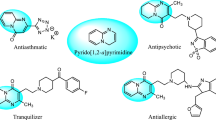
A fast, efficient, and green synthesis of pyrimido[4,5-d]pyrimidine derivatives has been achieved via one-pot three-component reaction starting from 6-amino-N,N-dimethyluracil, phenylisothiocyanate or phenylisocyanate, and aromatic aldehydes in the presence of polyethylene glycol-bound sulfonic acid as a catalyst in water as solvent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Multicomponent reactions (MCRs) have recently gained a considerable attention in organic synthesis, because they involve simultaneous reaction of more than two starting materials to yield a single product through one-pot reaction. Good selectivity, minimum waste production, low energy consumption, and high atom efficiency make MCRs suitable for the synthesis of varied drug candidates with augmented bioactivity.1
Nowadays, synthetic chemists try to avoid using hazardous solvents, toxic and expensive reagents in their work for the sake of environment and to choose environmentally benign reusable catalysts and solvents, instead. Among the environment friendly solvents for organic synthesis, water is particularly good because it is cheap, non-toxic, noncorrosive, non-flammable,2 – 5 offers good selectivity, and can be easily separated from the organic products.
Heterocyclic motifs play a prominent role in the design and synthesis of bioactive molecules. Pyrimidines and fused pyrimidines have been found to possess diverse biological activity.6 – 8 In particular, among pyrimido[4,5-d]-pyrimidines, a class of annulated uracils, a wide range of biologically active compounds like bronchodilators,9 antiallergic,10 cardiotonic,11 , 12 antihypertensive,13 and anticancer14 agents have been found.
The synthesis of pyrimido[4,5-d]pyrimidine derivatives is less investigated. The known methods include cycloaddition reactions,14 – 17 as well as three-component reactions.18 – 20 Some of them use AcOH,14 , 19 p-toluenesulfonic acid,20 and iodine17 as catalysts. One of the reported procedures20 features water as solvent while a few others15 , 16 , 18 are solvent-free. Recently, Wang et al.21 reported a synthesis of highly substituted pyrimido[4,5-d]pyrimidine derivatives involving 6 steps. However, these methods require extreme conditions like long reaction time, high energy, and complex synthetic pathways. To overcome these difficulties, we pursue to select a suitable, sustainable catalyst for the synthesis of pyrimido[4,5-d]pyrimidines.
For years, the attention of organic chemists has been focused on polymer-supported catalysts due to their low cost, high catalytic activity, easy work-up procedure, and recyclability.22 In the search for environmentally benign polymer-supported catalysts they have come up with polyethylene glycol-bound sulfonic acid (PEG–SO3H)23 – 28 which is attractive because of its acidic, non-volatile, noncorrosive, economical, and recyclable nature. This homogeneous catalyst has also been used for the synthesis of the heterocyclic compounds.29 , 30 Herein, we report the catalytic activity of PEG–SO3H for the synthesis of fused uracil derivatives.
Initially we performed the reaction of a mixture containing 6-amino-N,N-dimethyluracil (1) (1 mmol), phenylisothiocyanate (2, X = S) (1 mmol), and benzaldehyde
(3, Ar = Ph) (1 mmol) in water at a temperature of up to 70°C in the absence of catalyst to obtain compound 4a (Scheme 1). The reaction did not take place even after 3 h. In order to identify the optimal reaction conditions, we conducted the reaction in the presence of catalytic amount of PEG–SO3H both in aqueous and non-aqueous media.

Scheme 1
The PEG–SO3H-catalyzed synthesis of compound 4a was performed using different amounts of the catalyst in water. The results are summarized in Table 1. It was found that 10 mol % of PEG-SO3H catalyzed the reaction most efficiently in terms of yield and reaction time. A further increase of the amount of catalyst made no improvement in the yield. The role of solvent in the reaction was also investigated. Interestingly, the target compound was formed in good yields in both media, but the reaction time is less in aqueous medium because the catalyst is watersoluble.
In order to evaluate the efficiency of the present methodology, we have checked different acid catalysts for the synthesis of compound 4a as a model reaction, and results of this study are presented in Table 2. It is clear from the data shown that the present method is more efficient in both in terms of yields and reaction time and also compared with the aqueous p-TSA-catalyzed synthesis described earlier.20
Using these optimized conditions, we examined the scope of the reactions using isothiocyanate or isocyanate (2, X = S or O, respectively) with different aromatic aldehydes 3. The results are presented in Table 3. Because of the strong acidic nature of PEG–SO3H the electrophilicity of the aldehyde increases, which facilitates the attack by nucleophile and thus allows the reaction to be complete in a shorter period of time. In the synthesis of the target compounds 4a–l, isocyanate gave higher yields when compared to isothiocyanate. The average time taken for the synthesis of all the title compounds was 50–70 min. Aldehydes having electron-withdrawing groups react in a shorter time and with higher yield than those having electron-donating groups owing to the higher electrophilicity of the former.
The structure of the novel pyrimido[4,5-d]pyrimidine-2,4,7(1H,3H,6H)-triones 4g–l was fully characterized by IR, 1H and 13C NMR, and mass spectra, as well as elemental analysis. In the IR spectra, stretching frequencies at 1675–1690, 1711–1717, and 3218–3328 cm–1 confirmed the presence of carbonyl functional groups and NH proton in the molecule. In the 1H NMR spectra, the two singlets at 2.35–3.25 and 3.12–3.56 ppm can be assigned the protons of two methyl groups, and a broad singlet at 7.58–7.68 ppm corresponds to the NH proton. The mass spectra of compounds 4g–l showed the corresponding protonated molecular ion signals. The identity of 7-thioxopyrimido[4,5-d]-pyrimidine-2,4(1H,3H)-diones 4a–f described earlier20 was likewise confirmed by their spectral and physical characteristics.
Furthermore, we have also investigated the recyclability of PEG–SO3H catalyst. Four consecutive cycles of catalyst recovery and reaction showed almost the same catalytic activity. In order to recover the catalyst, water was added to the reaction mixture after completion of the reaction, and it was shaken to dissolve PEG–SO3H. The insoluble compound was filtered, extracted with ethyl acetate, and recrystallized from ethanol. The aqueous filtrate containing the catalyst was evaporated under reduced pressure, and the resulting solid was washed with diethyl ether and dried under reduced pressure. By using this recovered catalyst, the reaction was carried out for next three cycles for the synthesis of compound 4a, and in this process we have observed the smooth decline of the isolated product yield due to the decrease of the amount of PEG–SO3H catalyst (less than 10 mol %) due to the recovery loss after each cycle (Table 4).
A possible mechanism for the formation of product 4 is shown in Scheme 2. Primarily, the intermediate A is formed in situ from the reaction of 6-amino-N,N-dimethyluracil 1 with phenylisothiocyanate or phenylisocyanate 2.

Scheme 2
Then, nucleophilic attack of intermediate A to aldehyde 3 in the presence of PEG–SO3H catalyst gives intermediate B. Its subsequent cyclization and dehydration complete the reaction.
In summary, we have established an efficient one-pot three-component protocol for the synthesis of pyrimido[4,5-d]-pyrimidine derivatives by environmentally benign and economically viable PEG–SO3H catalyst in water as solvent. Furthermore, short reaction times, excellent yields, simple work-up procedure, and utilization of an inexpensive and reusable catalyst are the advantages of the present methodology. This methodology is a valuable addition to the existing repertory of methods for the synthesis of pyrimido[4,5-d]pyrimidine derivatives.
Experimental
FT-IR spectra were recorded on a Bruker ALPHA spectrometer. 1H and 13C NMR spectra were recorded on a Bruker AMX instrument (300 and 100 MHz, respectively) in DMSO-d 6 with TMS as internal standard. LC-MS spectra were recorded on an Acquity Ultra Performance Liquid Chromatography instrument coupled with an API 3000 mass spectrometer operating in electrospray positive ionization mode. Elemental analysis was performed on a Thermo Finnegan Flash EA 1112 instrument at University of Hyderabad, Hyderabad, India. Melting points were determined in open capillaries on Guna melting point apparatus and are uncorrected. Silica gel column chromatography was performed using Merck 7734 silica gel (60–120 mesh). TLC was done on Merck TLC plates. The chemicals were purchased from Sigma-Aldrich Chemicals.
Preparation of PEG–SO 3 H. Chlorosulfonic acid (0.11g, 10 mmol) was added to a solution of PEG (0.6 g, 1 mmol of SO3H groups) in CH2Cl2 (10 ml) at 0°C, and the resulting solution was stirred at room temperature overnight. Then, the solution was concentrated under vacuum, and ether was added to it. The resulting precipitate was filtered off and washed with ether to give 4.82 mg of PEG–SO3H as a white solid.
1,3-Dimethyl-5,6-diphenyl-7-thioxo-5,6,7,8-tetrahydropyrimido[4,5- d ]pyrimidine-2,4(1 H ,3 H )-dione (4a). A mixture of phenylisothiocyanate (2, X = S) (0.135 g, 0.89 mmol), 6-amino-N,N-dimethyluracil (1) (0.155 g, 1.00 mmol), and benzaldehyde (3, Ar = Ph) (0.106 g, 1.00 mmol) in water (10 ml) was refluxed in the presence of PEG–SO3H (0.6 g, 0.1 mmol). After the completion of the reaction (monitored by TLC, eluent n-hexane–AcOEt, 3:1), the reaction mixture was diluted with water (20 ml). The solid was separated by filtration and washed with water, dried, and recrystallized from ethanol. Yield 82%, mp 268–269°C (mp 269–270°C19). Compounds 4b–l were synthesized analogously.
1,3-Dimethyl-5-(4-methylphenyl)-6-phenyl-7-thioxo-5,6,7,8-tetrahydropyrimido[4,5- d ]pyrimidine-2,4(1 H ,3 H )-dione (4b). Yield 80%, mp 271–272°C (mp 270–271°C19). 5-(4-Chlorophenyl)-1,3-dimethyl-6-phenyl-7-thioxo-5,6,7,8-tetrahydropyrimido[4,5- d ]pyrimidine-2,4(1 H ,3 H )-dione (4c). Yield 84%, mp 274–275°C (mp 275–276°C19). 5-(4-Bromophenyl)-1,3-dimethyl-6-phenyl-7-thioxo-5,6,7,8-tetrahydropyrimido[4,5- d ]pyrimidine-2,4(1 H ,3 H )-dione (4d). Yield 78%, mp 278–279°C (mp 279–280°C19). 5-(4-Methoxyphenyl)-1,3-dimethyl-6-phenyl-7-thioxo-5,6,7,8-tetrahydropyrimido[4,5- d ]pyrimidine-2,4(1 H ,3 H )-dione (4e). Yield 86%, mp 271–272°C (mp 270–271°C19). 1,3-Dimethyl-(4-nitrophenyl)-6-phenyl-7-thioxo-5,6,7,8-tetrahydropyrimido[4,5- d ]pyrimidine-2,4(1 H ,3 H )-dione (4f). Yield 90%, mp 272–273°C (mp 273–274°C19).
1,3-Dimethyl-5,6-diphenyl-5,8-dihydropyrimido[4,5- d ]-pyrimidine-2,4,7(1 H ,3 H ,6 H )-trione (4g). Yield 86%, mp 224–225°C. IR spectrum ν, cm–1: 3218, 1717, 1690. 1H NMR spectrum, δ, ppm: 3.05 (3H, s, CH3); 3.12 (3H, s, CH3); 5.40 (1H, s, 5-CH); 7.22–7.38 (10H, m, H Ar); 7.58 (1H, s, NH). 13C NMR spectrum, δ, ppm: 28.6; 31.2; 105.3; 123.8 (2C); 124.3; 125.4; 126.5; 128.2 (2C); 129.4 (2C); 131.5 (2C); 137.7; 140.4; 146.5; 148.3; 151.2; 153.8. Mass spectrum, m/z: 363 [M+H]+. Found, %: C 66.12; H 4.88; N 14.28. C20H18N4O3. Calculated, %: C 66.29; H 5.01; N 15.46.
1,3-Dimethyl-5-(4-methylphenyl)-6-phenyl-5,8-dihydropyrimido[4,5- d ]pyrimidine-2,4,7(1 H ,3 H ,6 H )-trione (4h). Yield 85%, mp 218–219°C. IR spectrum ν, cm–1: 3323, 1711, 1684. 1H NMR spectrum, δ, ppm: 2.35 (3H, s, CH3); 3.40 (3H, s, CH3); 3.54 (3H, s, CH3); 5.56 (1H, s, 5-CH); 7.05–7.44 (9H, m, H Ar); 7.64 (1H, s, NH). 13C NMR spectrum, δ, ppm: 22.3; 27.8; 30.4; 37.2; 105.3; 121.2 (2C); 123.5; 126.8 (2C); 128.5 (2C); 130.4 (2C); 135.4; 139.4 (2C); 144.7; 148.4; 151.5; 154.7. Mass spectrum, m/z: 377 [M+H]+. Found, %: C 66.88; H 5.10; N 14.66. C21H20N4O3. Calculated, %: C 67.01; H 5.36; N 14.88.
5-(4-Chlorophenyl)-1,3-dimethyl-6-phenyl-5,8-dihydropyrimido[4,5- d ]pyrimidine-2,4,7(1 H ,3 H ,6 H )-trione (4i). Yield 88%, mp 226–227°C. IR spectrum ν, cm–1: 3325, 1715, 1678. 1H NMR spectrum, δ, ppm (J, HZ): 3.18 (3H, s, CH3); 3.48 (3H, s, CH3); 5.66 (1H, s, 5-CH); 7.06–7.40 (9H, m, H Ar); 7.65 (1H, s, NH). 13C NMR spectrum, δ, ppm: 28.5; 31.5; 36.4; 106.3; 125.4; 126.4 (2C); 126.7 (2C); 127.4; 128.2 (2C); 128.7 (2C); 130.4; 136.3; 142.4; 144.5; 149.8; 153.4. Mass spectrum, m/z: 397 [M+H]+. Found, %: C 60.32; H 4.18; N 14.02. C20H17ClN4O3. Calculated, %: C 60.53; H 4.32; N 14.12.
5-(4-Bromophenyl)-1,3-dimethyl-6-phenyl-5,8-dihydropyrimido[4,5- d ]pyrimidine-2,4,7(1 H ,3 H ,6 H )-trione (4j). Yield 84%, mp 237–238°C. IR spectrum ν, cm–1: 3316, 1714, 1680. 1H NMR spectrum, δ, ppm: 3.14 (3H, s, CH3); 3.52 (3H, s, CH3); 5.68 (1H, s, 5-CH); 7.05–7.42 (9H, m, H Ar); 7.60 (1H, s, NH). 13C NMR spectrum, δ, ppm: 29.2; 31.6; 35.4; 104.5; 123.3; 125.6 (2C); 126.3 (2C); 127.8 (2C); 128.1; 128.7 (2C); 129.4; 131.5; 138.4; 140.4; 148.4; 152.7. Mass spectrum, m/z: 441 [M+H]+. Found, %: C 54.32; H 3.72; N 12.58. C20H17BrN4O3. Calculated, %: C 54.44; H 3.88; N 12.70.
5-(4-Methoxyphenyl)-1,3-dimethyl-6-phenyl-5,8-dihydropyrimido[4,5- d ]pyrimidine-2,4,7(1 H ,3 H ,6 H )-trione (4k). Yield 90%, mp 220–231°C. IR spectrum ν, cm–1: 3325, 1712, 1675. 1H NMR spectrum, δ, ppm: 3.25 (3H, s, CH3); 3.56 (3H, s, CH3); 3.68 (3H, s, CH3O); 5.36 (1H, s, 5-CH); 7.08–7.34 (9H, m, H Ar); 7.66 (1H, s, NH). 13C NMR spectrum, δ, ppm: 28.7; 31.3; 36.4; 53.8; 104.7; 123.6; 125.8 (2C); 126.6; 127.8 (2C); 128.3 (2C); 128.7; 129.5; 131.2; 136.4; 140.7; 148.4; 151.2; 153.5. Mass spectrum, m/z: 393 [M+H]+. Found, %: C 64.10; H 5.04; N 14.16. C21H20N4O4. Calculated, %: C 64.28; H 5.14; N 14.28.
1,3-Dimethyl-5-(4-nitrophenyl)-6-phenyl-5,8-dihydropyrimido[4,5- d ]pyrimidine-2,4,7(1 H ,3 H ,6 H )-trione (4l). Yield 94%, mp 222–223°C. IR spectrum ν, cm–1: 3328, 1715, 1683. 1H NMR spectrum, δ, ppm: 3.20 (3H, s, CH3); 3.50 (3H, s, CH3); 5.44 (1H, s, 5-CH); 7.10–7.42 (9H, m, H Ar); 7.68 (1H, s, NH). 13C NMR spectrum, δ, ppm: 28.8; 31.5; 36.2; 106.2; 124.3; 125.1; 125.6 (2C); 126.8 (2C); 127.4; 128.5; 129.2 (2C); 131.4 (2C); 137.7; 141.8; 147.3; 152.4. Mass spectrum, m/z: 408 [M+H]+. Found, %: C 58.82; H 4.06; N 17.08. C20H17N5O5. Calculated, %: C 58.97; H 4.21; N 17.19.
The authors thank to Vikrama Simhapuri University, Nellore, for providing the lab facilities and University of Hyderabad, Hyderabad, for recording the NMR spectra and performing elemental analysis.
References
Dolle, R. E.; Le Bourdonnec, B.; Goodman, A. J.; Morales, G. A.; Thomas, C. J.; Zhang, W. J. Comb. Chem. 2008, 10, 753.
Aplander, K.; Hidestål, O.; Katebzadeh, K.; Lindström, U. M. Green Chem. 2006, 8, 22.
Liu, R.; Dong, C.; Liang, X.; Wang, X.; Hu, X. J. Org. Chem. 2005, 70, 729.
Stavber, G.; Zupan, M.; Jereb, M.; Stavber, S. Org. Lett. 2004, 6, 4973.
Hailes, H. C. Org. Process Res. Dev. 2007, 11, 114.
Pałasz, A.; Cież, D. Eur. J. Med. Chem. 2015, 97, 582.
Chobe, S. S.; Dawane, B. S.; Tumbi, K. M.; Nandekar, P. P.; Sangamwar, A. T. Bioorg. Med. Chem. Lett. 2012, 22, 7566.
Holla, B. S.; Kalluraya, B.; Sridhar, K. R.; Drake, E.; Thomas, L. M.; Bhandary, K. K.; Levine, M. J. Eur. J. Med. Chem. 1994, 29, 301.
Coates, W. J. EU Patent 351058; Chem. Abstr. 1990, 113, 40711.
Kitamura, N.; Onishi, A. EU Patent 163599; Chem. Abstr. 1984, 104, 186439.
Furuya, S.; Ohtaki, T. EU Patent 608565; Chem. Abstr. 1994, 121, 205395.
Heber, D.; Heers, C.; Ravens, U. Pharmazie 1993, 48, 537.
Raddatz, P.; Bergmann, R. DE Patent 3601731; Chem. Abstr. 1988, 109, 54786.
El-Moghazy, S. M.; Ibrahim, D. A.; Abdelgawad, N. M.; Farag, N. A. H.; EL-Khouly, A. S. Sci. Pharm. 2011, 79, 429.
Devi, I.; Borah, H. N.; Bhuyan, P. J. Tetrahedron Lett. 2004, 45, 2405.
Gohain, M.; Prajapati, D.; Gogoi, B. J.; Sandhu, J. S. Synlett 2004, 1179.
Moghaddam, M. F.; Reza Khodabakhshi, M.; Aminaee, M. Tetrahedron Lett. 2014, 55, 4720.
Prajapati, D.; Gohain, M.; Thakur, A. J. Bioorg. Med. Chem. Lett. 2006, 16, 3537.
Dabiri, M.; Arvin-Nezhad, H.; Reza Khavasi, H.; Bazgir, A. Tetrahedron 2007, 63(8), 1770.
Majumder, S.; Borah, P.; Bhuyan, P. J. Tetrahedron Lett. 2014, 55, 1168.
Wang, H.; Wang, C.; D. Bannister, T. Tetrahedron Lett. 2015, 56, 1949.
Benaglia, M.; Puglisi, A.; Cozzi, F. Chem. Rev. 2003, 103, 3401.
Kiasat, A. R.; Mehrjardi, M. F. Catal. Commun. 2008, 9, 1497.
Reddy, C. B.; Kumar, K. S.; Kumar, M. A.; Reddy, M. V. N.; Krishna, B. S.; Naveen, M.; Arunasree, M . K.; Reddy, C. S.; Naga Raju, C.; Reddy, C. D. Eur. J. Med. Chem. 2012, 47, 553.
Kiasat, A. R.; Mehrjardi, M. F. Synth. Commun. 2008, 38, 2995.
Wang, X. C.; Li, L.; Quan, Z. J.; Gong, H. P.; Ye, H. L.; Cao, X. F. Chin. Chem. Lett. 2009, 20, 651.
Quan, Z. J.; Da, Y. X.; Zang, Z.; Wang, X. C. Catal. Commun. 2009, 10, 1146.
Rani, V. J.; Vani, K. V.; Rao, C. V. Synth. Commun. 2012, 42, 2048.
Paul, S.; Asish, R. D. Catal. Sci. Technol. 2012, 2, 1130.
Wang, X.; Quan, Z.; Wang, F.; Wang, M.; Zhang, Z.; Li, Z. Synth. Commun. 2006, 36, 451.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2015, 51(8), 749–753
Rights and permissions
About this article
Cite this article
Badvel, S., Gopireddy, R.R., Shaik, T.B. et al. An efficient one-pot three-component synthesis of pyrimido[4,5-d]pyrimidine derivatives in aqueous medium. Chem Heterocycl Comp 51, 749–753 (2015). https://doi.org/10.1007/s10593-015-1769-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-015-1769-3