Abstract
In the present work, undoped and nickel doped zinc ferrite (ZnFe2−xNixO4) nano-composites were synthesized using a facile auto-combustion method using glycine as fuel. Effects of Ni dopant concentration and annealing process on structural and morphological properties were investigated by X-ray Diffraction (XRD), Raman spectroscopy, Fourier Transform InfraRed (FTIR) spectroscopy, and Scanning Electron Microscopy/Energy-dispersive X-ray spectroscopy (SEM/EDS). The formation of cubic spinel ferrites is confirmed by XRD analysis while asserting particles with a size range of 55–58 nm. Analysis of Raman spectra showed a transition of normal to inverse spinel-type with the increase in Ni content. Photocatalytic studies of as-synthesized nanoparticles using methylene blue (MB) demonstrated a strong correlation between photocatalytic efficiency and Ni doping. Ni-doped zinc-ferrites exhibited 98% photocatalytic efficiency at an optimum Ni doping concentration of 30%. As-synthesized ferrites have the potential to be used as an efficient, reusable, and magnetically removable photocatalyst system for removing organic pollutants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The immense exploitation of synthetic organic compounds for diverse industrial processes often created drastic environmental pollution worldwide [1]. Discharge of wastewater from large-scale industries is found to contain organic dye remnants which are reported to be extremely devastating to the entire ecosystem. Methylene blue (MB) is a typical aromatic dye that is usually employed in textile industries for the dyeing process of silk and cotton materials [2, 3]. Being stable, toxic, and non-degradable, these dye fractions even at low trace levels can deteriorate the quality of water significantly [4]. On potential exposure, human health could be endangered by its inherent carcinogenicity and tissue necrotic effects [5]. Besides, the photosynthesis of aquatic organisms could be hindered by the stable coloured nature of dye molecules. Extensive efforts based on adsorption, filtration, ion exchange, and chlorination have been put forward for addressing water remediation [6,7,8]. The development of advanced remediation strategies remains one of the global concerns owing to the increased threat of water contamination [9]. In this scenario, photocatalysis has acquired immense research attention where the organic contaminants can be completely degraded to nontoxic end products in an eco-friendly manner [10,11,12,13]. Besides, the formation of secondary toxic by products can be avoided by this technique. In addition to frequently employed photocatalysts based on titanium dioxide and zinc oxide, other systems like strontium/cerium bimetallic nanocomposites, pectin zirconium (IV) selenotungstophosphate nanocomposites are reported to be highly effective under ultra-violet or visible light sources [14, 15]. Still, different strategies have been put forward to address challenges such as achieving high efficiency, ease of recoverability and ability of sunlight driven catalysis.
Magnetic zinc ferrites (ZnFe2O4 or ZFO) are attracted enormous research attraction owing to their unique electric, magnetic and catalytic properties; while exhibiting high stability and ease of fabrication with reproducibility [16, 17]. These are n-type semiconductors with cubic structure and frequently used for various electronic applications [18,19,20]. Besides, these are characterized by a low bandgap of ~ 1.9 eV as determined in the work of Jang et al. and Borse et al. by using UV–Visible Diffuse Reflectance Spectroscopy (UV–Vis DRS), also characterized by a well suited electronic structure and broad absorption in the visible range making it suitable for many water redox reactions [21, 22]. Ferrite nanocomposites can be synthesized by various techniques such as hydrothermal, sonochemical, co-precipitation, and sol–gel auto combustion [23,24,25,26,27]. Among them, auto-combustion methods are mostly favourable owing to the simplicity, versatility, rapid completeness of reaction, the homogeneous addition of dopant could be ensured [28]. During the process, metal nitrates can be homogenously mixed in presence of fuel materials like hydrazides, urea, glycine, and citric acid. Magnetic recoverable ferrite composite was found to be quite appealing in the photocatalysis process as easy and complete separation of catalyst from the rest of the medium is possible using an external magnet. While conventional photocatalysts require mostly centrifugation or filtration for further removal, the catalyst recovery rate, and reusability could be much enhanced in the case of ferrites [29,30,31]. Besides, high surface area to volume ratio and quantum confinements of nano ferrites is most promising to get high efficient photocatalysts.
Recently studies have been reported on catalytic aspects of ZFO composites as the morphology and structural parameters can influence their performance. Nevertheless, few endeavours have investigated nickel–zinc ferrite nanocomposites materials for photocatalytic purposes. In the current work, ZFO samples were prepared by the auto-combustion method and annealed at 1000 °C. The effect of Ni dopant concentration on ZFO structure and morphology was studied in detail. It was found that doped samples present pure crystallographic and high efficiency regarding methylene blue decomposition.
2 Experimental
2.1 Materials
Base materials such as zinc nitrate (Zn(NO3)2⋅6H2O), ferric nitrate (Fe(NO3)3⋅9H2O), nickel nitrate (Ni(NO3)2⋅6H2O), glycine (C2H5NO2) from Sigma Aldrich, USA, and methylene blue from IBA scientific, IA were used for the present study.
2.2 Synthesis and Characterization of Zinc Ferrites
ZFO samples were prepared by dissolving and mixing the nitrates of Zinc, Iron, and nickel as precursors and glycine as fuel. Formation of the homogenous gel was ensured by applying stirring at 80 °C which finally undergone combustion [32]. The flame temperature during combustion can be from 600 to 1350 °C. After burning in the air and grinding, the burned gel turned to powder. All samples have then been annealed at 1000 °C under air. The Ni content in the ZnFe2−xNixO4 prepared samples was changed from x = 0.05 to 0.4 in weight percentage in the starting solutions.
The annealed samples were characterized by an X-Ray diffractometer (D8 advanced Bruker), Raman spectroscopy (RENISHAW) with laser edge 785 nm, FTIR (JASCO FT/IR-6300), Field Emission SEM/EDS (Thermo scientific Apreo C).
2.3 Photocatalytic Studies
The photocatalytic activity of as-prepared samples was evaluated using a photochemical reactor (Techinstro, India) equipped with a 450 W ultraviolet (UV) lamp. For this, 300 ml aqueous solution of methylene blue (0.02 × 10−3 mol/L) was taken in the reaction vessel followed by the addition of 0.1 g of ZFO doped by Ni (x = 0, 0.05, 0.15, 0.3, and 0.4 Adsorption–desorption equilibrium of dye on the photocatalyst surface was ensured by magnetic stirring in dark for 20 min. The reaction medium is then subjected to UV light and the temperature was kept steady during the reaction using an external water circulatory chiller unit. The decrease in absorption values of MB solutions was continuously monitored at each 20 min time interval using a UV–Visible spectrophotometer [33]. The degradation efficiency is calculated using the following equation:
where C0 and Ct are the initial and the final concentrations, respectively; A0 and At are the corresponding absorbance values of MB, which were measured at the highest absorbance peak of MB of 665 nm.
3 Results and Discussion
3.1 Structural Characterization
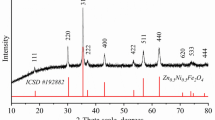
ZFO nanocomposites were successfully synthesized by auto-combustion method by varying the Ni content from 5 to 40% of ZnFe2−xNixO4 (x = 0, 0.05, 0.15, 0.3 and 0.4). The diffractograms of the synthesized samples were annealed at 1000 °C (Fig. 1a). The XRD spectra were analyzed by X’Pert high score software to check their structure allowed us according to find that the diffraction peaks of sample x = 0 are in good agreement with the ASTM card No. (01-073-1963) of pure Zinc Ferrite cubic phase with space group Fd3m which proves that the non-doped sample has a pure phase. The doped samples with x = 0.05, 0.15, 0.3 and 0.4 are in a good agreement with the ASTM card No. (01-073-1963) of pure Zinc Ferrite cubic phase with space group Fd3m. Extra peaks of secondary phases have been also observed [34]:
-
For: x = 0.05 and 0.15 the indexation of extra peaks consistent with ASTM card No. (00-008-0234) of Nickel Zinc Iron Oxide (Ni,Zn)Fe2O4 cubic phase with space group Fd3m as and ASTM card No. (01-073-0603) of α-Fe2O3 (hematite) rhombohedral nanoparticles.
-
For samples doped with x = 0.3 and 0.4 the extra phases fits with the ASTM card No. (00-008-0234) of Nickel Zinc Iron Oxide (Ni,Zn)Fe2O4 cubic phase with space group Fd3m as ZFO and ASTM card No. (00-001-1136) of ZnO hexagonal wurtzite nanoparticles.
The average crystallite size of zinc ferrites compositions was calculated from the XRD spectra using the Debye–Scherrer formula on the main peak (311) for all samples [35, 36]
where D is the crystalline size, λ denotes the wavelength of Cu-Kα X-ray radiation, β is the full width at half maximum of XRD peaks and θ is Bragg's angle. The variation of the crystal size of ZFO compositions was plotted as a function of Ni dopant concentration as shown in Fig. 1b. It can be seen that subsequent changes in Ni content impose a slight variation of crystal sizes from 55 to 57 nm. While lower and higher doping concentrations (5% and 40% respectively) resulted in slightly higher crystal size, the lowest crystal size was obtained for moderate Ni doping levels of 15 to 30%.
The characteristic Raman spectra of synthesized ZFO samples were represented in Fig. 2. It was reported that the positions of the peaks, Raman lines features, widths, energies, shape, and intensities get affected by the substitution leading to the changes in the lattice parameters and the cations positions in the lattice. The Raman shifts shows 5 modes (A1g + Eg + 3F2g) as normal spinel for samples with x = 0, 0.05 and 0.15 type and inverse spinel-type for x = 0.3 and x = 0.4 ones due to A1g mode represented as doublet peaks [37].
FTIR spectra of the synthesized ZFO samples were depicted in Fig. 3. Ferrites spinel oxides are reported to consist of two metal–oxygen (M–O) vibrations within the 350–600 cm−1 range. The characteristic absorption peaks are observed between 542 and 549 cm−1 due to the stretching vibrations of the Fe–O bonds in octahedral positions [38, 39]. Furthermore, the characteristic peaks observed between 401 and 415 cm−1 are due to the stretching vibrations of the Zn–O bonds in tetrahedral positions [40, 41]. The peak around 2900 cm−1 is not prominent still present in ZFO compositions; which represents C–H stretching vibrations. The absence of a peak around 3000 cm−1 indicates the absence of water moieties, mostly due to the annealing process. Further, the peak near 2300 cm−1 is mainly due to carbon dioxide molecules that adsorbed on the substrates.
3.2 Morphological Analysis
The SEM micrographs of ZFO samples showed surface morphology of as-prepared samples (Fig. 4). The grain size was found to be in the range of 103.5–652.5 nm for different compositions. Moreover, it could be noted that the particle size gets increased on the initial addition of Ni from 5 to 15%, thereafter it decreases. Some extent of agglomeration has clearly appeared in all compositions which might be attributed due to the exposure to higher temperatures during auto-combustion and annealing processes. The high surface area to volume ratio and inherent magnetic properties of nanoparticles can further facilitate the tendency for agglomeration. Our observation sounds well with previous studies conducted by Naik et al. for green synthesis of zinc ferrite nanoparticles using Limonia acidissima juice [42]. Moreover, the elemental analysis showed that as-synthesized zinc ferrites are composed of expected constituent elements such as Zn, Fe, O, and Ni [43]. This proved that the auto-combustion process produces pure zinc ferrites without any impurities (Fig. 5) where it represented the EDX of the pure ZFO and the Ni 30% doped one to check the Ni concentration.
3.3 Photocatalytic Activity
The photocatalytic behaviour of as-synthesized ZFO composites was investigated using methylene blue under UV light. The absorbance of MB subsequently decreased with increased during the reaction (Fig. 6a). It could be clearly seen that the photocatalytic performance of ZFO nanocomposites enhanced successively with Ni dopant up to 30% and thereafter decreases by 40% (Fig. 6b, c). After 200 min reaction, the degradation efficiencies of different composites were calculated and correlated with their corresponding crystal size (Fig. 7a). While bare ZFO exhibits ~ 95% degradation of MB, zinc ferrite composite with 30% Ni dopant was found to have the highest degradation efficiency of ~ 98%. Moreover, the rate constant of the reaction was also calculated for each nanocomposite as given in Fig. 7b. The highest rate constant of 2.38 × 10−2 was observed at 30% Ni content. The photocatalytic efficiency is then strongly correlated to the crystal size of nanocomposites. As the size is decreasing the specific surface area is increasing leading to a more exposed surface for dye adsorption. Besides, the reusability of the synthesized composites was evaluated by subjecting to photocatalysis for three cycles after subsequent washing with deionized water (Fig. 7c). The degradation efficiency of ZFO composites showed more than 85% reproducibility after three cycles. The observed reduction in efficiency might be due to the material loss during washing steps which could be improved by retrieving from the medium using an external magnetic field.
Figure 8 demonstrated the feasible photocatalytic mechanism of ZFO nanocomposites. When exposed to UV light, ZFO composites absorb enough energy to excite the electrons from valence band (VB) to conduction band (CB), forming free electrons (e−) in CB and holes (h+) in VB [44, 45]. The free electrons may be released into the aqueous solution of dye, which reacts with dissolved oxygen in the medium to form superoxide radicals (O2−). These can further attack water molecules or hydroxyl ions to form reactive hydroxide ions (OH.); that have the capacity to attack the dye molecules [19, 39]. Meanwhile, h+ in the valence band can also promote the formation of OH. radicals. Consequently, MB can decompose to nontoxic degradation products such as CO2 and H2O. The resultant changes in the MB solution were also depicted in the figure.
The proposed mechanism of photocatalysis of MB solutions using ZFO nanocomposites [46]
We have compared the photocatalytic efficiency obtained for as-prepared Ni-doped zinc ferrites with other systems used for methylene blue degradation (Table 1). A previous investigation by Jadhav et al.demonstrated the effect of Ni dopant on structural parameters and photocatalytic degradation of Rhodamine B [47]. They found that the photocatalytic effect increases with Ni content while using urea as fuel. In the present study, we have investigated the effect of Ni doping on structural and photocatalytic properties of ZFO nanocomposites by using glycine as a green fuel. We observed that the highest photocatalytic activity of 98% was obtained for the optimum Ni content of 30%. As auto-combustion process parameters could significantly affect the structure and properties of ZFO nanocomposites, the proper correlation between structural features and photocatalytic performance is highly necessitated in this context. The stability of the material has been proved by doing multiple photocatalysis cycles on the same sample.
4 Conclusions
In summary, ZFO nanocomposites with varying Ni dopant were successfully synthesized using auto-combustion followed by the annealing process. XRD revealed the formation of zinc ferrites crystallites with cubic spinel structure. The morphology of different zinc ferrites was evaluated using SEM. The degradation rate of MB solution increases successively with Ni doping up to 30% and thereafter decreases. With an optimum Ni doping (30%), photocatalytic activity up to 98% was achieved with as-synthesized ZFO nanocomposites, which can perform as an environmentally friendly, easily isolable, and recyclable photocatalyst for water treatments.
References
R.B. Baird et al., Standard Methods for the Examination of Water and Wastewater (American Public Health Association, Washington DC, 2017).
G. Muthuraman, T.T. Teng, Extraction and recovery of rhodamine B, methyl violet and methylene blue from industrial wastewater using D2EHPA as an extractant. J. Ind. Eng. Chem. 15(6), 841–846 (2009)
R.S. Razmara, A. Daneshfar, R. Sahrai, Determination of methylene blue and sunset yellow in wastewater and food samples using salting-out assisted liquid–liquid extraction. J. Ind. Eng. Chem. 17(3), 533–536 (2011)
S. Li et al., Fast photocatalytic degradation of dyes using low-power laser-fabricated Cu2O–Cu nanocomposites. RSC Adv. 8(36), 20277–20286 (2018)
K.M. Lee, S.B.A. Hamid, C.W. Lai, Multivariate analysis of photocatalytic-mineralization of eriochrome black T dye using ZnO catalyst and UV irradiation. Mater. Sci. Semicond. Process 39, 40–48 (2015)
G. Sharma et al., Guar gum-crosslinked-Soya lecithin nanohydrogel sheets as effective adsorbent for the removal of thiophanate methyl fungicide. Int. J. Biol. Macromol. 114, 295–305 (2018)
M. Wang et al., Enhanced removal of heavy metals and phosphate in stormwater filtration systems amended with drinking water treatment residual-based granules. J. Environ. Manag. 280, 111645 (2021)
D. Pathania et al., Fabrication of nanocomposite polyaniline zirconium(IV) silicophosphate for photocatalytic and antimicrobial activity. J. Alloys Compd. 588, 668–675 (2014)
P.C. Vandevivere, R. Bianchi, W. Verstraete, Treatment and reuse of wastewater from the textile wet-processing industry: review of emerging technologies. J. Chem. Technol. Biotechnol. 72(4), 289–302 (1998)
Y. Liao et al., Effects of heat treatment scheme on the photocatalytic activity of TiO2 nanotube powders derived by a facile electrochemical process. J. Alloys Compd. 509(3), 1054–1059 (2011)
A. Hajjaji et al., Photocatalytic activity of Cr-doped TiO2 nanoparticles deposited on porous multicrystalline silicon films. Nanoscale Res. Lett. 9(1), 1–6 (2014)
Y. Meor et al., Photocatalytic active nanorutile TiO2: synthesis characterization and photocatalysis tests. J. Nano Res. 26, 17–23 (2014)
A. Barnasas et al., Growth and characterization of nanostructured Ag-ZnO for application in water purification. J. Nano Res. 62, 75–86 (2020)
G. Sharma et al., Highly efficient Sr/Ce/activated carbon bimetallic nanocomposite for photoinduced degradation of rhodamine B. Catal. Today 33, 437–451 (2019)
G. Sharma et al., Preparation, characterization and antimicrobial activity of biopolymer based nanocomposite ion exchanger pectin zirconium (IV) selenotungstophosphate: application for removal of toxic metals. J. Ind. Eng. Chem. 20(6), 4482–4490 (2014)
B.I. Kharisov, H.R. Dias, O.V. Kharissova, Mini-review: ferrite nanoparticles in the catalysis. Arab. J. Chem. 12(7), 1234–1246 (2019)
N.M. Mahmoodi, Zinc ferrite nanoparticle as a magnetic catalyst: synthesis and dye degradation. Mater. Res. Bull. 48(10), 4255–4260 (2013)
H.R. Rajabi et al., Green synthesis of zinc sulfide nanophotocatalysts using aqueous extract of Ficus Johannis plant for efficient photodegradation of some pollutants. J. Mater. Res. Technol. 9(6), 15638–15647 (2020)
H.R. Rajabi et al., Fast sonochemically-assisted synthesis of pure and doped zinc sulfide quantum dots and their applicability in organic dye removal from aqueous media. J. Photochem. Photobiol. B 181, 98–105 (2018)
M. Roushani et al., Application of graphene quantum dots as green homogenous nanophotocatalyst in the visibl e-light-driven photolytic process. J. Mater. Sci. 28, 5135–5143 (2017)
J.S. Jang, S.J. Hong, J.S. Lee, P.H. Borse, O.S. Jung, T.E. Hong, H.G. Kim, Synthesis of zinc ferrite and its photocatalytic application under visible light. J. Korean Phys. Soc. 54(1), 204–208 (2009)
P.H. Borse, J.S. Jang, S.J. Hong, J.S. Lee, J.H. Jung, T.E. Hong, H.G. Kim, Photocatalytic hydrogen generation from water-methanol mixtures using nanocrystalline ZnFe2O4 under visible light irradiation. J. Korean Phys. Soc 55(4), 1472–1477 (2009)
J. Ye et al., Photocatalytic simultaneous removal of nitrite and ammonia via a zinc ferrite/activated carbon hybrid catalyst under UV–Visible irradiation. ACS Omega 4(4), 6411–6420 (2019)
R. Dom, R. Subasri, N.Y. Hebalkar, A.S. Chary, P.H. Borse, Synthesis of a hydrogen producing nanocrystalline ZnFe2O4 visible light photocatalyst using a rapid microwave irradiation method. RSC Adv. 2(33), 12782–12791 (2012)
N.D. Raskar et al., One step synthesis of vertically grown Mn-doped ZnO nanorods for photocatalytic application. J. Mater. Sci. 30(11), 10886–10899 (2019)
A. Gedanken, et al., Google Patents, Sonochemical coating of textiles with metal oxide nanoparticles for antimicrobial fabrics, 2019
Y.I. Kim, D. Kim, C.S. Lee, Synthesis and characterization of CoFe2O4 magnetic nanoparticles prepared by temperature-controlled coprecipitation method. Phys. B 337(1–4), 42–51 (2003)
A. Bhosale et al., Influential incorporation of RE metal ion (Dy3+) in yttrium iron garnet (YIG) nanoparticles: magnetic, electrical and dielectric behaviour. Ceram. Int. 46(10), 15372–15378 (2020)
D. Ponnamma et al., Synthesis, optimization and applications of ZnO/polymer nanocomposites. Mater. Sci. Eng. C 98, 1210–1240 (2019)
S. Singamaneni et al., Magnetic nanoparticles: recent advances in synthesis, self-assembly and applications. J. Mater. Chem. 21(42), 16819–16845 (2011)
Y. Bao et al., Magnetic nanoparticles: material engineering and emerging applications in lithography and biomedicine. J. Mater. Sci. 51(1), 513–553 (2016)
C.-C. Hwang, J.-S. Tsai, T.-H. Huang, Combustion synthesis of Ni–Zn ferrite by using glycine and metal nitrates—Investigations of precursor homogeneity, product reproducibility, and reaction mechanism. Mater. Chem. Phys. 93(2–3), 330–336 (2005)
M. Gaidi et al., Enhanced photocatalytic activities of silicon nanowires/graphene oxide nanocomposite: effect of etching parameters. J. Environ. Sci. 101, 123–134 (2020)
A. Guinier, Théorie et technique de la radiocristallographie. Dunod (1964)
W. Meng et al., Photocatalytic activity of highly porous zinc ferrite prepared from a zinc-iron (III)-sulfate layered double hydroxide precursor. J. Porous Mater. 11(2), 97–105 (2004)
S. Phumying et al., Nanocrystalline spinel ferrite (MFe2O4, M=Ni Co, Mn, Mg, Zn) powders prepared by a simple aloe vera plant-extracted solution hydrothermal route. Mater. Res. Bull. 48, 2060–2065 (2013)
R.S. Yadav et al., Structural, magnetic, optical, dielectric, electrical and modulus spectroscopic characteristics of ZnFe2O4 spinel ferrite nanoparticles synthesized via honey-mediated sol-gel combustion method. J. Phys. Chem. Solids 110, 87–99 (2017)
M. Rezaei et al., Selective and rapid extraction of piroxicam from water and plasma samples using magnetic imprinted polymeric nanosorbent: synthesis, characterization and application. Colloids Surf. A 586, 124253 (2020)
H.M. Rajabi et al., Comparison investigation on photocatalytic activity performance and adsorption efficiency for the removal of cationic dye: quantum dots vs. magnetic nanoparticles. J. Environ. Chem. Eng. 4(3), 2830–2840 (2016)
R. Rameshbabu et al., Synthesis and study of structural, morphological and magnetic properties of ZnFe2O4 nanoparticles. J. Supercond. Novel Magn. 27(6), 1499–1502 (2013)
A. Lassoued et al., Photocatalytic degradation of methyl orange dye by NiFe2O4 nanoparticles under visible irradiation: effect of varying the synthesis temperature. J. Mater. Sci. Mater. Electron. 29, 7057–7067 (2018)
M.M. Naik et al., Green synthesis of zinc ferrite nanoparticles in Limoniaacidissima juice: characterization and their application as photocatalytic and antibacterial activities. Microchem. J. 146, 1227–1235 (2019)
G. Padmapriya et al., Spinel NixZn1−xFe2O4 (0.0 ≤ x ≤ 1.0) nano-photocatalysts: synthesis, characterization and photocatalytic degradation of methylene blue dye. J. Mol. Struct. 1119, 39–47 (2016)
P. Dhiman et al., Nano FexZn1−xO as a tuneable and efficient photocatalyst for solar powered degradation of bisphenol A from aqueous environment. J. Clean. Prod. 165, 1542–1556 (2017)
G. Sharma et al., Fabrication and characterization of novel Fe0@Guar gum-crosslinked-soya lecithin nanocomposite hydrogel for photocatalytic degradation of methyl violet dye. Sep. Purif. Technol. 211, 895–908 (2019)
D. Chahar et al., Photocatalytic activity of cobalt substituted zinc ferrite for the degradation of methylene blue dye under visible light irradiation. J. Alloys Compd. 851, 156878 (2021)
S.A. Jadhav et al., Magneto-structural and photocatalytic behavior of mixed Ni–Zn nano-spinel ferrites: visible light-enabled active photodegradation of rhodamine B. J. Mater. Sci. 31, 11352–11365 (2020)
S. Gul et al., Al-substituted zinc spinel ferrite nanoparticles: preparation and evaluation of structural, electrical, magnetic and photocatalytic properties. Ceram. Int. 46(9), 14195–14205 (2020)
Acknowledgements
This work was supported by the directorate general of scientific research and technological development DGRSDT of Algeria. The authors would like to acknowledge the financial support from the University of Sharjah (Targeted Project Grant No. 2102143096).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hammouche, J., Gaidi, M., Columbus, S. et al. Enhanced Photocatalytic Performance of Zinc Ferrite Nanocomposites for Degrading Methylene Blue: Effect of Nickel Doping Concentration. J Inorg Organomet Polym 31, 3496–3504 (2021). https://doi.org/10.1007/s10904-021-01960-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-021-01960-z