Abstract
Purpose
The aim of this systematic review was to identify the interventions used to treat obstructive events, whether thrombotic or non-thrombotic, in long-term central venous catheters (LT-CVC) in cancer patients.
Methods
This review included clinical trials and observational studies reporting the drugs used to treat obstructive catheter events in cancer patients. The authors developed specific search strategies for CINAHL, Cochrane CENTRAL, LILACS, PubMed, Scopus, Web of Science, Google Scholar, Open Grey, and ProQuest. The authors evaluated methodological quality of included studies using criteria from Cochrane’s Collaboration Tool and the Methodological Index for non-randomized studies (MINORS). The quality of evidence was analyzed by using GRADE’s software.
Results
More than 9000 articles were found across the databases. After duplicates removed, the studies were selected in 2 phases. After that, only 15 studies were included. The drugs used to restoration of catheter function were urokinase (53.3%), alteplase (20%), tenecteplase (13.3%), reteplase (6.7%), recombinant urokinase (6.7%), and staphylokinase (6.7%). The results of meta-analysis of 14 studies showed an overall restoration rate of ~ 84%. The drug type meta-analysis demonstrates a success rate of ~ 84%, ~ 92%, and ~ 84% for urokinase, alteplase, and tenecteplase groups, respectively. The main methodological problem in included articles concerns the sample. The quality of evidence ranged from very low to high.
Conclusion
The most common interventions used to treat thrombotic catheter occlusion in cancer patients were urokinase and alteplase. No evidence was found about the treatment for non-thrombotic occlusion, thus elucidating an important gap to be investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Central venous catheters (CVC) are integral in the management of patients with chronic diseases, particularly those with cancer. CVC facilitate medical care by providing easy and safe venous access for blood tests, chemotherapy administration, parenteral nutrition, and other intravenous medications [1]. Related to the use of these types of vascular devices, catheter-related thrombosis, infection, and obstruction are the most frequent complications [2]. Occlusion may occur due to mechanical causes, drug precipitation, or parenteral nutrition, or as a result of a thrombotic process. There are numerous risk factors associated with the development of obstruction, such as catheter insertion site, number and size of lumens, and type of catheter [3]. Chemotherapy alone is a risk factor that raises the incidence of thrombotic occlusion of totally implanted catheter [4].
The incidence of catheter occlusion varies considerably according to the clinical conditions of the patients and studies conducted in children, and adults suggest that about 36% of catheters become occluded within a period of 1–2 years after implantation [5, 6]. Thrombotic occlusion was the main cause of catheter dysfunction, typically occurring within 1 week of catheter placement [1]. However, one study showed that the mean time to development of primary complications in long-term central venous catheters (LT-CVC) was 76.8 days, and in the case of thrombotic occlusion, the catheter dysfunction occurred on average 62.2 days after insertion [7].
Several strategies to resolve the obstruction have been described, including catheter removal, the use of interventional radiology techniques, and thrombolytic therapy [2]. Although most of LT-CVC are made with materials with minimal thrombogenicity, the need for removal of catheter has been reported in more than 25% of the cases [8]. Due to the risks and costs associated with CVC withdrawal and re-implantation, the clearance of occluded catheters using thrombolysis has become the first treatment option [9]. Although CVC occlusion is considered an important clinical problem, due not only to interrupt the antineoplastic therapy, the risks and costs of treatments for unblocking, differences in prevention, diagnosis, and treatment remain as a gap in evidence-based guidelines in this area [3].
A systematic review [10] that evaluates the interventions to restore patency of occluded CVCs has been previously published. However, this review used a small number of studies, all randomized clinical trials, generally addressing the management of thrombotic occlusion, regardless of the type of catheter, or the clinical condition of patient, as well as not address the management of non-thrombotic occlusion. In addition, new technologies and studies have been developed since its publication, and is necessary to update it.
Thus, the aim of this systematic review was to identify the interventions used in the treatment of obstructive events, whether thrombotic or non-thrombotic, in long-term central venous catheters in cancer patients.
Methods
Protocol and registration
This systematic review (SR) was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis PRISMA Checklist [11]. The protocol was registered at the International Prospective Register of Systematic Reviews [12] (PROSPERO) under number CRD42017074256.
PICO question
The guiding question for this SR was based on PICO (Population, Intervention, Control and Outcome) approach: “What are the different interventions (I) used to restore catheter patency (O) in cancer patients with obstructive long-term central venous catheters (P)?”
Eligibility criteria
Inclusion criteria
Retained articles where clinical trials or observational studies with cancer patients (adults and elderly) with occluded long-term central venous catheters (totally implanted catheters and tunneled catheters). Interventions to treat obstructive events including pharmacological and non-pharmacological substances. Catheter patency was considered by the ability to instill at least 5 mL of saline solution and to aspirate 3 mL of blood. There were no language or publication period restrictions.
Exclusion criteria
The studies were excluded in two phases. In phase-1 (titles and abstracts reading), the following criteria were applied: (1) studies with carries (children, adolescents, adults, and elderly) of short-term central venous catheters; (2) studies with hemodialysis catheter, peripherally inserted central catheter (PICC), apheresis catheter, or arterial catheter; (3) studies in children or adolescents; (4) studies evaluating interventions to prevent obstructive events or surgical interventions to treat obstructive events; (5) reviews of the literature, letters, case reports, and protocols.
In phase-2 (full-text reading) these additional criteria were added: (6) studies with different outcomes (not restoration of catheter patency); (7) duplicate studies; (8) studies that was not developed in cancer patients; (9) studies with incomplete data of the population or catheter type used.
Information sources
We developed search strategies for each of following electronic databases: PubMed/Medline, Cochrane CENTRAL, LILACS, Web of Science, Scopus, and CINAHL. A gray literature search was taken using Google Scholar, Open Grey, and ProQuest Thesis and Dissertations. The end search date was April 18, 2017 across all databases. It was later updated on January 22, 2018. Hand search on the reference list of included studies was also performed.
Search
Appropriate truncation and word combinations were selected and adapted for each database search (Appendix 1). All references were managed by reference manager software (EndNote X7, Thomson Reuters, New York, USA) and the duplicates were removed.
Study selection
The selection was completed in 2 phases. In phase-1, two reviewers (A.C.C.C, J.M.R) independently reviewed the titles and abstracts of all citations identified on electronic databases. Articles did not appear to meet the inclusion criteria were discarded. In phase-2, the same reviewers applied the inclusion criteria to the full text of the articles. The reference list of selected studies was critically assessed by both examiners. Any disagreement, in the first or second phase, was resolved by discussion until an agreement between the two authors was attained. When they did not reach a consensus, the third author (C.I.V) became involved to make a final decision.
Data collection process and data items
Two reviewers (A.C.C.C, J.M.R) independently collected data from the selected studies. The third reviewer (C.I.V) assessed the accuracy of the information collected. For all the included studies, the following characteristics were recorded: study characteristics (author, year, country of publication, and study design), sample characteristics (type and size), intervention characteristics (drug type, doses, infusion time, follow-up time), outcome characteristics (efficacy [restoration of catheter patency] and safety), and main conclusions. If the required data were not complete or the data presented could not be extrapolated, attempts were made by email to contact the authors to retrieve the missing information.
Risk of bias in individual studies
Risk of bias of selected studies was evaluated using the Cochrane Collaboration’s tool [13] for randomized clinical trials and the Methodological Index for non-randomized studies (MINORS) [14] for non-randomized clinical trials and observational studies. Two reviewers (A.C.C.C, J.M.R) independently assessed the quality of each included study. Risk of bias was judged as “low,” “high,” or “unclear” when the Cochrane Collaboration’s tool [13] was used. When MINORS [14] was used, items were scored with “0” (not reported), “1” (reported but inadequate), or “2” (reported and adequate). The global ideal score being 16 for non-comparative studies and 24 for comparative studies. Disagreements between both reviewers were resolved by a third reviewer (C.I.V).
Summary measures
The efficacy outcomes were expressed by the percentage of catheter patency restored from the total sample of included studies.
Synthesis of results
Statistical pooling of data using meta-analysis was planned whenever trials were considered combinable and relatively homogeneous in relation to design, interventions, and outcomes. Heterogeneity within studies was evaluated either by considering clinical (differences about participants, type of interventions and results), methodological (design, and risk of bias), and statistical characteristics (effect of studies) or by using inconsistency indexes (I2) statistical test [15]. Meta-analysis was performed with the aid of MedCalc Statistical Software version 14.8.1 (MedCalc Software, Ostend, Belgium). Heterogeneity was calculated by I2, following the appropriate Cochrane Guidelines and a value greater than 50% was considered an indicator of substantial heterogeneity among studies and the random effect might be used [15]. When I2 is lower than 50%, fixed effect is recommended. MedCalc provides both fixed and random effect for each analysis, so we choose between both based on I2. The significance level was set at 5%.
Confidence in cumulative evidence
A summary of overall strength of evidence available was performed using “Grading of Recommendations Assessment, Development and Evaluation” (GRADE) [16]. A summary of findings table was produced via GRADEpro software (McMaster University, Hamilton, Canada).
Results
Study selection
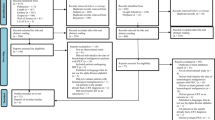
In phase-1, 9661 articles were found across the six electronic databases. After duplicates removed, 75 of the 4685 studies were selected for phase-2. A gray literature search was performed identifying 15 articles, whereas none met the inclusion criteria. The references list of included studies were screened, and 12 additional articles were included. After three consecutive attempts by email, in a period of a month, we did not get answers from the experts, and articles were not included through this type of search. Subsequently, 87 studies were for full-text reading, and 72 of these articles were excluded (Appendix 2). Therefore, only 15 studies fulfilled the eligibility criteria and were included in the qualitative synthesis. Of those, 14 were adequate to use for the meta-analysis. A flowchart of the process of identification, inclusion, and exclusion of studies is shown in Fig. 1.
Study characteristics
From the 15 selected studies, 13 of them were clinical trials [4, 9, 17,18,19,20,21,22,23,24,25,26,27] and 2 were observational studies [2, 28] with evaluation due to patient’s charts. Sample sizes ranged from 4 [28] to 171 [25] LT-CVC, in a total of 949 catheters in 1613 patients included. Among the LT-CVC used, 138 (14.5%) were tunneled catheters and 811 (85.5%) were totally implanted catheters, with different types of occlusion: partial occlusion (9 studies, 60%) and partial or total occlusion (6 studies, 40%). The main purpose of LT-CVC was the infusion of chemotherapy, with the most prevalent types of cancer included breast cancer (130, 21%), hematological cancer (114, 18%), and solid tumors (79, 13%).
The drugs used for the restoration of catheter patency were urokinase [2, 17, 19,20,21, 23, 26, 28] (8, 53.3%), recombinant urokinase [22] (1, 6.7%), alteplase [4, 21, 27] (3, 20%), tenecteplase [18, 25] (2, 13.3%), reteplase [24] (1, 6.7%), and staphylokinase [9] (1, 6.7%). Two studies compared the efficacy of urokinase in relation to urokinase plus heparin [23] and alteplase [21]. Other studies have compared placebo with recombinant urokinase (r-UK) [22] and tenecteplase [18]. Other trials evaluated the efficacy of different drugs without control group.
The aim of the included studies was to evaluate the efficacy and safety of the drugs in restoring the catheter patency, at different doses and at different infusion times. The restoration rate ranged from 40 to 100%, since the administration time ranged from 15 min to 24 h. The number of doses used ranged from 1 to 8 doses. There were no adverse events associated with drugs reported in the articles; only one study [22] reported minor bleeding events in 5% of patients receiving r-UK. All included studies addressed the treatment of thrombotic occlusion. No studies about the management of non-thrombotic catheter occlusion in cancer patients have been found, only in other populations (mainly use of parenteral nutrition in patients with metabolic and gastrointestinal disorders). The summary of the descriptive characteristics of the included articles is provided in Table 1.
Risk of bias of individual studies
Three randomized clinical trials (RCT) were classified as having an unclear risk of bias in these domains: “selection bias,” “performance bias,” “detection bias,” and “other bias.” Regarding to selection bias, the studies [22, 23] were classified as unclear because the authors did not explain how was performed the random sequence generation and allocation concealment. In relation to performance bias, the study [23] did not elucidated if the personnel was blinded about the intervention used. Due to detection bias, the study [23] did not described how blinding of outcome assessment was made. And some studies [21,22,23] did not bring sufficient information to evaluate if other bias were present, so we judged them as other bias.
Two RCT were classified as having a high risk of bias, one study [22] in the domain “Reporting bias” as it did not report all the results obtained according to infusion times used and other study [18] in the domain “Other bias” due to report of possible conflict of interest. In the case of non-randomized studies, three [4, 9, 27] of them were considered as having a high risk of bias because they obtained 8 points (50% of the total score), and 4 studies [24,25,26, 28] were classified as having a low risk of bias cause they obtained between 12 and 14 points.
Figure 3 describes the Cochrane Risk of Bias Tool [13] for RCT. Appendix 3 provides more information about the MINORS [14] scores.
Results of individual studies
Most of the included articles used urokinase (UK) as an intervention for the restoration of catheter function. Bjeletich [17] is the study with the longest publication time (1987). Despite the small number of catheters included, it was the only who used the drug at the initial 10,000 IU dose, where the use was performed within the home care setting and found a 100% restoration rate.
Son et al. [28] used the UK at a dose of 5000 IU, with a 50% successful restoration rate, with the lowest number of catheters included (n = 4). The same dose was used by Whigham et al. [26]; however, there were a greater number of doses used, in addition to the use of an extra dose of 40,000 IU of UK in an extended infusion of 12 h. Thus, it was a gain in the efficacy of thrombolysis, with a total restoration rate of 98.7%.
Chang et al. [2] used the UK at 15000 IU and 20,000 IU, with an overall restoration rate of 70%. Haire et al. [19, 20] used a dose of 40,000 IU in prolonged infusion (12 h [19] and 6 h [20]) in catheters with occlusion refractory to the use of 5000 IU of urokinase. Restoration rates were 96.7% and 79%, respectively. Horne et al. [23] used the same UK dose and the same prolonged infusion regimen used in the Haire’s studies [19, 20] comparing to the use of a heparin (320 IU)-associated UK (40,000 IU) solution. However, there was no difference on the efficacy between the two interventions (76% in both groups).
Whigham et al. [27] used alteplase at a dose of 1 mg/mL in up to 4 doses and was successful in restoring catheter function in 92.9% of the cases. Massmann et al. [4] used alteplase at a dose of 3 mg/3 mL in up to 2 doses, obtaining an overall restoration rate (92.7%) very similar to that found in the study described [27] previously. Haire et al. [21] used 2 mg/2 mL of alteplase in a comparative study with urokinase at a dose of 10,000 IU, finding a statistically significant difference between the efficacy of the drugs used (89% versus 59%, p = 0.013).
Haire et al. [22] used recombinant urokinase at a dose of 5000 IU and compared with placebo (saline solution). Although the author reports that r-UK is more effective than placebo in restoring patency (54% versus 30%, p = 0.002), when considering all catheter types, in the specific case of totally implanted catheters, no difference was observed between the groups (40% versus 28%, p = 0.34).
Gabrail et al. [18] used tenecteplase (TNK) at a dose of 2 mg/2 mL in two groups, where one started with infusion of placebo (saline solution), obtaining a restoration rate, after administration of one or two doses, of 90%. Tebbi et al. [25] used TNK at the same dose in a study without a control group, observing a success rate of 80%.
Liu et al. [24] used reteplase at a dose of 0.4 IU/2 mL in upto two doses, and found a success rate of 94.6%. Verhamme et al. [9] used a new drug, staphylokinase (SY 162), at doses of 0.15 mg, 0.30 mg, and 0.45 mg, obtaining restoration rates of 50% (0.15 mg) and 88% (0.30 mg and 0.45 mg).
Synthesis of results
From the 15 studies included, 14 of them were grouped to perform the meta-analysis. The heterogeneity found among the studies was high (86.37%), so the random model was chosen for the statistical analysis. The result of the meta-analysis, when considering all interventions, showed an overall restoration frequency of ~ 84% (CI 76.51 to 90.41) (total sample = 888 catheters) (Fig. 2a).
When the meta-analysis by drug type was performed, the articles were divided in three groups: A (urokinase), B (alteplase), and C (tenecteplase). The heterogeneity between the studies in the group A meta-analysis was high (80.49%) and the results demonstrated an overall restoration rate of ~ 84% (CI 71.10 to 93.89) (total sample = 224 catheters) (Fig. 2b). The heterogeneity between the studies in the group B meta-analysis was 0%, indicating high homogeneity among them. The results showed an overall restoration rate of ~ 92% (CI 88.25 to 94.96) (total sample = 294 catheters) (Fig. 2c). In the group C meta-analysis, the heterogeneity found between the studies was low (38.02%) and the results indicated a success rate of ~ 84% (CI 77.32 to 90.01) (total sample = 234 catheters) (Fig. 2d).
Risk of bias within studies
Although the articles have different designs, the main methodological problem concerns the sample. Most of the included studies, including clinical trials, used convenience samples, without randomization of participants and absence of control group (Fig. 3).
Confidence in cumulative evidence
The quality of evidence using GRADE’s Summary of Findings Table ranged from very low to high. This variation was directly related to the risk of bias and imprecision presented by included articles, as well as by the heterogeneity found among them (Table 2).
Discussion
Fibrinolytic agents have been used successfully for more than two decades in restoring the patency of occluded catheters [29]. This systematic review investigated the available evidence of the interventions used in the treatment of obstructive events in LT-CVC in cancer patients. Among the interventions used to restore the catheter function, urokinase was the most frequent, followed by alteplase.
Until 1999, urokinase was the only pharmacological agent approved to treat the thrombotic catheter occlusion. The dose approved by FDA for catheter clearance is 5000 IU, which represents 1.6% of the approved dose for the treatment of pulmonary embolism in adults [30]. The UK binds to plasminogen and converts it into an active enzyme, plasmin, that when exposed to the clot causes lysis [31]. In this review, studies using the UK, including its recombinant form (r-UK), showed restoration rates from 40 to 100%, noting that the most successful studies were conducted more than 20 years ago. This condition can be explained in part by the scarce use of imaging tests to diagnose the type of occlusion that affected the catheters included in these studies and to assess the type of clearing obtained with the therapy used, often being only a partial resolution of the problem.
Alteplase (rt-PA) is a recombinant analog derived from human tissue plasminogen activator, secreted by vascular endothelial cells. It has higher fibrin specificity than the UK and shorter plasma half-life (UK 16 min, rt-PA 5 min) [30]. The dose approved by FDA for catheter clearance is 2 mg/2 mL, which corresponds to 2% of the dose used to treat pulmonary thromboembolism in adults, similar to the proportion adopted for the UK dose. Alteplase is not easily removed from clots, which may maintain its local effect longer and require a smaller total dose for a restoration of catheter function [30]. In this review, studies using rt-PA showed success rates from 89 to 92.9%.
Other recombinant forms of tissue plasminogen activators (TPA) have been described in the articles. However, their use for clearance of catheter has not been approved yet, being an off-label use. Reteplase is a TPA variant indicated in the treatment of acute myocardial infarction in adults. It has lower affinity for fibrin than alteplase and longer plasma half-life (13–16 min). Although alteplase binds strongly to the fibrin matrix and accumulates on the surface of the thrombus, reteplase exhibits clot penetration and plasminogen activation within [24]. Tenecteplase is a recombinant form of TPA with a mechanism of action similar to that of alteplase. It is characterized by an increase in resistance to inhibitor of plasminogen activation when compared to alteplase [18]. A single study [24] evaluated the use of reteplase, finding an overall restoration rate of ~ 95%. And two studies [18, 25] evaluated the use of tenecteplase, achieving success in restoration between 80 and 90%.
Staphylokinase, a fibrinolytic agent with high fibrin specificity, is another drug which is being used off-label in restoring the catheter patency. As staphylokinase infusion in humans is also associated with the immunogenic response, like streptokinase, several molecular modifications have been developed to decrease the immunogenicity of this substance, preserving its potency and specificity to fibrin [9]. A single study [9] evaluated the use of the SY162 variant in restoring catheter patency, finding a success rate from 50 to 88%, depending on the dose used.
Regarding to the time of infusion, a huge heterogeneity was found, depending on the dose. In the case of urokinase, the administration time ranged from 15 min to 24 h. For alteplase, the infusion time ranged from 2 to 24 h. And for the other TPA, the time ranged from 2 to 4 h.
Only one study [22] reported adverse event possibly related to the drug (r-UK) characterized as “minor hemorrhagic events.” Thus, it can be inferred that thrombolytic therapy is potentially safe when used in the restoration of catheter patency in cancer patients.
By evaluating the data from qualitative analysis, a trend of superiority in the efficacy of TPA was observed in relation to urokinase, which was the most used drug among the studies. However, when the data from meta-analysis was evaluated, an overall restoration frequency is very similar between the drugs analyzed, with a slight superiority of alteplase in relation to other interventions.
Thus, for better use in clinical practice, due to heterogeneity in the concentration of drugs, the time of administration of these medications could be an important factor to be considered, since a short infusion time optimizes the use of human and material resources, besides avoid delays in treatment and possible complications.
Another factor that may be determinant for the current use of these substances is the costs associated with therapy. However, one study [32] demonstrated the use of thrombolysis (with alteplase) in the clearance of LT-CVC represents significantly lower costs than the catheter replacement. This study found cost-benefit in the use of thrombolysis not only in reducing length of stay, but also in decrease the use of other services such as radiology, laboratory, nursing care, and general material resources.
aled a high heterogeneity (80.49%) when the urokinase group was evaluated. This is due to the different study designs included, different concentrations and number of doses used, and different sample sizes. The great heterogeneity found in this group had a direct impact on the evaluation of heterogeneity when all interventions were considered, with an even higher heterogeneity (86.37%).
Most of the included studies had a risk of bias related to study population (absence of randomization, blinding, and control group) and due to the high heterogeneity between the doses used and infusion times, the quality of evidence was not robust. Thus, the evidence found tend to confirm the success of thrombolytic therapy in general. However, there is insufficient data for optimal drug, dosage, and time of administration standardization to restore catheter patency.
Some methodological limitations of this review should be listed. Reference studies using alteplase and r-UK with large and representative samples that met the main outcome of this review (restoration of catheter patency) were excluded. As they included both adult and pediatric populations, as well as different catheter types, it was not possible to reliably extract the data that met our inclusion criteria.
As we did not find articles on the treatment of non-thrombotic catheter occlusion in cancer patients, it is not possible to describe with certainly which interventions could be used and their effects on the restoration of catheter function.
Therefore, we suggest additional studies on the management of obstructive events in LT-CVC in cancer patients, in order to standardize and guide current clinical practice.
Conclusion
In view of evidence obtained, the most common interventions used for the treatment of thrombotic catheter occlusion in cancer patients were urokinase and alteplase. The use of thrombolytic therapy seems to be safe, and in relation to efficacy, there was a relative superiority of alteplase in relation to the other drugs in the restoration of catheter patency. However, it was not possible to establish optimal concentration, number of doses, and infusion time for an effective restoration.
Regarding to the treatment of non-thrombotic catheter occlusion in cancer patients, no evidence was found about the interventions used, thus elucidating an important gap to be investigated.
References
Williams A (2018) Catheter occlusion in home infusion. J Infus Nurs 41(1):52–57. https://doi.org/10.1097/NAN.0000000000000259
Chang DH, Mammadov K, Hickethier T, Borggrefe J, Hellmich M, Maintz D, Kabbasch C (2017) Fibrin sheaths in central venous port catheters: treatment with low-dose, single injection of urokinase on an outpatient basis. Ther Clin Risk Manag 13:111–115. https://doi.org/10.2147/TCRM.S125130
Baskin JL, Reiss U, Wilimas JA, Metzger ML, Ribeiro RC, Pui CH, Howard SC (2012) Thrombolytic therapy for central venous catheter occlusion. Haematologica 97(5):641–650. https://doi.org/10.3324/haematol.2011.050492
Massmann A, Jagoda P, Kranzhoefer N, Buecker A (2015) Local low-dose thrombolysis for safe and effective treatment of venous port-catheter thrombosis. Ann Surg Oncol 22(5):1593–1597. https://doi.org/10.1245/s10434-014-4129-0
Toril Rubio M, Rodríguez BMA (2017) Revisión sistemática de las complicaciones de los dispositivos de administración de tratamiento al paciente oncológico. Enferm Glob 16(46):544–561. https://doi.org/10.6018/eglobal.16.2.251571
Baskin JL, Pui CH, Reiss U, Wilimas JA, Metzger ML, Ribeiro RC, Howard SC (2009) Management of occlusion and thrombosis associated with long-term indwelling central venous catheters. Lancet 374:159–169. https://doi.org/10.1016/S0140-6736(09)60220-8
Tolar B, Gould JR (1996) The timing and sequence of multiple device-related complications in patients with long-term indwelling catheters. Cancer 78:1308–1313. https://doi.org/10.1002/(SICI)1097-0142(19960915)78:6<1308::AID-CNCR20>3.0.CO;2-3
Moll S, Kenyon P, Bertoli L, de Maio J, Homesley H, Deitcher SR (2006) Phase II trial of alfimeprase, a novel-acting fibrin degradation agent, for occluded central venous access devices. J Clin Oncol 24(19):3056–3060. https://doi.org/10.1200/JCO.2006.05.8438
Verhamme P, Goossens G, Maleux G, Collen D, Stas M (2007) A dose-finding clinical trial of staphylokinase SY162 in patients with long-term venous access catheter thrombotic occlusion. J Thromb Thrombolysis 24(1):1–5. https://doi.org/10.1007/s11239-006-0006-4
van Miert C, Hill R, Jones L (2012) Interventions for restoring patency of occluded central venous catheter lumens. Cochrane Database of Syst Rev 4:CD007119. https://doi.org/10.1002/14651858.CD007119.pub2
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
Costa ACC, Ribeiro JM, Vasques CI et al (2017) Interventions for treatment of thrombotic occlusion in long-term central venous catheters: a systematic review. PROSPERO CRD42017074256. http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD4201074256
Higgins JP, Altman DG, Gotzsche PC et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. BMJ 343:d5928. https://doi.org/10.1136/bmj.d5928
Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J (2003) Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg 73:712–716. https://doi.org/10.1046/j.1445-2197.2003.02748.x
Higgins J, Green S (2011) Cochrane handbook for systematic reviews of interventions Version 5.1.0. The Cochrane Collaboration, 2011. URL http://handbook.cochrane.org
Schünemann H, Brożek J, Guyatt G (2013) GRADE handbook for grading quality of evidence and strength of recommendations: the GRADE Working Group. URL guidelinedevelopment.org/handbook
Bjeletich J (1987) Declotting central venous catheters with urokinase in the home by nurse clinicians. NITA 10(6):428–430
Gabrail N, Sandler E, Charu V, Anas N, Lim E, Blaney M, Ashby M, Gillespie BS, Begelman SM (2010) TROPICS1: a phase III, randomized, double-blind, placebo-controlled study of tenecteplase for restoration of function in dysfunctional central venous catheters. JVIR 21(12):1852–1858 https://doi.org/10.1016/j.jvir.2010.09.002
Haire WD, Lieberman RP, Lund GB, Edney J, Wieczorek BM (1990) Obstructed central venous catheters: restoring function with a 12-hour infusion of a low-dose urokinase. Cancer 66(11):2279–2285. https://doi.org/10.1002/1097-0142(19901201)66:11<2279::AID-CNCR2820661105>3.0.CO;2-O
Haire WD, Lieberman RP (1992) Thrombosed central venous catheters: restoring function with 6-hour urokinase infusion after failure of bolus urokinase. JPEN 16(2):129–132. https://doi.org/10.1177/0148607192016002129
Haire WD, Atkinson JB, Stephens LC, Kotulak GD (1994) Urokinase versus recombinant tissue plasminogen activator in thrombosed central venous catheters: a double-blinded, randomized trial. Thromb Haemost 72(4):543–547
Haire WD, Deitcher SR, Mullane KM, Jaff MR, Firszt CM, Schulz GA, Schuerr DM, Schwartz LB, Mouginis TL, Barton RP (2004) Recombinant urokinase for restoration of patency in occluded central venous access devices: a double-blind, placebo-controlled trial. Thromb Haemost 92(3):575–582. https://doi.org/10.1160/TH03-11-0686
Horne MK III, Mayo DJ (1997) Low-dose urokinase infusions to treat fibrinous obstruction of venous access devices in cancer patients. J Clin Oncol 15(7):2709–2714. https://doi.org/10.1200/JCO.1997.15.7.2709
Liu CY, Jain V, Shields AF, Heilbrun LK (2004) Efficacy and safety of reteplase for central venous catheter occlusion in patients with cancer. JVIR 15:39–44. https://doi.org/10.1097/01.RVI.0000106385.63463.EC
Tebbi C, Costanzi J, Shulman R, Dreisbach L, Jacobs BR, Blaney M, Ashby M, Gillespie BS, Begelman SM (2011) A phase III, open-label, single-arm study of tenecteplase for restoration of function in dysfunctional central venous catheters. JVIR 22(8):1117–1123. https://doi.org/10.1016/j.jvir.2011.02.034
Whigham CJ, Greenbaum MC, Fisher RG, Goodman CJ, Thornby JI, Thomas JW (1999) Incidence and management of catheter occlusion in implantable arm ports: results in 391 patients. JVIR 10:767–774. https://doi.org/10.1016/S1051-0443(99)70112-0
Whigham CJ, Lindsey JI, Goodman CJ, Fisher RG (2002) Venous port salvage utilizing low dose tPA. Cardiovasc Intervent Radiol 25(6):513–516. https://doi.org/10.1007/s00270-002-2615-4
Son JT, Min SY, Kim JI, Choi PW, Heo TG, Lee MS, Kim CN, Kim HY, Yi SY, Lee HR, Roh YN (2014) Thrombolytic therapy using urokinase for management of central venous catheter thrombosis. Vasc Spec Int 30(4):144–150. https://doi.org/10.5758/vsi.2014.30.4.144
Deitcher SR, Fraschini G, Himmelfarb J, Schuman E, Smith TJ, Schulz GA, Firszt CM, Mouginis TL (2004) Dose-ranging trial with a recombinant urokinase (urokinase alfa) for occluded central venous catheters in oncology patients. JVIR 15(6):575–579. https://doi.org/10.1097/01.RVI.0000124950.24134.19
Ponec D, Irwin D, Haire WD, Hill PA, Li X, McCluskey E, COOL Investigators (2001) Recombinant tissue plasminogen activator (alteplase) for restoration of flow in occluded central venous access devices: a double-blind placebo-controlled trial – the cardiovascular thrombolytic to open occluded lines (COOL) efficacy trial. JVIR 12(8):951–955. https://doi.org/10.1016/S1051-0443(07)61575-9
Haire WD (2001) Techniques in dosing for thrombolysis of occluded central venous catheters. Tech Vasc Interv Radiol 4(2):127–130. https://doi.org/10.1016/S1089-2516(01)90008-3
Ernst FR, Chen E, Lipkin C, Tayama D, Amin AN (2014) Comparison of hospital length of stay, costs, and readmissions of alteplase versus catheter replacement among patients with occluded central venous catheter. J Hosp Med 9(8):490–496. https://doi.org/10.1002/jhm.2208
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
We declare that we have had full control of the primary data extracted for this study, and we agree to allow the journal to revise the data, if it will be requested.
Rights and permissions
About this article
Cite this article
da Costa, A.C.C., Ribeiro, J.M., Vasques, C.I. et al. Interventions to obstructive long-term central venous catheter in cancer patients: a meta-analysis. Support Care Cancer 27, 407–421 (2019). https://doi.org/10.1007/s00520-018-4500-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-018-4500-y