Abstract
Purpose
Using Virchow’s triad as a framework, we sought to identify risk factors independently associated with symptomatic peripherally inserted central venous catheter (PICC)-related large vein thrombosis (PRLVT) in neurological intensive care patients.
Methods
A retrospective cohort study and detailed chart review were performed for 431 consecutive PICCs placed in patients admitted to our neurological intensive care unit between March 2008 and February 2010. Variables theorized to potentially increase the risk of PRLVT were abstracted from the medical record. Each variable was then tested for its independent association with PRLVT.
Results
During the study period, 431 PICCs were placed with an incidence rate for symptomatic thrombosis of 8.4%. In adjusted analysis, catheter placement in a paretic arm (OR, 9.85; 95% CI, 4.42–21.95), surgery longer than 1 h during dwell time of the catheter (OR, 3.26; 95% CI, 1.48–7.17), a history of venous thromboembolism (OR, 6.66; 95% CI, 2.38–18.62), and mannitol use (OR, 3.27; 95% CI 1.27–8.43) were independently associated with the development of thrombosis.
Conclusions
Alterations in blood flow and consistency, but not vessel injury, appear associated with symptomatic thrombosis following placement of PICCs in neurological intensive care patients. Mannitol use and placement in a paretic arm are potentially modifiable risk factors. Given the high incidence rate of symptomatic thrombosis, future studies should focus on comparing cumulative complications of centrally inserted venous catheters and PICCs in intensive care patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Central venous access is indicated for a wide variety of reasons in intensive care patients. Traditionally, this has been achieved through centrally inserted central venous catheters (CICVCs); however, peripherally inserted central venous catheters (PICCs) are being increasingly utilized because of the ease of insertion and a lower rate of insertion-related mechanical complications [1]. While there are certainly fewer insertion-related complications with PICCs, there is no convincing evidence that cumulative complication rates are reduced with PICCs. The most significant concern is PICC-related large vein thrombosis (PRLVT), especially in intensive care patients. Studies have reported symptomatic PRLVT in up to 20% of patients, with asymptomatic PRLVT being reported in as many as 50% of patients [1–9]. Though studies have evaluated all hospitalized patients or patients following discharge from the ICU, there have been no studies completed evaluating the incidence rate of PRLVT or attempting to identify independent risk factors for PRLVT in intensive care patients [3, 10]. One prospective study in ICU patients was halted early because of an unacceptably high rate of thrombosis [9].
Complications of PRLVT thrombosis include pain and swelling, compromised future venous access, late post-thrombotic syndrome, and venous thromboembolism (VTE) including pulmonary embolism [11–13]. One subpopulation of intensive care patients where VTE is particularly worrisome is neurological intensive care patients, as these patients often have compelling contraindications to systemic anticoagulation. Due to these contraindications, VTE carries significant risk of morbidity and mortality [14–20].
Based on the current literature, risk factors for development of PRLVT are not well understood. Identification of modifiable risk factors for PRLVT would offer opportunities to decrease thrombosis and improve outcomes. We hypothesized that increased expression of Virchow’s triad would increase the risk of developing a PRLVT. Accordingly, we sought to identify risk factors related to alterations in blood flow, blood consistency, or vessel injury that were independently associated with symptomatic PRLVT in neurological intensive care patients.
Methods
Study design
This cohort study was approved by the University of Michigan Institutional Review Board, and data were obtained by retrospective chart review. Information technology personnel designed and implemented a search paradigm to query information systems to identify all patients admitted to the neurological intensive care unit (ICU) from March 2008 through February 2010. This patient list was cross-referenced with the electronic order entry system to identify all patients who underwent PICC insertion during their ICU stay. All patients underwent preliminary chart review to ensure a PICC had been placed. A PICC “line” was defined similar to other studies in which any catheter exchanged or replaced within 24 h in the same arm was counted as one line of continuous duration [9].
Symptomatic
PRLVT was determined by reviewing the duplex ultrasound reports for every patient who had a PICC line placed. Symptomatic PRLVT was defined as an event that prompted duplex ultrasonography of the ipsilateral extremity in which an acute, proximal large vein thrombosis was confirmed in association with the PICC or within 5 days of PICC removal. In patients with a history of ipsilateral upper extremity deep venous thrombosis, new symptoms must have prompted the duplex ultrasound and acute thrombosis must have been confirmed. All patients with suspected PRLVT were evaluated using duplex ultrasonography per our ICU protocol, and no patients were treated presumptively without confirmation. All venous duplex ultrasonography studies were interpreted by board-certified radiologists as part of clinical care. Ultrasounds were not re-interpreted as part of this study. Deep venous thrombosis was defined as thrombosis of the brachial, axillary, or subclavian vein, and extensive clots were defined as clots in or proximal to the axillary vein. Similar to other studies, the basilic and cephalic veins above the elbow were considered large veins involved in symptomatic thrombosis
PICC insertion and maintenance
Data on line placement and maintenance are provided in the Electronic Supplemental Material (ESM)-1.
Outcome of interest
The primary outcome was the occurrence of a symptomatic PRLVT.
Explanatory variables
Based on previous literature or pathophysiology, we chose to investigate the following: (1) risk factors associated with altered blood flow: catheter diameter, congestive heart failure, placement in a paretic arm (defined as ≤2/5 Medical Research Council strength for longer than 48 h), and any surgery lasting longer than 1 h during dwell time of the line (detailed chart review was utilized rather than procedural codes to ensure surgery occurred during the dwell time of the line); (2) risk factors for abnormal blood consistency: hypercoagulable state (hereditary thrombophilias or acquired prothrombotic states), previous VTE, cancer, and the use of VTE prophylaxis; and (3) risk factors for vessel injury: number of attempts (all punctures where the wire was advanced, all exchanges, or advances), number of manipulations (pull-backs), and catheter duration. Definitions of hypercoagulable states and other variables of interest are detailed in ESM-2.
Statistical analysis
Statistical analysis was performed using commercially available software (SPSS version 18, IBM Corporation, Somers, NY). We include only the first PICC line placement for any individual patient in our analysis. The assumption was made that repeated observations and outcomes in the same patient would correlate, and randomly choosing or selecting subsequent PICC line placements would violate the independent group assumption. Variables were screened for normality using normality plots and the Shapiro-Wilks test. Quantitative data with normal distributions were expressed as mean ± standard deviation, and data not meeting the normality assumption were expressed as medians and interquartile ranges. Univariate comparison of continuous variables with a normal distribution was assessed using two-sample t tests, and continuous variables not meeting the normality assumption were assessed using the Mann-Whitney U test. All categorical data were assessed by chi-square test or Fisher's exact test, as appropriate. For univariate analysis, PRLVT was expressed as a dichotomous outcome (yes/no). Incidence rate of PRLVT was expressed per catheter days and per line.
Logistic regression was used to test bivariate and multivariate associations between our variables of interest and the dichotomous outcome of PRLVT. Age and duration of use were treated as continuous variables. Catheter insertion side (left vs. right), catheter location (brachial/cephalic vs. basilic), catheter diameter (6F vs. 5F), attempts (>1 vs. 1), manipulations (≥1 vs. 0), and all other variables were treated as categorical variables.
Multivariate logistic regression was used to assess independent association between our variables of interest and the development of thrombosis. In our primary model, we planned to adjust for any confounding variables not in the causal pathway with a significant bivariate association with thrombosis (p < 0.05). Given the limited number of outcomes, we planned only to correct for variables with the strongest bivariate association with thrombosis. However, since controversy exists in the literature with regard to the risk factors for PRLVT, we planned a priori to adjust for all explanatory variables and any exploratory variables of interest with a bivariate association with thrombosis in alternative models adjusting for confounders with the strongest bivariate association with thrombosis. Due to the low number of patients with a prothrombotic state or not receiving subcutaneous heparin for VTE prophylaxis, these variables were not included in our analysis. A p value <0.05 was considered statistically significant.
Results
Baseline characteristics
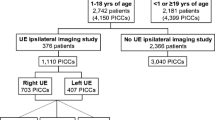
During the study period, a total of 501 PICCs were placed in the neurological ICU; however, this number was reduced to 431 “unique” PICC line placements after adjusting for lines that were immediately replaced in the same arm after inadvertent removal and after excluding subsequent lines in a single patient. The incidence rate of symptomatic PRLVT was 8.4% of PICC lines and 5.5 PRLVTs per 1,000 catheter days. Baseline demographics between those who developed a PRLVT and those who did not can be seen in Table 1. Results of descriptive and univariate analysis of procedure-related variables can be seen in Table 2. Of the identified PRLVTs in this study, 70% were deep venous thromboses and 64% were extensive. Data on surgical procedures are provided in ESM-3.
Explanatory variables associated with altered blood flow
Placement in a paretic arm [odds ratio (OR), 7.57; 95% confidence interval (CI), 3.70–15.48], surgery longer than 1 h (OR, 3.01; 95% CI, 1.50–6.06), and a history of congestive heart failure (OR, 2.62; 95% CI, 1.01–6.83) were associated with the occurrence of a PRLVT. In adjusted analysis, PICC placement in a paretic arm (OR, 9.85; 95% CI, 4.42–21.95) and surgery longer than 1 h during dwell time of the catheter (OR, 3.26; 95% CI, 1.48–7.17) were independently associated with development of a PRLVT (Table 3). Catheter diameter (6F vs. 5F) was not associated with occurrence of a PRLVT in bivariate (OR, 1.54; 95% CI, 0.74–3.23) or multivariate (OR, 1.005; 95% CI, 0.448–2.225) models after adjusting for mannitol use, placement in a paretic arm, or surgery longer than 1 h, nor did it significantly modify the OR for these covariates.
Explanatory variables associated with altered blood consistency
A history of VTE was associated with development of a PRLVT (OR, 4.23; 95% CI, 1.75–10.24); however, a history of cancer (OR, 0.98; 95% CI, 0.39–2.45) or coagulopathy (OR, 0.57; 95% CI, 0.13–2.47) was not. In adjusted analysis, a history of VTE remained independently associated with development of a PRLVT (OR, 6.66; 95% CI, 2.38–18.62) (Table 3). A history of cancer and coagulopathy were not significant in adjusted analysis, and they did not significantly modify the OR of the covariates of mannitol use, placement in a paretic arm, or surgery longer than 1 h.
Explanatory variables associated with vessel injury
The number of attempts (OR, 1.09; 95% CI, 0.53–2.24), manipulations (OR, 0.88; 95% CI, 0.44–1.75), and catheter duration (OR, 1.01; 95% CI, 0.98–1.03) were not associated with PRLVT in bivariate analysis. The findings remained unchanged in adjusted analysis and did not significantly modify the OR of the covariates of mannitol use, placement in a paretic arm, or surgery longer than 1 h.
Other variables of interest
In unadjusted analysis, male sex (OR, 2.14; 95% CI, 1.04–4.39) and mannitol use (OR, 3.43; 95% CI, 1.49–7.87) during dwell time of the PICC line were associated with PRLVT. In adjusted analysis, mannitol use remained independently associated with PRLVT (OR, 3.27; 95% CI, 1.27–8.43) (Table 3).
Discussion
We sought to identify clinical factors that increase the risk of developing a PRLVT in neurological intensive care patients. We hypothesized that increased expression of components of Virchow’s triad—venous stasis, hypercoagulability, and vessel injury—would increase the risk of developing a PRLVT. Our results support this hypothesis.
Of the factors hypothesized to increase venous stasis, placement of the PICC line in a paretic arm and surgery longer than 1 h during dwell time of the PICC were found to be significantly associated with development of a PRLVT in multivariate logistic regression analysis. Despite the fact that no study has previously identified PICC placement in a paretic arm as a risk factor, our investigation found this to have the strongest association with PRLVT. Our results corroborate the findings of another recent study showing that surgery longer than 1 h is a risk factor for PRLVT [3]. By using detailed chart review and not relying on procedural codes, we were able to ensure surgery was performed during dwell time of the line and prior to the diagnosis of thrombosis. Given the majority of our surgeries were neurosurgical or surgical tracheostomies and that blood transfusions were only required in only 6% of surgeries, our data are in line with previous data suggesting that venous stasis associated with prolonged paresis or anesthesia is a risk factor for development of deep venous thrombosis.
Contrary to our hypothesis that increased diameter of the catheter would create increased venous stasis and ultimately increase PRLVT, we did not find that catheter diameter had an independent association with PRLVT. Several previous studies demonstrated an increased risk of thrombosis as catheter diameter increased, but this has not been consistently proven across all studies [3, 4, 21, 22]. One recent study showed that insertion of a 5F or 6F catheter was a risk factor for developing symptomatic PRLVT when compared to 4F catheters. In that study, no difference was found between 5F and 6F catheters [3]. In our study, all catheters were either 5F or 6F. Thus, our study does not completely exclude the possibility that increasing catheter diameter increases the risk of developing a PRLVT, but it does support previous data in that there is likely no significant difference between the commonly used 5F and 6F catheters. This, however, is probably not a truly modifiable risk factor in intensive care patients as these patients require larger catheters, and single-lumen 4F catheters are rarely acceptable. Since we do not routinely measure vessel-to-catheter ratio, which may be a more reflective value than simply catheter size, it remains to be proven that PRLVT may be reduced by improved patient selection and catheter size.
Our data also supported the supposition that alterations in blood consistency or coagulability are involved in PRLVT. Consistent with previous studies, we found a history of VTE to be a significant risk factor for developing a subsequent PRLVT. We also found mannitol therapy to be a strong independent risk factor for development of a PRLVT. We propose that this may be due to dehydration from mannitol therapy ultimately resulting in increased serum viscosity. This change in viscosity may promote thrombosis, leading to the observed increase in PRLVT. This risk factor has not been identified previously.
Interestingly, none of the variables related to vessel injury were associated with the development of a PRLVT. Specifically, patients requiring more than one attempt at catheter placement or requiring line manipulations, each of which have the potential to cause more vessel damage, did not appear to have higher odds of developing a PRLVT. Similarly, increased duration of use was not associated with development of a PRLVT in our cohort. The median dwell time until diagnosis of PRLVT was 8 days in a unimodal distribution. These data are in line with a recent cohort study of hospitalized patients, and suggest that PICC lines are not injuring the endothelium and causing dysfunction over time [3]. Mechanisms involved with alteration of blood flow or consistency early in the ICU stay seem to play a larger role in formation of a PRLVT.
The significance of PRLVT and upper extremity deep venous thrombosis in general has previously come into question [11, 12]. All thromboses detected in our cohort were symptomatic as we did not perform routine duplex ultrasonography during the study period. Additionally, 70% of the PRLVTs in our study were deep venous thromboses and 64% were extensive. Upper extremity deep venous thrombosis is reported to result in symptomatic pulmonary embolus in approximately 9% of cases and asymptomatic pulmonary embolus in up to 33% of cases [11, 12]. No specific data are available regarding catheter-associated thrombosis. In addition, no data exist to compare the risk or significance of thrombosis between CICVCs and PICCs.
The most significant limitation of our study is its retrospective nature. However, a number of variables not previously explored were examined, adding value to the study. Recent prospective trials were limited in the variables examined or to examining only incidence rate of PRLVT, and did not specifically address critically ill patients who may have different risk factors and different incidence rates of PRLVT compared to other patient populations. Another limitation is that we could only evaluate symptomatic PRLVT and surely missed a large portion of asymptomatic thrombosis, which is reported to occur more frequently than symptomatic thrombosis. Finally, others have noted that suboptimal position of the catheter tip is associated with thrombosis formation; however, we were unable to assess this as it occurred so infrequently in our cohort. Our findings suggest that a prospective study is needed in this patient population to determine the incidence rate of PRLVT, evaluate risk factors for PRLVT, and compare outcomes against CICVCs.
Conclusions
Our data suggest that increased expression of components of Virchow’s triad increase the risk of developing a symptomatic PRLVT. Specifically, in our study, surgery longer than 1 h during dwell time of the PICC line, placement of the PICC line in a paretic arm, mannitol therapy, and history of a previous VTE all were significantly associated with developing a PRLVT. As the use of PICC lines continues to increase in intensive care patients, a prospective, randomized trial is needed to compare not only mechanical complications related to insertion, but also cumulative complications between PICCs and CICVCs. Novel, potentially modifiable risk factors identified in this study include mannitol therapy and placement in a paretic arm. While these findings should be replicated in an independent, prospectively followed cohort, it may be prudent to avoid these risk factors in neurological intensive care patients whenever possible.
References
Bernardi E, Piccioli A, Marchiori A, Girolami B, Prandoni P (2001) Upper extremity deep vein thrombosis: risk factors, diagnosis, and management. Semin Vasc Med 1:105–110
Chemaly RF, de Parres JB, Rehm SJ, Adal KA, Lisgaris MV, Katz-Scott DS, Curtas S, Gordon SM, Steiger E, Olin J, Longworth DL (2002) Venous thrombosis associated with peripherally inserted central catheters: a retrospective analysis of the Cleveland Clinic experience. Clin Infect Dis 34:1179–1183
Evans RS, Sharp JH, Linford LH, Lloyd JF, Tripp JS, Jones JP, Woller SC, Stevens SM, Elliott CG, Weaver LK (2010) Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest 138:803–810
Grove JR, Pevec WC (2000) Venous thrombosis related to peripherally inserted central catheters. J Vasc Interv Radiol 11:837–840
Lobo BL, Vaidean G, Broyles J, Reaves AB, Shorr RI (2009) Risk of venous thromboembolism in hospitalized patients with peripherally inserted central catheters. J Hosp Med 4:417–422
Paauw JD, Borders H, Ingalls N, Boomstra S, Lambke S, Fedeson B, Goldsmith A, Davis AT (2008) The incidence of PICC line-associated thrombosis with and without the use of prophylactic anticoagulants. JPEN J Parenter Enteral Nutr 32:443–447
Paz-Fumagalli R, Miller YA, Russell BA, Crain MR, Beres RA, Mewissen MW (1997) Impact of peripherally inserted central catheters on phlebitic complications of peripheral intravenous therapy in spinal cord injury patients. J Spinal Cord Med 20:341–344
Schmittling ZC, McLafferty RB, Bohannon WT, Ramsey DE, Hodgson KJ (2004) Characterization and probability of upper extremity deep venous thrombosis. Ann Vasc Surg 18:552–557
Trerotola SO, Stavropoulos SW, Mondschein JI, Patel AA, Fishman N, Fuchs B, Kolansky DM, Kasner S, Pryor J, Chittams J (2010) Triple-lumen peripherally inserted central catheter in patients in the critical care unit: prospective evaluation. Radiology 256:312–320
Bonizzoli M, Batacchi S, Cianchi G, Zagli G, Lapi F, Tucci V, Martini G, Di Valvasone S, Peris A (2011) Peripherally inserted central venous catheters and central venous catheters related thrombosis in post-critical patients. Intensive Care Med 37:284–289
Moser KM, Fedullo PF, LitteJohn JK, Crawford R (1994) Frequent asymptomatic pulmonary embolism in patients with deep venous thrombosis. JAMA 271:223–225
Prandoni P, Polistena P, Bernardi E, Cogo A, Casara D, Verlato F, Angelini F, Simioni P, Signorini GP, Benedetti L, Girolami A (1997) Upper-extremity deep vein thrombosis. Risk factors, diagnosis, and complications. Arch Intern Med 157:57–62
Elman EE, Kahn SR (2006) The post-thrombotic syndrome after upper extremity deep venous thrombosis in adults: a systematic review. Thromb Res 117:609–614
Cheng JS, Arnold PM, Anderson PA, Fischer D, Dettori JR (2010) Anticoagulation risk in spine surgery. Spine (Phila Pa 1976) 35:S117–S124
Gerber DE, Grossman SA, Streiff MB (2006) Management of venous thromboembolism in patients with primary and metastatic brain tumors. J Clin Oncol 24:1310–1318
Jenkins EO, Schiff D, Mackman N, Key NS (2010) Venous thromboembolism in malignant gliomas. J Thromb Haemost 8:221–227
Khaldi A, Helo N, Schneck MJ, Origitano TC (2011) Venous thromboembolism: deep venous thrombosis and pulmonary embolism in a neurosurgical population. J Neurosurg 114:40–46
Kim KS, Brophy GM (2009) Symptomatic venous thromboembolism: incidence and risk factors in patients with spontaneous or traumatic intracranial hemorrhage. Neurocrit Care 11:28–33
Lazio BE, Simard JM (1999) Anticoagulation in neurosurgical patients. Neurosurgery 45:838–848
Taniguchi S, Fukuda I, Daitoku K, Minakawa M, Odagiri S, Suzuki Y, Fukui K, Asano K, Ohkuma H (2009) Prevalence of venous thromboembolism in neurosurgical patients. Heart Vessels 24:425–428
Abdullah BJ, Mohammad N, Sangkar JV, Abd Aziz YF, Gan GG, Goh KY, Benedict I (2005) Incidence of upper limb venous thrombosis associated with peripherally inserted central catheters (PICC). Br J Radiol 78:596–600
Allen AW, Megargell JL, Brown DB, Lynch FC, Singh H, Singh Y, Waybill PN (2000) Venous thrombosis associated with the placement of peripherally inserted central catheters. J Vasc Interv Radiol 11:1309–1314
Conflict of interest
The authors have no conflicts of interest or financial disclosures to declare.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wilson, T.J., Brown, D.L., Meurer, W.J. et al. Risk factors associated with peripherally inserted central venous catheter-related large vein thrombosis in neurological intensive care patients. Intensive Care Med 38, 272–278 (2012). https://doi.org/10.1007/s00134-011-2418-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-011-2418-7