Abstract
Comprehensive density functional theory calculations at B3LYP/6-311++G(d, p) level have been undertaken on a new type of organic Brønsted acids based on perchloric and triflic acid in order to design superacids. The deprotonation enthalpy (ΔH acid ) and Gibbs free energy (ΔG acid ) were used to calculate the acidity of the proposed structures. For the acidity comparison, a systematic estimation of aromaticity in the superacids and their anions have been studied using structural (harmonic oscillator model of aromaticity) and magnetic (nucleus-independent chemical shift) criteria. The calculated gas phase ΔH acid for the proposed acids were 319–249 kcal/mol which are less than the most mineral acids. Tautomers of proposed organic acids were also investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The protonation and deprotonation reactions are very important in the chemical and biochemical processes [1]. The acidity of a compound is usually defined as the proton loss and creation of an anionic species. The negative charge in the anion can be stabilized by various parameters such as the proper solvation. Due to low solubility, anion solvation is not successfully occurred in the most organic solvents. Hence, the acidity decreases sharply. The use of inorganic acids is severely restricted due to their corrosive properties and difficulty of working with them. Some organic syntheses involve processes in which the formation of carbocations is required. Carbocations are unstable species and very strong acids are required for their production and stabilization such as perchloric acid and trifluoromethanesulfonic (triflic) acid [2,3,4]. Superacids are used as effective catalyst in the organic syntheses such as alkylation, acylation, isomerization, polymerization, and coal industry [5]. A superacid is a compound with acidity greater than that of pure 100% sulfuric acid [6, 7]. Till now, many theoretical attempts have been made to design the organic superacids [8,9,10,11,12,13,14,15,16,17,18,19,20]. Anion stability has been considered in most of the proposed methods. The negative charge on the conjugate base of the designed acid may be stabilized by electron-withdrawing substituents, resonance, and intramolecular hydrogen bonding [21,22,23]. Recently, our research team has been able to design the organic superacids based on fluorosulfuric, dithionic, peroxydisulfuric, and nitric acid, whose acidity falls in the range of the superacids [24,25,26]. In the continuation of these research works, the present study investigates acidity of the compounds (a-f) based on perchloric and triflic acid by using density functional theory (DFT).
Computational details
All geometric optimizations and frequency calculations for the all designed molecules have been carried out by using Gaussian 09 suite [27]. The DFT calculations have been performed using the B3LYP method in conjunction with 6-311++G(d, p) basis set. Benchmarking the computational method is presented below. All structures were confirmed to be local energy minima by computing their analytical vibrational frequencies.
The deprotonation enthalpy (ΔHacid) and Gibbs free energy (ΔGacid) for the studied compounds in the gas phase (reaction 1) can be considered as suitable and reliable parameters for comparing their acidities (Eqs. (1) and (2)).
H and G in these equations describe the enthalpy and Gibbs free energy for the studied species at 298 K, respectively. The enthalpy and Gibbs free energy for proton (H+) are given as 1.48 and − 6.27 kcal/mol, respectively.
Results and discussion
The strategy used to design the organic acids based on perchloric and trifluoric acid is to replace one of the oxygen atoms of perchloric and triflic acid with a vinyl cyclopentadiene (Cp) group (Schemes 1 and 2).
Upon deprotonation of the designed acids, the negative charge is expected to be placed on Cp. According to the Hückel rule, flat rings containing conjugated π bonds are aromatic in the case of having 4n+2 electrons. The negative charge on Cp makes it aromatic (Scheme 3) [3].
Evaluation of aromaticity for this ring in the designed acids relative to their conjugate bases can be another criterion for the acidity comparison. It is expected that the aromaticity indices of Cp increase in the anion with respect to its corresponding conjugate acid. Literature review illustrates that two significant and general indices are implemented for the aromaticity evaluation including (1) harmonic oscillator model of aromaticity (HOMA) [28, 29] and (2) nucleus-independent chemical shift (NICS) [30]. HOMA is a structural index which is defined for an all-carbon ring using Eq. (4) [31].
In this relation, Ri and Ropt stand for the C–C bonds in the studied aromatic ring and benzene, respectively, which is estimated to be 1.388 Å for the later one. Also, n indicates the number of carbons in the studied ring and α as a normalization factor which is equal to 257.7. The NICS index as a magnetic parameter is also a reliable benchmark for determining the aromaticity of the studied compounds. The NICS value accounts for the negative magnetic coverage at the center of the ring and 1 Å above it. The negative value of NICS points out to the aromaticity. In this study, both HOMA and NICS indices have been calculated, compared, and discussed for the acid and the conjugate base.
Recently, we have successfully used B3LYP for calculating the PAs of some organic superacids [24,25,26]. Other studies have employed B3LYP as appropriate methodology for predicting the acidity of organic acids [11, 32, 33]. It should be noted that one of the objectives of this study is to find a computationally accurate and reliable method; the structure of molecule d1 and its anion (d-H)- have been investigated using DFT, HF, and MP2 methods with the same basis set of 6-311++G(d, p). Table 1 lists the selected bond lengths and acidity of molecule d1.
As can be seen from Table 1, the calculated bond lengths via the two methods of B3LYP and MP2 are close to each other with a slight difference. A plot of the DFT vs MP2-computed bond lengths (Fig. 1) shows a linear relationship with r2 = 0.996. Besides, the obtained values of ΔHacidvia the three abovementioned methods are nearly identical for molecule d1. All the computations in this study were carried out using the DFT-B3LYP method as this method has increased in popularity due to the relatively high accuracy of the results compared with the relatively low computational cost.
As can be seen from Schemes 1 and 2, C–H tautomers can also be considered for the designed superacids a-f in addition of the O–H isomer. As would be observed, the O–H isomers are unstable compared with the C–H ones and meet higher acidity. The most stable tautomer among the superacids of a-c set is their isomer 2 (a2, b2, c2). In addition to O–H and C–H tautomers for superacids c, N–H ones can also be considered (c5, c6). These isomers are unstable than C–H ones to the amount of 7–9 kcal/mol. It is noteworthy that tautomerism is also possible in the superacids d-f. The remarkable point about the stability of superacids based on triflic acid is that they do not have particular order like the perchloric acid–based counterparts. Among the superacids of group d, the structure d3, e4 in group e, and N–H tautomer (f5) in group f are more stable isomers.
The data of ΔHacid, ΔGacid, NICS, and HOMA associated with the superacids a1-c7 are given in Table 2.
Comparison of the acidity of molecule a1 containing a Cp with that of perchloric acid shows that their acidities are approximately equal. The high acidity of a1 is due to the stability of anion (a-H)-. Comparison of the aromaticity indices of the neutral molecules of group a with respect to their corresponding conjugate base ((a-H)- anion) in Table 2 is a suitable criterion for the anion stability and basicity decrease of the conjugate base. As reflected in Table 2, the significant increase of HOMA and NICS indices confirms the formation of the aromatic cyclopentadienyl anion. The electronegative and electron-withdrawing substituents such as fluorine and cyanide group have the ability of stabilizing the negative charge through inductive and resonance effects. Placing 4 fluorine atoms in Cp increases the acidity of molecule b1 relative to a1 by 13.01 kcal/mol. Among the C-H tautomers of the superacids of group b, molecule b3 meets the highest acidity over the other ones. As illustrated by the aromaticity data in Table 2, the (b-H)- anion is more stable than the (a-H)– one and its NICS value is more aromatic to the amount of − 1.35 ppm. The fluorine atom possesses a high electronegativity, but due to its small atomic radius and the presence of 7 electrons in its valance layer, it has low electron-accepting capability, so it cannot stabilize the anion created by superacids as expected. The cyanide group is a unique group in terms of the electron acceptance, due to the sp hybridization of its carbon atom and binding to the electronegative nitrogen [34]. However, the important point about this group is the negative charge stability via resonance. As can be seen from Scheme 4, the negative charge is placed on the electronegative nitrogen through the resonance which leads to the anion stability as a consequence. The molecules in group c contain four cyanide groups, with acidities far higher than those of groups a and b. Hence, all of this group’s acids are considered as superacids and their protonated enthalpy (ΔHacid) is below 266 kcal/mol and lower than that of perchloric acid to the amount 32 kcal/mol. The significant increase in NICS and HOMA data for Cp in the (c-H) – anion relative to the neutral state indicates the complete negative charge stability.
Placing two Cp groups on perchloric acid creates superacid c7 with ΔHacid = 262.59 kcal/mol. The comparison of c7 molecule’s acidity with that of the most stable isomer of group c (c2) indicates that adding Cp more than one case has negligible effect on their acidity. The superacid c2 with small molecular size has acidity near that of c7 molecule.
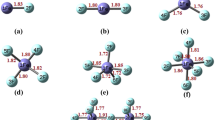
With these interesting and encouraging results, the acidic properties of triflic acid’s derivatives containing a Cp have been examined by implementing the DFT-B3LYP/6-311++G(d, p) method and the obtained results are briefly presented in the following text. The acidity data and aromaticity indices are given in Table 3 for the organic triflic superacids d-f. Figure 2 illustrates some the optimized structure corresponding to the organic superacids d-f.
Placing a Cp group with a double bond on the triflic acid led to acid d1 whose acidity is higher to the amount of 11.06 kcal/mol. Placing four fluorine atoms instead of the four hydrogen atoms of cyclopentadiene results into the molecules of group e with remarkable acidity. Further comparisons clarify that the acidity of similar isomers in this group is prominently higher than that of the perchloric acid–based acids. For example, the acidity of molecule e1 is 11.47 kcal/mol higher than that of b1. Sharp increasing HOMA and NICS indices in the acids of group e indicates the complete stability of anion (e-H)-. The acidity amount highly increases by placing four CN groups in Cp. The remarkable point about the triflic acid–based superacids containing cyanide group (f) is that the stable isomer in them is N–H tautomer (f5) which is more stable than O–H (f1) one to the amount of 13.2 kcal/mol. In the present study, f1 is the strongest designed superacid with ΔHacid as 249.26 kcal/mol, which is stronger than most of the superacids designed and reported within the scientific sources. The NICS value associated with Cp in (f-H)- anion is equal to − 12.37 ppm which is higher than the standard value (− 10 ppm) for the benzene ring.
It should be noted that the acidity of the proposed structures relative to triflic acid can be reported using the reaction shown in Scheme 5. In this reaction, a negative data of ΔH means that f3 is a stronger acid than triflic acid.
It is interesting to note that by carefully examining the NICS data, it was revealed that Cp in neutral superacids d1, e1, and f1 derivatives has strong aromaticity. On the other hand, less changes were observed in NICS and HOMA data of them compared with their corresponding conjugate bases. In fact, the aromaticity indices have not notably increased in the corresponding anions (Scheme 6).
One possible reason for the aromaticity index increment in the neutral form of these acids is the resonance phenomenon. The double bond between the sulfur and carbon atom in their structures is somewhat single bond (Scheme 7).
In order to prove and confirm this phenomenon, one can compare their bond length with the anion form in the optimized structures. In the case of such phenomena and resonance existence, there should be less change in the C–S bond length of their anion. Evaluating of these structures by GaussView software, indicates a slight increase in C–S bond length.
Conclusion
In conclusion, our protocol to superacids is to construct perchloric and triflic acid derivatives with one or two vinyl cyclopentadiene groups. Upon protonation of the molecules, the negative charge is delocalized in the aromatic cyclopentadienyl ring. The aromaticity of the 5-membered ring leads in stability of the conjugate bases. Both fluorine and cyanide groups on cyclopentadiene ring can be utilized as electron-withdrawing substituents that can stabilize the anions. The deprotonation enthalpy for most of the designed organic superacids a-f is smaller than 300 kcal/mol indicating that generally, they are stronger acids than the mineral acids. It should be said that the comparison of the designed compounds’ acidity with organo fluorosulfuric derivatives [26] points out to the weaker acidity of the present derivatives while they are stronger acids than organonitric acids (Scheme 8) [24].
References
Warren JJ, Mayer JM (2015). Biochem 54:1863–1878
Dalpozzo R, Bartoli G, Sambri L, Melchiorre P (2010). Chem Rev 110:63501–63551
Carey FA, Sundberg RJ (2007) Chemical bonding and molecular structure, Advanced Organic Chemistry, Part A: Structure and Mechanisms4th edn. Springer Science Business Media, LLC
Vollhardt KPC, Schore NE (2018). Organic chemistry: structure and function, 8th ed. New York
Olah GA, Prakash GKS, Molnár Á, Sommer J (2009) Superacid Chemistry. John Wiley & Sons, Inc
IUPAC (1997) Compendium of chemical terminology. In: McNaught AD, Wilkinson A (eds) the “Gold Book”2nd edn. Blackwell Scientific Publications, Oxford
Gillespie RJ, Peel TE, Hammett (1973). J Am Chem Soc 95:5173–5178
Valadbeigi Y, Rouhani M (2019). Theor Chem. https://doi.org/10.1016/j.comptc.2019.112550
Vianello R, Maksić ZB (2010). J Organomet Chem 75:7670–7681
Mó O, Yáñez M, Alkorta I, Elguero J (2013). J Mol Model 19:4139–4145
Czapla M, Skurski P (2015). J Phys Chem A 119:12868–12875
Koppel IA, Burk P, Koppel I, Leito I, Sonoda T, Mishima M (2000). J Am Chem Soc 122:5114–5124
Maksic’ ZB, Vianello R (2004). New J Chem 28:43–846
Srivastava AK, Kumar A, Misra N (2017). J Fluor Chem 197:59–62
Yáñez M, Mó O, Alkorta I, Elguero J (2013). Chem Eur J 19:11637–11643
Vianello R, Maksić ZB (2005). Tetrahedron Lett 46:3711–3713
Valadbeigi Y, Kurtén T (2019). Comput Theor Chem 1153:34–43
Lipping L, Leito I, Koppel I, Krossing I, Himmel D, Koppel IA (2015). J Phys Chem A 119:735–743
Zhou FQ, Xu WH, Li JF, Zhao RF, Yin B (2017). Inorg Chem 56:11787–11797
Valadbeigi Y, Vianello R (2018). Int J Quantum Chem 118:25754–25764
Si MK, Ganguly B (2017). New J Chem 41:1425–1429
Vianello R, Liebman JF (2004) Maksic´ ZB. Chem Eur J 10:5751–5760
Bayat A, Fattahi A (2019). J Phys Org Chem. https://doi.org/10.1002/poc.3919
Saeidian H, Mashhadian S, Mirjafary Z, Rouhani M (2019). Comput Theor Chem 1157:11–18
Saeidian H, Shams B, Mirjafary Z (2019). Struct Chem 30:787–793
Shams B, Saeidian H (2018). Comput Theor Chem 1135:48–55
Frisch JM, et al. (2013). Gaussian 09, Gaussian, Inc. Wallingford CT.
Krygowski TM, Szatylowicz H, Stasyuk OA, Dominikowska J, Palusiak M (2014). Chem Rev 114:6383–6422
Vianello R, Maksić ZB (2007). Struct Chem 18:821–826
Porannea RG, Stanger A (2015). Chem Soc Rev 44:6597–6615
Krygowski TM, Cyrański MK (2001). Chem Rev 101:1385–1420
Valadbeigi Y (2017). Chem Phys Lett 681:50–55
Vianelloa R, Maksic ZB (2005). Tetrahedron 61:9381–9390
Luna A, Mó O, Yáñez M, Guillemin JC, Gal JF, Maria PC (2007). Int J Mass Spectrom 267:125–133
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saeidian, H. Design of organic superacids based on triflic and perchloric acid by using DFT-B3LYP calculations. Struct Chem 31, 851–859 (2020). https://doi.org/10.1007/s11224-019-01444-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-019-01444-4