2014) Screening for novel risk factors related to peripherally inserted central catheter-associated complications. J Hosp Med. 2014. doi:10.1002/jhm.2207
De Cicco M, Matovic M, Balestreri L, et al. (1995) Antithrombin III deficiency as a risk factor for catheter-related central vein thrombosis in cancer patients. Thromb Res 78(2):127–137. http://ac.els-cdn.com.elibrary.jcu.edu.au/0049384895000410/1-s2.0-0049384895000410-main.pdf?_tid=bbfb9dc0-2f7a-11e4-8e64-00000aab0f02&acdnat=1409316671_a759fbf43df618af570121f0583cb1b7. Accessed 7 May 2014
King MM, Rasnake MS, Rodriguez RG, Riley NJ, Stamm JA (2006) Peripherally inserted central venous catheter-associated thrombosis: retrospective analysis of clinical risk factors in adult patients. South Med J 99(10):1073–1077. doi:10.1097/01.smj.0000240707.22171.12
Evans RS, Sharp JH, Linford LH et al (2010) Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest 138(4):803–810. doi:10.1378/chest.10-0154
Saber W, Moua T, Williams EC et al (2011) Risk factors for catheter-related thrombosis (CRT) in cancer patients: a patient-level data (IPD) meta-analysis of clinical trials and prospective studies. J Thromb Haemost 9(2):312–319. doi:10.1111/j.1538-7836.2010.04126.x
Linnemann B, Lindhoff-Last E (2012) Risk factors, management and primary prevention of thrombotic complications related to the use of central venous catheters. Vasa. 41(5):319–332. doi:10.1024/0301-1526/a000217
Verso M, Agnelli G, Kamphuisen P, et al. (2008) Risk factors for upper limb deep vein thrombosis associated with the use of central vein catheter in cancer patients. Intern Emerg Med. 2008/06/01 2008;3(2):117-122. doi: 10.1007/s11739-008-0125-3
Seeley MA, Santiago M, Shott S (2007) Prediction tool for thrombi associated with peripherally inserted central catheters. J Infus Nurs 30(5):280–286. doi:10.1097/01.NAN.0000292570.62763.3f
Conflict of interest
None of the authors have any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leung, A., Heal, C., Perera, M. et al. A systematic review of patient-related risk factors for catheter-related thrombosis. J Thromb Thrombolysis 40, 363–373 (2015). https://doi.org/10.1007/s11239-015-1175-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-015-1175-9