Abstract
Co0.7Zn0.3Fe2O4 nanorods were synthesized from hydrazine precursor by co-precipitation technique. Infrared and thermogravimetric–differential thermogravimetric curves of the precursor indicated the bridging bidentate nature of hydrazine and three-step thermal decomposition. The as-synthesized cobalt zinc ferrite nanorods were characterized by powder X-ray diffraction, scanning electron microscopy, energy dispersive spectroscopy, transmission electron microscopy, vibrating sample magnetometry and UV–diffuse reflection spectroscopy which proposed the phase structure, morphology, magnetic and optical properties. Co0.7Zn0.3Fe2O4 nanorods showed a sensible photocatalytic activity on Congo red, Malachite green, Methylene blue, Methyl red, Rhodamine B and Rose bengal under solar light at different time intervals and were magnetically separated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cobalt zinc ferrites have been widely investigated due to their specific electrical [1], optical [2] and magnetic [3, 4] properties. They are gaining attraction because of their applications in microwave absorption [5], sensors [6], magnetic resonance imaging [7] and drug delivery. To date, several methods have been developed to synthesize cobalt zinc ferrites, such as combustion method [8], co-precipitation method [9], microwave combustion method [10], sol–gel auto-combustion method [2], microwave hydrothermal route [11], solution combustion method [12] and modified citrate gel method [13]. Above all, the co-precipitation method is a versatile approach because it is inexpensive and does not require harsh experimental conditions.
Nowadays, the presence of organic dyes in water bodies is cited as one of the critical global problems due to industrialization [14,15,16]. Because of that, much attention has been paid by researchers to develop various novel technologies for water purification [17, 18]. Among those, photocatalytic degradation of synthetic dyes using nanocatalyst is an effective technique [19]. Meanwhile, ferrites are also effective in the degradation of many potential organic contaminants [20,21,22,23,24,25,26]. Hitherto, the photodegradation of organic dyes using cobalt zinc ferrite nanoparticles have been scarcely reported [20, 27].
Since utilization of solar energy is an ideal pathway for this purpose [28, 29], our present study is focused on the photocatalysis of cobalt zinc ferrite nanoparticles under solar light synthesized from the hydrazinated precursor [30] by co-precipitation technique. The photocatalytic activity of as-said nanoparticle is analyzed for the degradation of Congo red (CR), Malachite green (MG), Methylene blue (MB), Methyl red (MR), Rhodamine B (RhB) and Rose bengal (RB) in aqueous solution at different time intervals under bright solar light.
Experimental
Materials
All of the chemical reagents were purchased from Merck, India, in analytical reagent (AR) grade and used without further purification. Distilled water was used for all of the solutions. The following reagents were used: 2,4-dichlorophenoxyacetic acid, hydrazine hydrate (99%), iron sulfate heptahydrate, cobalt nitrate hexahydrate, zinc nitrate hexahydrate, ethanol, diethylether, concentrated hydrochloric acid, carbontetrachloride, CR, MG, MB, MR, RhB and RB.
Synthesis of Co0.7Zn0.3Fe2O4 nanorods
Co0.7Zn0.3Fe2O4 nanorods were prepared from the inorganic precursor through co-precipitation technique by the addition of an aqueous solution (50 ml) of hydrazine hydrate (0.0199 mol) and 2,4-dichlorophenoxyacetic acid (0.01 mol) to the corresponding aqueous solution (50 ml) containing ferrous sulphate heptahydrate (0.01 mol), cobalt nitrate hexahydrate (0.0061 mol) and zinc nitrate hexahydrate (0.0012 mol). The mixed solutions were stirred magnetically for half an hour. The formed yellow precipitate was filtered and washed with water, alcohol, diethylether and air-dried. This precursor was thermally decomposed in air for 1 hour which led to the formation of Co0.7Zn0.3Fe2O4 nanorods.
Characterization
Infrared (IR) spectra were recorded by the KBr disk technique using a Shimadzu spectrophotometer. Simultaneous thermogravimetry–differential thermogravimetry analysis (TG–DTA) was carried out in air using a Universal V4.5A instrument. Scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS) images were performed on a HITACHI Model S-3000H. X-ray diffraction (XRD) patterns were recorded using an X-ray diffractometer (X’pert PRO model) using Cu-Ka radiation, at 40 keV in the 2-h range of 20–90°. Transmission electron microscopy (TEM) micrographs were collected on a Joel/JEM 2100 instrument. The UV–diffuse reflectance spectrum (UV–DRS) was operated on a Shimadzu UV–DRS spectrophotometer. The room-temperature magnetic parameter measurement was performed using a Lakeshore VSM 7410 magnetometer. The adsorption and photocatalytic activity studies were carried out on a Shimadzu UV-2550 UV–Vis spectrophotometer.
Photocatalytic test
The photocatalytic activity of Co0.7Zn0.3Fe2O4 nanorods was evaluated by the degradation of CR, MG, MB, MR, RhB and RB under bright solar light. The Co0.7Zn0.3Fe2O4 nanorods as a catalyst (200 mg) were added to a flask containing 100 ml of dye solutions with an initial concentration of 500 mg l−1 under magnetic stirring for 1 hour in the dark to attain adsorption–desorption equilibrium. After that, the suspensions were kept under solar light and the samples were collected at different time intervals (0, 10, 20, 30, 40, 50 and 60 min). The concentrations of dyes were determined at wavelengths of 496, 664, 615, 532, 550 and 554 nm for CR, MB, MG, MR, RB and RhB, respectively.
Results and discussion
Characterization of the precursor
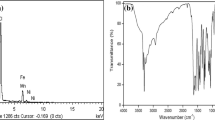
The hydrazine content (5.7%) in the precursor was determined by titration using KIO3 as titrant, by volumetric analysis under Andrew’s condition [31]. The percentages of cobalt (0.78%), zinc (0.34%) and iron (2.52%) in the precursor were confirmed by EDS analysis (Fig. 1). From these data, the molecular stoichiometric composition of the precursor was assigned as Co0.7Zn0.3Fe2(C6H3Cl2OCH2COO)4·2N2H4 and confirmed by thermal analysis.
Figure 2 shows the infrared spectrum of the precursor. Three bands in the region 3255–3340 cm−1 are due to the N–H stretching frequency. The N–N stretching frequency observed at 948 cm−1 confirmed the bidentate bridging nature of hydrazine [32]. The separation of asymmetric and symmetric stretching frequency of carboxylate found at 240 cm−1, which documented monodentate linkage [32] of the carboxylate group. The band at 454 cm−1 indicated the presence of a metal/oxygen bond [33].
TG–DTA curves of the precursor showed four steps of mass loss and endothermic peaks (Fig. 3). A very small endothermic peak was observed as the initial step. The mass loss of 5.74% corresponds to the elimination of two hydrazine molecules with increase from room temperature to 180 °C. The strong endotherm observed in the region 180–310 °C is due to the beginning of decarboxylation with a 39.4% mass loss. In the region 310–580 °C, complete decarboxylation takes place. Thus, the precursor Co0.7Zn0.3Fe2(C6H3Cl2OCH2COO)4·2N2H4 was thermally decomposed in air to yield Co0.7Zn0.3Fe2O4 nanorods [34].
Characterization of Co0.7Zn0.3Fe2O4 nanorods
Figure 4 shows XRD pattern of Co0.7Zn0.3Fe2O4 nanorods. The characteristic peaks coincide exactly with the JCPDS card nos. 73-1963 (ZnFe2O4) and 22-1086 (CoFe2O4). The sharp and intense XRD peaks revealed their nanocrystalline nature [35] as confirmed by the selected area electron diffraction (SAED) pattern (Fig. 6d). The average crystallite size of the nanorods calculated from Scherrer's formula is 14 nm.
The IR spectrum of Co0.7Zn0.3Fe2O4 nanorods (Fig. 5) showed the characteristic peaks of tetrahedral and octahedral Fe–O stretching bands at 530 and 420 cm−1, respectively. The other characteristic bands of N–N and N–H stretching frequencies observed in the precursor (Fig. 2) had vanished. Thus, the formation of Co0.7Zn0.3Fe2O4 nanorods from the precursor was further confirmed.
Figure 6a presents an SEM image of Co0.7Zn0.3Fe2O4 nanorods. As seen in the image, agglomeration in the particles exhibits irregular nanorod-like morphology interconnected with each other. EDS spectra (Fig. 6b) confirmed that the nanorods are mainly composed of Co, Zn, Fe and O. Figure 6c shows a TEM image of the nanorods, indicating a particle size of around 20 nm, which closely matched the size calculated by Scherrer’s equation. The SAED pattern (Fig. 6d) exhibits spots forming rings corresponding to (311), (400), (331), (440), (444) and (642) planes indicated polycrystalline nature of the sample.
Figure 7 illustrates the room-temperature magnetic parameter measurement of Co0.7Zn0.3Fe2O4 nanorods. A saturation magnetization (Ms) of 14.5 emu g−1, remanent magnetization (Mr) of 2.2 emu g−1 and a coercivity (Hc) of 306.41 Oe were observed. The hysteresis loop reveals that the soft magnetic nature and excellent magnetic properties were maintained to some extent.
The UV–DRS of Co0.7Zn0.3Fe2O4 nanorods (Fig. 8) exhibits strong absorption between 200 and 800 nm and an absorption edge at 418 nm. The optical band gap (Eg) can be calculated as 2.9 eV. The low band gap energy and particle size were the driving forces for acceptable photocatalytic activity of the nanorods under solar light.
Photocatalytic properties and adsorption kinetics
The photodegradation of dyes (CR, MB, MG, MR, RB and RhB) under bright solar light using Co0.7Zn0.3Fe2O4 nanorod as a catalyst was evaluated. In the photocatalytic test, the reaction time differs with respect to dyes: 50 min (CR and MB), 40 min (MG, RhB and RB) and 30 min (MR). The photocatalytic degradation of dyes was shown to follow the order: CR > RB > MB > MR > RhB > MG (Fig. 9a). It can be clearly seen that 58% of CR and RB molecules in the solution degraded after 40 min. The catalyst was separated by an external magnet for recycling (Fig. 9b).
Figure 9c reveals the relationship between the time and the equilibrium adsorption (qe) of various dyes on Co0.7Zn0.3Fe2O4 photocatalyst. Equation (1) is used to calculate the equilibrium uptake:
where qe is the amount of dye adsorbed by catalyst at equilibrium, C0 is the initial concentration of the dye solution in mg l−1, Ce is the equilibrium concentration of the dye solution in mg l−1, V is the volume of the solution in ml and W is the mass of catalyst in milligrams taken for the experiments. It is obvious that there was significant adsorption of RB and MB, while there was a moderate adsorption of RhB, CR, MG and MR.
Equation (2) represents the adsorption kinetics as explained by a second-order model:
where k2 is the rate constant for second-order adsorption (g mg−1 min−1), and qe and k2 were determined from the slope and intercept of plot t/qt versus t (Fig. 9d) for various dyes. The linear plot with correlation coefficient R2 concluded that the pseudo-second-order adsorption model was suitable for fitting the adsorption kinetics.
Conclusion
A Co0.7Zn0.3Fe2O4 photocatalyst was synthesized by thermal decomposition technique from its precursor Co0.7Zn0.3Fe2(C6H3Cl2OCH2COO)2·2N2H4. The chemical formula was fixed by the data observed from EDS, IR and TG–DTA of the precursor. The TG–DTA showed three-step decomposition which led to the formation of Co0.7Zn0.3Fe2O4 photocatalyst. The sharp and intense XRD peaks suggested a polycrystalline nature and the crystallite size calculated as 14 nm from the Debye–Scherrer formula was confirmed by TEM. Nanorods with agglomeration were observed in the SEM image while EDS showed the presence of the expected elements. The soft magnetic nature of the photocatalyst was observed from the VSM hysteresis loop. From the photodegradation of CR, MB, MG, MR, RB and RhB, it can be concluded that Co0.7Zn0.3Fe2O4 nanorods are an efficient magnetic photocatalyst and magnetically separable for the recycling process.
References
D.K. Pradhan, S. Kumari, V.S. Puli, P.T. Das, D.K. Pradhan, A. Kumar, J.F. Scott, R.S. Katiyar, Phys. Chem. Chem. Phys. 19, 210 (2016)
I.M. Abdulmajeed, L.S. Khalil, Int. J. Curr. Eng. Technol. 6, 976 (2016)
F.S.M. Doulabi, M. Mohsen-Nia, Iran. Polym. J. 22, 9 (2013)
T. Tatarchuk, M. Bououdina, W. Macyk, O. Shyichuk, N. Paliychuk, I. Yaremiy, B. Al-Najar, M. Pacia, Nanoscale Res. Lett. 12, 141 (2017)
X. Huang, J. Zhang, W. Rao, T. Sang, B. Song, C. Wong, J. Alloys Compd. 662, 409 (2016)
N. Somaiah, T.V. Jayaraman, P.A. Joy, D. Das, J. Magn. Magn. Mater. 324, 2286 (2012)
Z. Ghasemian, D. Shahbazi-Gahrouei, S. Manouchehri, Avicenna J. Med. Biotechnol. 7, 64 (2015)
M.H. Yousefi, S. Manouchehri, A. Arab, M. Mozaffari, R. Amiri, J. Amighian, Mater. Res. Bull. 45, 1792 (2010)
P. Yaseneva, M. Bowker, G. Hutchings, Phys. Chem. Chem. Phys. 13, 18609 (2011)
M. Sundararajan, L.J. Kennedy, J.J. Vijaya, J. Nanosci. Nanotechnol. 15, 6719 (2015)
K. Praveena, K. Sadhana, Int. J. Sci. Res. Pub. 5, 1 (2015)
R. Rani, S.K. Sharma, K.R. Pirota, M. Knobel, S. Thakur, M. Singh, Ceram. Int. 38, 2389 (2012)
E. Girgis, M.M.S. Wahsh, A.G.M. Othman, L. Bandhu, K.V. Rao, Nanoscale Res. Lett. 6, 1 (2011)
R. Kant, Nat. Sci. 4, 22 (2012)
M. Balakrishnan, S.A. Antony, S. Gunasekaran, R.K. Natarajan, Indian J. Sci. Technol. 1, 1 (2008)
Z. Carmen, S. Daniela, Organic pollutants ten years after the Stockholm convention—environmental and analytical update, ed. By T. Puzyn (In Tech, 2012), p. 55
X. Qu, P.J.J. Alvarez, Q. Li, Water Res. 47, 3931 (2013)
A. Ahmad, S.H. Mohd-Setapar, S.C. Chuo, A. Khatoon, W.A. Wani, R. Kumar, M. Rafatulla, RSC Adv. 5, 30801 (2015)
E. Casbeer, V.K. Sharma, X.-Z. Li, Sep. Purif. Technol. 87, 1 (2012)
G. Fan, J. Tong, F. Li, Ind. Eng. Chem. Res. 51, 13639 (2012)
K. Zaharieva, V. Rives, M. Tsvetkov, Z. Cherkezova-Zheleva, B. Kunev, R. Trujillano, I. Mitov, M. Milanova, Mater. Chem. Phys. 160, 271 (2015)
S.D. Jadhav, P.P. Hankare, R.P. Patil, R. Sasikala, Mater. Lett. 65, 371 (2011)
P. Guo, M. Lv, G. Han, C. Wen, Q. Wang, H. Li, X.S. Zhao, Materials 9, 806 (2016)
P.P. Hankare, R.P. Patil, A.V. Jadhav, K.M. Garadkar, R. Sasikala, Appl. Catal. B 107, 333 (2011)
L. Han, X. Zhou, L. Wan, Y. Deng, S. Zhan, J. Environ. Chem. Eng. 2, 123 (2014)
K.N. Harish, H.S.B. Naik, P.N.P. Kumar, R. Viswanath, ACS Sustain. Chem. Eng. 1, 1143 (2013)
C. Lin, X. Xie, C. Li, Q. Yan, Mater. Sci. Semicond. Process. 82, 46 (2018)
Z. Zhang, X. Jiang, B. Liu, L. Guo, N. Lu, L. Wang, J. Huang, K. Liu, B. Dong, Adv. Mater. 30, 1705221 (2018)
Z. Zhang, J. Huang, Y. Fang, M. Zhang, K. Liu, B. Dong, Adv. Mater. 29, 1606688 (2017)
S. Rangasamy, K. Kalimuthu, M. Rakkiasamy, Synth. React. Inorg. Metal-Org. Nano-Metal Chem. 45, 482 (2015)
A.I. Vogel, Vogel’s Text book of Quantitative Chemical Analysis, 5th edn. (Longmann, London, 1989), p. 384
A. Braibanti, F. Dallavalle, M.A. Pellinghelli, E. Leporati, Inorg. Chem. 7, 1430 (1968)
M.G. Naseri, E.B. Saion, M. Hashim, A.H. Shaari, H.A. Ahangar, Solid State Commun. 151, 1031 (2011)
U.B. Gawas, V.M.S. Verenkar, Thermochim. Acta 556, 41 (2013)
R.C.C. Monteiro, A.A.S. Lopes, M.M.R.A. Lima, J.P.B. Veiga, J. Non-Cryst. Solids 491, 124 (2018)
Acknowledgements
The authors are thankful to Kongunadu Arts and Science College, Coimbatore, for providing facilities. We acknowledge the provision of the necessary facilities by SAIF Cochin, IIT Madras, and Karunya University, Coimbatore.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Raina, O., Manimekalai, R. Photocatalysis of cobalt zinc ferrite nanorods under solar light. Res Chem Intermed 44, 5941–5951 (2018). https://doi.org/10.1007/s11164-018-3465-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-018-3465-2