Abstract
In this paper, a back-propagation neural network has been utilized to study on the correlation between impact sensitivity and molecular properties of 33 nitramine molecules. By using density functional theory method B3P86/6-31G**, all the molecular properties have been calculated. Eight different sets of molecular properties, including (HOMO−LUMO)*BDE, E, BDE/E, HOMO−LUMO, BDE*μ, R 2, ΔE, and BDE, have been used to train and test the network. Based on the test results, the correlation order between the molecular properties and impact sensitivity has been achieved. The correlation order shows that the input set with the descriptor ΔE(atomization energy) can obtain better results than any other descriptor for nitriamines, which surely accounts for a comparatively stronger correlation between ΔE(atomization energy) and impact sensitivity for nitramines we have studied in this work.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The study on the correlation between impact sensitivity of energetic materials and molecular structures has recently been an ongoing area of research in explosive theory [1]. The so-called impact sensitivity h 50%is measured experimentally by drop weight impact test, where a height of 50% probability in causing an explosion is measured when hit by a hammer with a standard weight [2, 3]. Since storage, synthesis, and application of energetic materials are significantly affected by impact or shock sensitivity, the researches about the impact sensitivity is of primary importance. Once we get the ability to predict potential explosives using computational methods, it will provide significant cost savings, increase the safety of storage and testing of these compounds, and provide for greater control of the performance of these explosives [4]. Presently, the usage of quantum mechanical methods as a predictive tool for assessing relationships between molecular properties of various explosive molecules and their impact sensitivities has been suggested in several previous works. Correlations between impact sensitivity and chemical composition reported earlier by Kamlet and Adolph were probably the most widely applied in the area of energetic materials research [5]. These demonstrated that for families of high-energy molecules with similar decomposition mechanisms, there were approximately linear relationships between log h 50%and OB100, the latter was a measure of “oxygen balance”; MNDO/3 molecular orbital method was used by Owens [6] to calculate the energy for bond rupture for a number of energetic molecules and the results showed that the weakest bond was generally that between the NO2and the remainder of the molecule. In addition, his calculation results indicated a correlation between the magnitude of the energy barrier and the susceptibility to detonation; Politzer [7] investigated shock-sensitivity relationships for nitramines and nitroaliphatics, and showed that shock sensitivity was related to the strengths of all the N–NO2and C–NO2bonds, taken in conjunction with overall molecular size; Rice and co-workers [8, 9] pointed out that for nitroaromatic molecules there was a correlation between the bond dissociation energy of the weakest bond and sensitivity. Moreover, they investigated the relation between impact sensitivity and charge distribution in energetic molecules; Xiao and co-workers [10–12] once proposed a principle of the smallest bond order to identify the relative magnitudes of impact sensitivity of most energetic compounds. For polynitroaromatic series, Jinshan and Gang [1] showed that the Mulliken bond order of C–NO2linkage correlated well with the observed values of h 50%; Zeman found a logarithmic relationship between the drop energy E drand heats of fusion for 33 polynitro compounds [13]. The correlation between impact sensitivity and approximated heats of detonation of several nitramines was examined by Edwards et al. [4]. Besides, many other properties have been found to correlate with impact sensitivities. These properties include molecular electronegativities [14, 15], vibrational states [16, 17], molecular weights and detonation gas concentrations [18], parameters related to oxidation numbers [19], partial atomic charges [15, 20–24], heats of reaction [25, 26], heats of explosion [27], bond orders [28, 29], and activation energies [27–31]. Although works listed above succeeded in finding a certain kind of correlations between some of the specific molecular properties and the impact sensitivity, many of the correlations almost existed in a certain type of explosive and the number of explosives in each type was usually small. In addition, most of the works simply examined the correlation between one or two molecular properties and impact sensitivity of explosive compounds. Consequently, in order to make a further study on the correlation between impact sensitivity and molecular properties, investigations should be carried out on how molecular properties affect impact sensitivity for the same type of energetic materials with similar molecular structure. In the present work, DFT method B3P86/6-31G**is adopted to calculate molecular properties of 33 nitramine explosives, and artificial neural networks method is utilized to research the correlation order between different molecular properties and impact sensitivity.
The neural network is a nonlinear function of many parameters that maps particular inputs (in our case, molecular properties) to an output (in our case, impact sensitivity). The network is made up of simple, yet highly connected, processing elements called nodes [32]. They are computer-based emulations of the brain’s information-processing capability [3]. Neural networks have been applied to various engineering problems, such as robotics, pattern recognition, speech, etc. [33]. In addition, the use of neural network methods has grown constantly in a variety of applications in chemistry and physics since their first utilization for the prediction of protein secondary structure. For example, neural networks have been used to calculate the ground-state eigenenergy of two-dimensional harmonic oscillators [34], to solve nonhomogenous ordinary and partial differential equations [35], and to obtain the electronic correlation energy for atoms and diatomic molecules [36]. But for the explosive engineering, there exist few reports about the impact sensitivity based on neural networks method, although Nefati [3] and Cho et al. [37] predicted the impact sensitivity of various types of explosive molecules via neural networks. However, they both provided better impact sensitivity values by employing dozens of compositional and topological descriptors, which is not very realistic for explosive synthesis engineering, and which property can correlate with the sensitivity best is still not clear. In the current work, we will choose five fundamental molecular descriptors in each input set so as to examine the sensitivities more conveniently. Since the fundamental molecular properties were easier to calculate, correlation study should be used to identify fundamental molecular properties that indicate sensitivity best. In addition, their works did not include the correlation order between impact sensitivity and molecular properties, which will be studied in the following part of this paper.
Energetic secondary nitramines are currently a subject of increasing interest in high-energy materials research. Of particular importance are efforts aimed at gaining insight into how molecular features of nitramines influence their impact sensitivities.
In this paper, we will utilize the back-propagation neural network (BP) to analyze the correlation between impact sensitivity of nitramines and molecular properties. In particular, the correlation order between various molecular properties and impact sensitivity will also be examined.
Theoretical methods
Calculation method
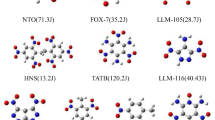
Thirty-three nitramine molecules as shown in Table 1were investigated in this paper. All the experimental impact sensitivity values were taken from [38]. In this work, by using Gaussian 98 hybrid DFT B3P86 method, the Becke-style three-parameter density functional theory [39] with the Perdew’s 86 (P86) [40] in combination with the basis set 6-31G**[41], the geometry was optimized and then the molecular structures were calculated. The calculated molecular properties included the sum of electronic and zero-point energy (E), zero-point vibration energy (ZPE), dipole moment (μ), electronic spatial extent (R 2), highest occupied molecular orbital (HOMO), and lowest unoccupied molecular orbital (LUMO). Furthermore, the atomization energy of various explosive molecules (ΔE) and the bond dissociation energy of the weakest X–NO2bond (BDE) were also calculated in this paper.
One can expect that the properties listed above examined for correlations contain many “obvious” choices and may be already well described in previous works. That is true when only one or two molecular properties are examined. But until recently, the correlation order of different molecular properties with impact sensitivity has not been studied for the same type of energetic materials with similar molecular structure, and which property correlates with sensitivity best is still not known when different properties are considered. Here, we would like to present a detailed study on the correlation between different molecular properties and impact sensitivity for nitramines.
Back-propagation neural network
In the field of structure–activity or structure–property relationships, the back-propagation neural networks, particularly the three-layer networks, have gained wide acceptance [3]. So a fully connected three-layer architecture, which had an input layer, a hidden layer, and an output layer, was adopted in our neural network.
A back-propagation neural network, which was first developed by Rumelhart and McClelland [42], “learns” by repeatedly passing data through neurons and adjusting their weights and biases to minimize the mean-squared error (MSE), \(\sum {(t_i } - o_i )^2 /n\)(iis a training observation and nis the total number of input vectors), until the output (o i ) predicted by network matches the target (t i ) or given property point values.
The neural network can be trained to perform a particular task using a variety of learning algorithms. In this paper, we chose BP algorithm implementing Levenberg–Marquardt variation for the network, whose training speed was much faster than that of a gradient descent algorithm [43].
By using BP neural network, emphasis was primarily laid on finding out the molecular descriptor, which had the strongest correlation with impact sensitivity of nitramines. Thus, impact sensitivity h 50%was an obvious choice of the only output neuron. Similarly, selecting the proper molecular descriptors of nitramine molecules, which were to be used as the input neurons of the neural network, was also our focus. After a preliminary evolutionary multiple linear regression treatment, BDE/E, BDE*μ, HOMO−LUMO, μ, R 2, BDE, ΔE, (HOMO−LUMO)*BDE and the number of C, H, N, O atoms in each CHNO nitramine molecule, which indicated the value of bond dissociation energy divided by total energy, bond dissociation energy multiplied by dipole moment, energy gap, electronic spatial extent, bond dissociation energy, atomization energy, and energy gap multiplied by bond dissociation energy, respectively, were chosen as input descriptors. In order to find out the correlation order between molecular descriptors and impact sensitivity of nitramine molecules, the descriptors mentioned above were divided into eight different sets, each were combined with the number of C, H, N, O atoms comprising each CHNO nitramine molecule. In this case, eight different input sets, with the number of C, H, N, O atoms being the common descriptors in each set, were constructed, as summarized in Table 2.
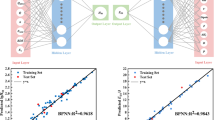
Usually, the difficulty in finding optimum neural-network architecture resided in deciding on the number of hidden layers and the number of neurons in them. By trial and error, we found that only one hidden layer containing two neurons yielded better overall results than those with more neurons in hidden layers despite the fact that these latter networks could give slightly better fitting of the data set. Therefore, the 5-2-1 structure, which was 5 neurons in input layer, 2 neurons in hidden layer, and 1 neuron in output layer, was adopted for our neural network as depicted in Fig. 1.
During training and test process, we divided 33 nitramine molecules into two groups, a training group and a test group, respectively. The training group was used to help the net learn the relationship between the given input vectors and the target output vector. Meanwhile, the test set consisting of a few compounds, some of which may be dissimilar to the training group, were chosen to validate the network’s generation ability. In order to accelerate the convergent speed, input groups must be normalized. The normalized formula is
where \(x_i ^\prime\)is the data after normalization, x i is the original data, uand s dare respectively the average value and standard error of the original data [44]. Now, various types of threshold functions are used in neural networks [45], the most common being the sigmoid function, which is incorporated as a transfer function in the MATLAB Neural Network Toolbox 3.0, installed in a PC computer. In this paper, the log sigmoid transfer function, log(1/1+e−z) was used to calculate the outputs of the neurons in hidden layer and output layer. The training parameters were generated randomly. The learning rate was optimized by trial and error and the best value obtained was 0.05. The reasonable output error was 0.001. The neural network was trained for 2000 epochs (using incremental updating or randomization of the training data order to avoid local minima), which were required to reach a maximum error of 0.001. In Fig. 2, the maximum error of the network could be observed as a function of the number of propagation cycles during the training process. As can be seen, the estimate of the network accuracy was asymptotically decreasing functions. After about 60 cycles, the network converged to a maximum error of 0.00099. Further training could not improve the performance of the network, and at this point training was stopped.
After training process, a few more nitramine molecules, which belonged or did not belong to the input groups, were chosen as test group to validate the generation ability of the network. The reasoning for this specific construction was to allow the test group to contain molecules which were dissimilar to the training group, so that the test and prediction results would exhibit distinct outliers. The test procedure was repeated five times starting from different initial random sets of weights, a technique known to weaken the detrimental effects of local minima on the error surface [46], and the mean-square errors were calculated, as shown in Fig. 3. The mean values, which were obtained by employing set 6, were used as the final values presented in Table 1. Meanwhile, the predicted impact sensitivity values of nitramines calculated by employing set 1 were also listed in Table 1for comparison. Two plots of predicted versus experimental h 50%values obtained by employing input set 6 and set 1 are depicted in Figs. 4and 5to illustrate our conclusion more intuitively.
Results and discussion
As shown in Fig. 3, MSE values were in the range from 0.782 to 1.200. The smallest MSE value was from the set 6 with ΔE, and the biggest MSE value was from the set 1 including (HOMO−LUMO)*BDE. It indicated that with the same network architecture and training parameters, molecular descriptor ΔEhad the strongest correlation while (HOMO−LUMO)*BDE had the slightest correlation with impact sensitivity of the nitramines studied in this work. It is noteworthy that the correlation order studied here was in fact between input sets and impact sensitivity. Since corss-correlation of descriptors in each input set was very slight and each set had four common descriptors, the difference of MSE values in training and test process can be thought mainly coming from very different descriptor in each input set. Thus, we considered the correlation order was mainly decided by the very different descriptor in each set.
Two plots of predicted versus experimental h 50%values obtained by employing set 6 and set 1 are displayed in Figs. 4and 5, respectively. Solid lines indicate perfect agreement between experiment and prediction. As shown in Fig. 5, we could see that the predicted values in set 1, in which the molecular descriptor (HOMO−LUMO)*BDE was included, were mostly larger than the experimental data. Nevertheless, in Fig. 4, the predicted values in set 6, in which ΔEwas contained, were in excellent agreement with the experimental values. It clearly indicated that ΔEhad the strongest correlation with impact sensitivity while (HOMO−LUMO)*BDE had the slightest correlation with impact sensitivity. Predicted values in other six sets were all worse than set 6, but better than set 1.
In quantum chemistry, atomization energy (ΔE) was defined as the difference between molecular energy and the sum of energies of atoms comprising the molecule. For instance such as CH4N4O2,
For nitramine explosives, whose structures were mainly made up of carbon chains, the molecular descriptor ΔEhad the strongest correlation with impact sensitivities. This conclusion was in agreement with Edward et al.’s work [4].
BDE here was defined as the difference between the zero-point-corrected energy of parent nitramine molecule and that of the products of the unimolecular dissociation in which an NO2group was removed. For example, for CH4N4O2, the BDE was
It was noteworthy that for nitramines studied in this work, the breaking of the R–NO2may be the rate-controlling step in the initiation of detonation, which again supported the Owens’ conclusion [6]. Based on chemical reaction kinetics, Arrhenius equation showed that the reaction rate constant depended on the ratio of activation barrier to temperature, so the activation energy may be a key factor in impact initiation and sensitivity of a material. As BDE/Ehad a certain relationship with activation energy, a strong correlation might exist between impact sensitivity and BDE/E, which indicated that bond dissociation energy in unit molecular energy. In this paper, descriptor BDE/Ehad the second strongest correlation with impact sensitivity of nitramines, with MSE value 0.833.
As depicted in Fig. 3, the correlation order after ΔEand BDE/Ewas as follows: R 2, BDE, μ, BDE*μ, HOMO−LUMO, and (HOMO−LUMO)*BDE. For the last one, the difference between LUMO and HOMO was defined as electronic transition level, which had influence on molecular properties. The lower the HOMO was, the larger was the molecular ionization energy. LUMO was related to molecular affinity, the smaller it was, the more the energy of system decreased when electrons came into the orbital. (HOMO−LUMO)*BDE and HOMO−LUMO had the weakest correlation with impact sensitivity, which indicated that the initial process of detonation correlated slightly with electronic excitation for nitramines we studied.
Conclusion
In this paper, by using DFT B3P86/6-31G**method, the electronic structures of 33 nitramine molecules were calculated. Based on the calculated molecular properties, we performed BP neural network to study on the correlation between impact sensitivity of nitramine explosives and molecular structures. Eight different input sets were constructed to train the network, and a few more molecules were chosen to test the generation ability of the network. Best results were obtained by employing set 6, which contained the molecular descriptor ΔE. The correlation order between impact sensitivity and the descriptors was obtained as follows: ΔE, BDE/E, R 2, BDE, μ, BDE*μ, HOMO−LUMO, and (HOMO−LUMO)*BDE. It demonstrates that the atomization energy ΔEis the best index of impact sensitivity for nitramine explosives, and BDE/Ethe second. Whether this conclusion is general for other chemical families needs to be further studied.
References
Jinshan L, Gang Z (1997) Chin J Explos Propellants 2:56–58
Kamlet MJ, Adolph HG (1979) Propellants Explos 4:30–34
Nefati H, Cense J-M, Legendre J-J (1996) J Chem Inform Comput Sci 36:804–810
Edwards J, Eybl C, Johnson B (2004) Int J Quant Chem 100:713–719
Kamlet MJ (1976) Proceedings of the 6th symposium (international) on detonation, San Diego, CA, pp 312–322
Owens FJ (1996) J Mol Struct (Theochem) 370:11–16
Politzer P (1991) Chem Phys Lett 181:78–82
Rice BM, Samir S, Owens FJ (2002) J Mol Struct (Theochem) 583:69–72
Rice BM, Hare JF (2002) J Phys Chem A 106:770–776
Xiao HM, Wang ZY, Yao JM (1985) Acta Chim Sin 1:16–20
Xiao HM, Li YF (1995) Sci Chin B 38:538–542
Xiao HM, Li YF, Feng PL (1987) Proceedings of the international symposium, October, 434
Zeman S, Krupka M (2003) Propellants Explos Pyrotech 28:301–307
Mullay JA (1987) Propellants Explos Pyrotech 12:60–63
Mullay J (1987) Explos Pyrotech 12:121–124
Fried LE, Ruggiero AJ (1994) J Phys Chem 98:9786–9791
McNesby KL, Coffey CS (1997) J Phys Chem B 101:3097–3104
Adolph HG, Holden JR, Cichra DA (1981) Technical report NSWC TR 80-495. Naval Surface Weapons Center, Dahlgren, VA
Jain SR (1987) Propellants Explos Pyrotech 12:188–195
Delpuech A, Cherville J (1978) Propellants Explos 3:169–175
Delpuech A, Cherville J (1979) Propellants Explos 3:121–128
Delpuech A, Cherville J (1979) Propellants Explos 3:61–65
Owens FJ, Jayasuriya K, Abrahmsen L, Politzer P (1985) Chem Phys Lett 116:434–438
Murray JS, Lane P, Politzer P, Bolduc PR (1990) Chem Phys Lett 168:135–140
Wu CJ, Fried LE (1998) Proceedings of the 11th symposium (international) on detonation, Office of Naval Research: Arlington, VA, pp 490–497
Vaullerin M, Espagnacq A, Morin-Allory L (1998) Propellants Explos Pyrotech 3:237–242
Fukuyama I, Ogawa T, Miyake A (1986) Propellants Explos Pyrotech 11:140–143
Xiao HM, Fan JF, Gu ZM, Dong HS (1998) Chem Phys 226:15–24
Fan J, Gu Z, Xiao HM, Dong H (1998) J Phys Org Chem 11:177–184
Fan J, Xiao H (1996) J Mol Struct (Theochem) 365:225–229
Zhao-Xu C, Heming X (2000) Int J Quantum Chem 79:350–357
Hu SR (1992) Discussion about neural network. National University of Defense Technology, Changsha, China
Hu LH, Wang XJ, Wong L, Chen GH (2003) J Chem Phys 119:11501–11507
Darsey JA, Noid D, Upadhyaya BR (1991) Chem Phys Lett 177:189–194
Lagaris IE, Likas A, Fotiadis DI (1997) Comput Phys Commun 104:1–14
Silva GM, Acioli PH, Pedroza AC (1997) J Comput Chem 18:1407–1414
Cho SG, No KT, Goh EM, Kim JK, Shin JH, Joo YD, Seong S (2005) Bull Kor Chem Soc 26:399–408
Bulusu SN (1989) Chemistry and physics of energetic materials. Kluwer Academic, Dordrecht, The Netherlands
Becke AD (1993) J Chem Phys 98:5648–5652
Perdew JP (1986) Phys Rev B 33:8822–8824
Dunning TH Jr (1989) J Chem Phys 90:1007–1023
Rumlhart D, McClelland J (1986) Parallel distributed processing, vol 1. Bradford Books/MIT Press, Cambridge, MA
Johson-Estrepo B (2003) J Chem Inform Comput Sci 43:1513–1519
Xin W (2000) Application and design of neural network based on MATLAB. Science Publishing House, Beijing, China
Lippmann RP (1987) IEEE ASSP Mag 4:2–7
Hensen LK, Salamon P (1990) IEEE Trans Pattern Anal Mach Intell 12:993–1001
Acknowledgement
The authors gratefully acknowledge the financial support provided by the National Natural Science Foundation of China (grant no. 10376021).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jun, Z., Xin-lu, C., Bi, H. et al. Neural networks study on the correlation between impact sensitivity and molecular structures for nitramine explosives. Struct Chem 17, 501–507 (2006). https://doi.org/10.1007/s11224-006-9101-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-006-9101-6