Abstract
In order to examine the acidity of organic sulfuric acid derivatives in gas phase, comprehensive density functional theory (DFT) calculations at B3LYP/6-31++G(d,p) level have been undertaken. In the title compounds, one of the oxygen atoms is substituted by an 1,3-cyclopentadiene group. The focus of this paper is on DFT studies of two classes of sulfuric acid derivatives: dithionic and peroxydisulfuric acids. Tautomers of proposed organic sulfuric acids are also investigated. DFT calculations indicate that the acidity of the proposed acids without any electron withdrawing groups on the ring was more than sulfuric acid.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acidity is defined as ability of a compound to lose H+, resulted negative charge. Proton transformation has great importance in different chemical and biochemical process [1]. Superacids are very useful chemicals in organic synthesis and material sciences such as alkylation, acylation, isomerization, polymerization, cracking, and coal industrial [2,3,4]. Superacids are used widely for preparation of carbocations which are important intermediates in organic synthesis. A magic acid (a mixture of Lewis and Brønsted acid) are also utilized for stabilization of unstable carbocations [5]. Generally, a superacid has greater acidity than 100% pure sulfuric acid [6]. Remarkable investigations by computational chemists have been made to tailor neutral small organic molecules as superacid [7,8,9,10,11,12,13,14,15]. The key point for design and fabrication of superacids is stabilization of the corresponding conjugate base in order to make the anion less basic. Four methods are utilized in the literature for design of a superacid: (1) delocalization of negative charge by substituent effects such as field/inductive and π-electron resonance effects [16, 17], (2) increasing of polarizability by using superacceptor groups [18], and (3) strong hydrogen bonds in the conjugate base [19]. The fourth one is based on aromatic stabilization of negative charge in the corresponding conjugate bases. Recently, acidity of some organic Brønsted superacids bearing cyclopentadiene and polycyclic aromatic hydrocarbon derivatives with electron withdrawing groups such as -CN and -F has been investigated by researchers [8, 20,21,22,23,24]. The reported ΔHacid values for the strongest superacids bearing cyclopentadiene are about 220–275 kcal/mol.
Sulfuric acid, H2SO4, is the mostly used and central substance in chemical industries [25, 26] and it is an important reagent in chemical laboratories too. Sulfuric acid reacts with most chemicals to result the corresponding sulfate. In the present study, new protocol is used to design of the strongest neutral organic acid based on two classes of sulfuric acid derivatives: dithionic 1 and peroxydisulfuric acid 2 (Fig. 1). Replacing an oxygen atom doubly bonded to sulfur atom on these acids with cyclopentadiene leads to new small interesting organic compounds which can behave as superacids. The present strategy for design of highly acidic molecules involves stabilization of negative charge in the corresponding conjugate anions through strong intramolecular hydrogen bond and making aromaticity.
Computational details
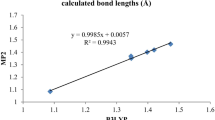
Geometry optimizations and frequency calculations for all proposed molecules were performed by the use of Gaussian 09 program [27]. DFT calculations with the Becke three-parameter hybrid functional (DFT-B3LYP) were performed, using 6-31++G(d,p) basis set. It has been demonstrated that DFT and its hybrid B3LYP functional [28, 29] gives a satisfactory description of the deprotonation process. There are many benchmark studies confirming the accuracy and reliability of DFT/B3LYP [30, 31]. Bryantsev and co-workers [31] compared the performances of different methods including MP2, B3LYP, X3LYP, and M06-L. They found that M06-L gives more accurate results for neutral systems while B3LYP exhibits more accurate energies for systems including ions. As one of our goals is to find an accurate method that is computationally efficient, we have also focused on MP2 with 6-31++G(d,p) basis sets. Three studied molecules (Table 1) were optimized using MP2 method. The geometrical parameters of the molecules obtained by the MP2 and B3LYP were compared in Table 1. Comparison of the geometrical parameters reveals that the results obtained by the two methods are in good accordance.
Deprotonation reaction enthalpy (ΔHacid), Gibbs free energy (ΔGacid), nucleus-independent chemical shift (NICS) [32, 33], and the harmonic oscillator model of aromaticity (HOMA) index [14, 34] of the studied compounds can be considered as trustable parameters to compare the acidity of the chemicals and stability of the corresponding conjugated bases.
Results and discussion
Scheme 1 shows chemical structure of dithionic acid derivatives of cyclopentadiene and their corresponding conjugated bases. After deprotonation, the negative charge of the base is delocalized in the five membered ring and so it is expected more aromaticity than their corresponding acids. The aromaticity parameters, NICS and HOMA indices of the acids, and their conjugated bases are inserted in Table 2. As shown in Scheme 1, different dithionic acid derivatives as well as the corresponding prototropic tautomers were considered with their relative energies in kcal/mol. C-H tautomers are more stable than the corresponding dithionic acid derivatives. It is worthy to note that gas-phase acidity of the tautomers reduces by 0.2–20 kcal/mol than dithionic acid derivatives (Table 2). The calculated ΔHacid and ΔGacid data of dithionic acid derivatives and their prototropic tautomers are collected in Table 2. The NICS values for these acids are generally between − 2.1 and − 8.8 ppm, while these values for their conjugate bases are in the range of − 6.2 to 11.2 ppm. The HOMA indices of the corresponding conjugate base of dithionic acid derivatives 1 are in the range of 0.48–0.75.
Replacing a (=O) group of dithionic acid with a cyclopentadiene motif resulted stable small organic acids (1a-a′′′), in which 1a shows ΔHacid = 284.0 kcal/mol, that is 27.5 kcal/mol lower than that of H2SO4 (ΔHacid = 311.5 kcal/mol) [35].
These findings prompted us to investigate effect of cyano substituents on stabilization of conjugate base. Cyano motif has an electron-accepting nature and requires very less steric conditions for keeping of negative charge of conjugate base [35]. DFT calculations show that all isomers in category (b) exhibit strong acidity to superacid behavior, ΔHacid = 247–257 kcal/mol. After deprotonation of (b), the negative charge is in the resonance with the strong electron-accepting –CN groups. Sharply increasing of HOMA index for the conjugate bases of (b), comparing to the corresponding acids supports this point. Substituting four –CN groups into organic acid 1a, gave superacid 1b with deprotonation enthalpy of 247.8 kcal/mol, 36.2 kcal/mol which is less than for 1a. This compound is more acidic than the most organic superacids which have been reported in literature. It should be noted that there are two different acidic hydrogens on the proposed organic dithionic acids (1b′-b′′′): C-H and O-H. DFT calculations on structure 1b′′′ show that hydrogen on carbon is about 24.3 kcal/mol more acidic than hydrogen on oxygen. After deprotonation of these acids (C-H), it is expected an aromatic anion as the corresponding conjugate base and the negative charge is in the resonance with the strong electron- accepting cyano groups.
By more substituting cyclopentadiene group into dithionic acid 1, compounds 1c and 1c′, the acidity decreases a little and the calculated deprotonation enthalpy increases to 286 and 309.1 kcal/mol, respectively. As expected the second cyclopentadiene has no effect on delocalization of negative charge at the conjugate base. We carried out B3LYP/6-31++G(d,p) investigation on structure (d), in which two (=O) groups of dithionic acid were replaced with two cyclopentadiene rings and one of the ring bears four –CN substituents. This strategy results in acidity enhancement comparing to category (c) and making aromaticity of (1d′-H)− (Table 2). Tetracyano substituted cyclopentadiene facilitates the conjugation of π-electrons in (1d′-H)− structure. Dramatic enhancement in the NICS index of (1d′-H)− comparing to 1d, supports this result.
Compounds (c) and (d) have two same acidic hydrogens. They can donate proton twice. Enthalpy of the second deprotonation is more than the first one. The difference in deprotonation enthalpy for series (c) is about 80–90 kcal/mol (Table 2). The first conjugate base is capable of forming intra-hydrogen bond after losing H+ ion, making it more stable than the second conjugate base. Figure 2 compares the bond lengths of 1b and its conjugate base, (1b-H)−. As shown, in the base form distance of S=O......HO hydrogen bond is about 2.58 Å and partial charge on the H and O atoms are observed. Large and apposite charges make stronger hydrogen bond in short distance. After deprotonation, anion has a larger change in bond lengths than neutral acid (Fig. 2).
As mentioned in literature, the extension of conjugate system increases the acidity of poly enols [20]. So, a structure improvement of dithionic acid 1 is made through replacing an oxygen atom, doubly bonded to sulfur with 1H- and 2H-indene which leads to new small organic compounds (e) and (f), respectively (Scheme 2).
The calculated ΔHacid, ΔGacid, HOMA values for superacids (e) and (f), as well as NICSfive(1) and NICSsix(1) data are inserted in Table 3. The subscript of five and six show that NICS values were calculated at 1 Å above the five and six membered rings, respectively.
B3LYP/6-311++G(d,p) calculations on the tetrasubstituted structure of 1e′ and 1f′′ (as stable tautomers) reveals that they exhibit strong acidity to superacid behavior, ΔHacid = 268.7 and 263.2 kcal/mol, respectively. As shown in Table 3, dithionic acids bearing 1H-indene group (e) are more acidic than compound (f). It is interesting to note that HOMA index of five membered ring in (1f′′-H)− was increased sharply in compared with its acid 1f′′; but reverse trend was observed in the HOMA index of six membered of (1f′′-H)− and 1f′′ (Table 3). The best way to explain this finding is comparing the structure of (1f′′-H)− vs. 1f′′. Anion (1f′′-H)− is a bicyclic aromatic hydrocarbon which its resonance stabilization energy per ring is less than that of 1f′′. Acid 1f′′ has only an identifiable benzene ring and a five membered ring with an alkene bond which can not take part in π-electron delocalization. Therefore, HOMA index of six membered ring in (1f′′-H)− was decreased in comparing to 1f′′ (Scheme 3).
With these valuable results about organic dithionic superacid derivatives, attention was focused on peroxydisulfuric acid 2. The chemical structure of the proposed organic peroxydisulfuric acids 2, C-H tautomers, their relative energies in kcal/mol as well as their conjugated base are shown in Scheme 4. As shown in Scheme 4, C-H tautomer 2a′ is about 112 kcal/mol more stable than O-H tautomer 2a.
The calculated ΔHacid, ΔGacid, HOMA, and NICS values for acids peroxydisulfuric 2, are provided in Table 4. ΔHacid for unsubstituted 2a (less stable isomer) is 213.5 kcal/mol, showing a very strong superacid. It was seen that the acidity of 2a′ is increased by substituting cyano groups (4 CN) on cyclopentadiene ring, 2b. DFT calculations show that ΔHacid value for tetracyano substituted 2b is 265.7 kcal/mol (Table 4).
NICS and HOMA values for conjugate bases of peroxydisulfuric acids 2 in comparing to their acids were confirmed that the negative charge of the anions was delocalized well by the cyclopentadiene motif.
Conclusion
In the present study, for the first time, two new class of organic dithionic and peroxydisulfuric acids as well as their some prototropic tautomers containing cyclopentadiene motif was designed and their acidities were investigated by B3LYP/6-31++G(d,p) calculations in gas phase. Some of these proposed structures are more acidic than H2SO4, FSO3H, and even CF3SO3H. After deprotonation, the negative charge is delocalized in the aromatic cyclopentadiene group. NICS and HOMA indices of the corresponding conjugate base of the acids were also supported this finding. Substitution of electron withdrawing groups CN on aromatic rings, enhanced the acidity which fall into the defined range of superacidity.
References
Corma A (1995). Chem Rev 95:559–614
Olah GA, Prakash SK, Molnar A, Sommer J (2008) Superacid Chemistry. John Wiley & Sons, New York
Dalpozzo R, Bartoli G, Sambri L, Melchiorre P (2010). Chem Rev 110:3501–3551
Busca G (2007). Chem Rev 107:5366–5410
Olah GA, Rasul G, York C, Prakash GKS (1995). J Am Chem Soc 117:11211–11214
Hall NF, Conant JB (1927). J Am Chem Soc 49:3062–3070
Si MK, Ganguly B (2017). New J Chem 41:1425–1429
Vianello R, Liebman JF, Maksić ZB (2004). Chem Eur J 10:5751–5760
Lipping L, Leito I, Koppel I, Krossing I, Himmel D, Koppel IA (2015). J Phys Chem A 119:735–743
Yanai H, Taguchi T (2015). J Fluor Chem 174:108–119
Alcamí M, Mó O, Yáňez M (2002). J Phys Org Chem 15:174–186
Kheirjou S, Abedin A, Fattah A (2012). Comput Theor Chem 1000:1–5
Valadbeigi Y (2016). Int J Quantum Chem 117:190–196
Shams B, Saeidian H (2018). Comput Theor Chem 1135:48–55
Rocha AS, Costa GC, Martinhon PT, Sousa C, Rocha AB (2017). Mater Chem Phys 186:138–145
Yagupolskii LM, Petrik VN, Kondratenko NV, Souvali L, Kaljurand I, Leito I, Koppel IA (2002). J Chem Soc Perkin Trans 2:1950–1955
Koppel IA, Taft RW, Anvia F, Zhu SZ, Hu LQ, Sung KS, DesMarteau DD, Yagupolskii LM, Yagupolskii YL, Ignatev NV, Kondratenko NV, Volkovskii AY, Vlasov VM, Notario R, Maria PC (1994). J Am Chem Soc 116:3047–3057
Koppel IA, Burk P, Koppel I, Leito I (2002). J Am Chem Soc 124:5594–5600
Tian Z, Fattahi A, Lis L, Kass SR (2009). J Am Chem Soc 131:16984–16988
Valadbeigi Y (2017). Chem Phys Lett 681:50–55
Vianello R, Maksic ZB (2008). New J Chem 32:413–427
Vianello R, Maksić ZB (2005). Eur J Org Chem 16:3571–3580
Vianello R, Maksić ZB (2005). Tetrahedron Lett 46:3711–3713
Vianello R, Maksić ZB (2005). Tetrahedron 61:9381–9390
Davenport WG, King MJ (2006) Sulfuric acid manufacture: analysis, control and optimization. Elsevier, Amsterdam
Chenier PJ (1987) Survey of industrial chemistry. John Wiley & Sons, New York
Frisch JM, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Nakatsuji H, Caricato M, Li X, Hratchian HP, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima R, Honda Y, Kilao O, Nakai H, Verven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroveror VN, Kobayashi R, Normand J, Ragavachari K, Rendell A, Burant JC, Tomasi SJ, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Strattmann RE, Yazyev O, Austin AJ, Cammi R, Ochetrski JW, Martin RL, Morokuma K, Zakrazawski VG, Votn GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresmanet JB (2013) Gaussian, Inc., Wallingford CT, Gaussian 09, Gaussian, Inc.
Merrill GN, Kass SR (1996). J Phys Chem A 100:17465–17471
Burk P, Koppel IA, Koppel I, Leito I, Travnikova O (2000). Chem Phys Lett 323:482–489
Tirado-Rives J, Jorgensen W (2008). J Chem Theor Comput 4:297–306
Bryantsev VS, Diallo MS, van Duin ACT, Goddard III WA (2009). J Chem Theor Comput 5:1016–1026
Valadbeigi Y (2017). Comput Theor Chem 1102:44–50
Wolinski K, Hilton JF, Pulay P (1990). J Am Chem Soc 112:8251–8260
Radenković S, Antić M, Đorđević S, Braïda B (2017). Comput Theor Chem 1116:163–173
Koppel IA, Burk P, Koppel I, Leito I, Sonoda T, Mishima M (2000). J Am Chem Soc 122:5114–5124
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Saeidian, H., Shams, B. & Mirjafary, Z. Comprehensive DFT calculations on organic sulfuric acid derivatives to design of powerful neutral organic superacids. Struct Chem 30, 787–793 (2019). https://doi.org/10.1007/s11224-018-1222-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-018-1222-1