Abstract
A large library of pyrano[2,3-d]pyrimidines and pyrido[2,3-d]pyrimidines was synthesized through the reaction between various aromatic aldehydes, malononitrile and pyrimidine derivatives in the presence of Al-HMS-20 as the catalyst in EtOH at room temperature after 12 h. This multi-component approach is a very efficient and economical benign process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With increasing concerns about environmental pollution problems, researchers have started to pay more attention to the detrimental effects of non-green processes on the environment. Currently, mesoporous silicate acid catalysts have gained great interest in chemical synthesis because environmentally benign chemical processes have been greatly needed in light of the paradigm shift to green chemistry [1]. Mesoporous silicates due to larger pores in comparison with acidic zeolites could be good hosts for bulky organic substrates, as such substrates have more efficient contact with acidic sites on the interior surfaces of mesopores [2]. HMS is a hexagonal mesoporous silica with a particular wormlike pore structure. It has a simple preparation method using cheap primary alkyl amines which can be extracted without pollution [3]. After the introduction of mesoporous FSM-16 [4, 5] and MCM-41 [6] in the early 1990s, a series of mesoporous aluminosilicates have been reported which have remarkable acidic properties [7]. Purely mesoporous silica does not have enough acidity, but the acidity can be improved through the insertion of foreign metal ions into its structure during the synthesis [8, 9].
Pyrano[2,3-d]pyrimidines and pyrido[2,3-d]pyrimidines have attracted great attention owing to their diverse biological properties [10]. Pyrimidine-containing heterocyclic compounds have shown antitumor, antibacterial, antihypertensive, vasodilator, bronchodilator, hepatoprotective, cardiotonic, and antiallergic activities [11–16]. Also, some of them have shown antimalarial, antifungal, analgesic, and herbicidal properties [17, 18]. The derivatives of pyrano-pyrimidines have been reported to display biological activities, especially antipyretic, anti-inflammatory, and gastroprotective [19]. Pyrido-pyrimidine derivatives are of great interest due to their anti-tumor, antifolate, antibacterial, and growth regulator activities [20]. Thus, development of new greener approaches for the synthesis of these important scaffolds is still of interest [21].
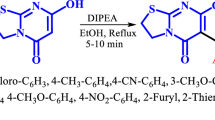
Here, a new route for the synthesis of pyrano[2,3-d]pyrimidines 4a–l and pyrido[2,3-d]pyrimidines 6a–h was developed in the presence of Al-HMS-20 as the heterogeneous recyclable catalyst, in EtOH at room temperature (Scheme 1).
Experimental
The chemicals used in this work were purchased from Merck and Fluka Chemical. Al-HMS-20 was prepared as reported in the literature [20]. Melting points were measured on an electrothermal 9100 apparatus and are uncorrected. The specific surface area and pore volume of the samples were measured in an ASAP-2010 Micromeritics (USA) using low temperature N2 physisorption isotherms. Before the analysis, the sample was evacuated at 350 °C under vacuum conditions. The acidity of Al-HMS was measured by TPD of ammonia in a TPD/TPR analyzer (2900 Micromeritics) with a thermal conductivity detector. The Si/Al ratio of Al-HMS was determined by XRF (XRF-8410 Rh 60 kV).
Typical procedure for preparation of 7-amino-2,4-dioxo-5-phenyl-1,3,4,5-tetrahydro-2H-pyrano[2,3-d]pyrimidine-6-carbonitrile (4a)
To a magnetically stirred solution of benzaldehyde (0.11 g, 1.00 mmol) and malononitrile (0.07 g, 1.00 mmol) in EtOH (5 mL), barbituric acid (0.13 g, 1.00 mmol) and Al-HMS-20 (0.03 g) were added at room temperature and the reaction mixture was stirred for 12 h. Upon completion, the reaction mixture was filtered off for separation of the catalyst, and the catalyst was washed with acetone (2 × 3 mL) [some of the products were washed with DMSO (3 mL) and crystallized in EtOH/DMSO/H2O]. Then, product 4a as a colorless crystal (0.26 g, yield 92 %) was crystallized in acetone/EtOH mixture resulting from the washing process.
Results and discussion
The catalytic activity of Al-HMS-20 [22] with BET surface area 1,289 m2/g, pore volume 2.1 cm3/g, and total acidity 0.845 mmol NH3/g was evaluated for the synthesis of pyrano[2,3-d]pyrimidines via a three-component reaction of aromatic aldehydes 1, malononitrile 2 and pyrimidines 3. For optimization of reaction conditions, the reaction of benzaldehyde 1a, malononitrile 2 and barbituric acid 3a as a model reaction was investigated. In a pilot experiment, 3a (1 mmol) and Al-HMS-20 was added to the round bottom flask containing 1a and 2 in solvent (5 mL), and the mixture was stirred for 12 h at room temperature. The progress of the reaction was monitored by TLC. As shown in Table 1, it was found that 0.03 g of Al-HMS-20 in EtOH is the best reaction condition.
To evaluate the use of this interesting approach, a variety of aldehydes and pyrimidines were examined for which a good library of pyrano[2,3-d]pyrimidines 4 was obtained in fairly high yields (Table 2).

The efficiency of the catalyst was studied for the synthesis of pyrido[2,3-d]pyrimidines 6a–h in three-component reaction of aromatic aldehydes 1, malononitrile 2 and 6-amino-uracils 5 in EtOH at room temperature. The results are very promising and good yields observed in this reaction conditions (Table 3).

Recyclability of the catalysts was also examined. For this reason, the catalyst, which was recovered from the reaction between benzaldehyde (1a), malononitrile (2) and Barbituric acid (3a), were washed with acetone (2 × 5 mL), dried in the oven (40 °C, 6 h), and used again. This procedure was carried out four times. The results of these successive reactions are shown in Table 4. It is clear that, no decrease in the reactivity or performance can be seen for after successive use of the catalyst, Al-HMS-20.
Using Al-HMS-20, we want to solve the problems of using the homogeneous catalyst such as separation and regeneration. Successively, we overcame these problems by utilizing Al-HMS as a heterogeneous recoverable catalyst in EtOH. Thus, this process could be interesting for large-scale synthesis.
Conclusions
In conclusion, we have developed an efficient method for the synthesis of pyrano[2,3-d]pyrimidines and pyrido[2,3-d]pyrimidines under mild reaction conditions in the presence of Al-HMS-20 in EtOH as a solvent with good yields. The present route has the advantages such as good yields and recyclability of the catalyst without loss of performance. The work-up procedure is very simple, and the products do not require further purification. Good yields and recyclability of the catalyst for this multi-component reaction makes it an interesting alternative to other approaches.
References
P.T. Anastas, J.C. Warner, Green Chemistry: Theory and Practice (Oxford University Press, London, 1999)
C.T. Kresge, M.E. Leonowicz, W.J. Roth, J.C. Vartuli, J.S. Beck, Nature 359, 710 (1992)
K. Szczodrowski, B. Prélot, S. Lantenois, J.-M. Douillard, J. Zajac, Microporous Mesoporous Mater. 124, 84 (2009)
M. Onaka, N. Hashimoto, Y. Kitabata, R. Yamasaki, Appl. Catal. A. Gen. 241, 307 (2003)
T. Chiranjeevi, G.M. Kumaran, J.K. Gupta, G.M. Dhar, Thermochim. Acta 443, 87 (2006)
T. Yanagisawa, T. Shimizu, K. Kuroda, C. Kato, Bull. Chem. Soc. Jpn. 63, 988 (1990)
S. Inagaki, A. Koiwai, N. Suzuki, Y. Fukushima, K. Kuroda, Bull. Chem. Soc. Jpn. 69, 1449 (1996)
J.Y. Ying, C.P. Mehnert, M.S. Wong, Angew. Chem. Int. Ed. 38, 56 (1999)
M.J. Gracia, E. Losada, R. Luque, J.M. Campelo, D. Luna, J.M. Marinas, A.A. Romero, Appl. Catal. A. Gen. 349, 148 (2008)
D. Fenn (ed.), The Pyrimidines (Wiley, New York, 1994)
D. Heber, C. Heers, U. Ravens, Pharmazie 48, 537 (1993)
E.M. Griva, S. Lee, C.W. Siyal, D.S. Duch, C.A. Nichol, J. Med. Chem. 23, 327 (1980)
M.M. Ghorab, A.Y. Hassan, Phosphorus Sulfur Silicon Relat. Elem. 141, 251 (1998)
S. Furuya, T. Ohtaki, Eur. Patent 608565, 1994
W.J. Coates, Eur. Patent 351058, 1990
N. Kitamura, A. Onishi, Eur. Patent 163599, 1984
J. Davoll, J. Clarke, E.F. Elslager, J. Med. Chem. 15, 837 (1972)
G. Levitt, US Patent 4339267, 1982
O. Bruno, S. Schenone, A. Ranise, E. Barocell, M. Chiavarini, V. Ballabeni, S. Bertoni, Arzneim. Forsch. 50, 140 (2000)
D.A. Ibrahim, N.S.M. Ismail, Eur. J. Med. Chem. 46, 5825 (2011)
M. Kidwai, A. Jain, S. Bhardwaj, Mol. Divers. 16, 121 (2012)
R. Mokaya, W. Jones, J. Catal. 172, 211 (1997)
Acknowledgment
We gratefully acknowledge financial support from the Research Council of Shahid Beheshti University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sabour, B., Peyrovi, M.H. & Hajimohammadi, M. Al-HMS-20 catalyzed synthesis of pyrano[2,3-d]pyrimidines and pyrido[2,3-d]pyrimidines via three-component reaction. Res Chem Intermed 41, 1343–1350 (2015). https://doi.org/10.1007/s11164-013-1277-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-013-1277-y