The microreview summarizes the data published over the last 10 years on the methods of preparation and properties of 6-(aryldiazenyl)-4H-chromenes, a new promising class of compounds incorporating the azo and 4H-chromene fragments in their structure. The material is systematized according to the structure of the starting reagents.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction

Functionally substituted chromenes, in particular 2-amino-4H-chromene-3-carbonitriles, represent one of the most popular classes of heterocyclic compounds. The great interest in the chemistry of substituted chromenes is reflected in an impressive number of recent reviews1,2,3,4,5,6,7,8,9,10,11,12,13,14,15 and is owing to the exceptional accessibility of 2-amino-4Hchromene-3-carbonitriles and the wide spectrum of their biological activity. Azo compounds represent another wellknown and readily available class of compounds. Despite their long history, azo compounds are still the object of close attention as biologically active molecules,16,17 as markers in biomedical research,19,20,21 and also because of their unique photochemical and optical properties.22,23,24 In recent years, a number of studies have been dedicated to the synthesis and study of the properties of substituted 6-(aryldiazenyl)-4H-chromenes, a new class of compounds that are of interest both for their optical properties and possible biological activity. The simultaneous presence in their molecule of 4H-chromene and azo pharmacophore fragments in some cases results in a synergistic effect. This microreview presents the most significant studies of the chemistry of 6-(aryldiazenyl)-4H-chromenes published over the last 10 years.

Synthesis on the basis of 5-(aryldiazenyl)-2-hydroxybenzaldehydes

The products of azo coupling of aryldiazonium salts with salicylic aldehydes seem to be the most convenient starting reagents for the preparation of target arylazochromenes. Thus, chromenes 1 were obtained by the reaction of aldehydes 2 with 2 equiv of malononitrile in the presence of piperidine.25,26 Compound 1 (Ar = 4-ClC6H4) possesses a pronounced antibacterial and fungicidal activity.26

When malononitrile and dimedone were introduced into the reaction instead of 2 equiv of malononitrile, chromenes 3 were formed which showed in vitro anticancer activity on MCF-7 cells.27


 Victor V. Dotsenko was born in Voroshilovgrad (Luhansk) in 1976. He holds the degree of Doctor of Sciences in Chemistry (2015). His research interests include chemistry of O,S,Se,N-heterocycles, chemistry of active methylene nitriles and thioamides, biologically active compounds.
Victor V. Dotsenko was born in Voroshilovgrad (Luhansk) in 1976. He holds the degree of Doctor of Sciences in Chemistry (2015). His research interests include chemistry of O,S,Se,N-heterocycles, chemistry of active methylene nitriles and thioamides, biologically active compounds.
 Ekaterina A. Varzieva was born in Nalchik in 1994 and is currently a graduate student at the Department of Organic Chemistry and Technology of the Kuban State University. Her research interests include chemistry of heterocyclic compounds, chemistry of organosilicon compounds, epoxy compounds.
Ekaterina A. Varzieva was born in Nalchik in 1994 and is currently a graduate student at the Department of Organic Chemistry and Technology of the Kuban State University. Her research interests include chemistry of heterocyclic compounds, chemistry of organosilicon compounds, epoxy compounds.
Synthesis on the basis of 5-(aryldiazenyl)-2-hydroxybenzaldehydes (continued) 
When malononitrile is replaced by dimedone,28 barbituric acid,28 or 4-hydroxycoumarin,29 4H-chromenes are also formed as the products. Azo compounds 4 obtained in this way exhibit28 moderate anti-inflammatory and antioxidant activity.
The three-component condensation of H2C(CN)2, P(OEt)3, and aldehydes 2 in the presence of basic ionic liquids (IL) led to (4H-chromen-4-yl)phosphonic acid esters 5.30 Molecular docking results for the BCL2 apoptosis regulator indicated a potential anticancer activity of compounds 5.
A multicomponent synthesis of 4-pyrazolyl-4Н-chromenes 6 by the reaction of aldehydes 2, malononitrile, hydrazine, and ethyl acetoacetate in the presence of meglumine in H2O31 or by a mechanochemical reaction in the presence of Fe3O4-based magnetic nanoparticles32 was described. It is likely that the use of such exotic catalysts is not strictly necessary; however, this is the sole example of the synthesis of chromenes 6 in the literature. Compounds 6 exhibit intense fluorescence with an emission maximum at 582–586 nm.31


Synthesis on the basis of 4-(aryldiazenyl)phenols

Cyclization reactions based on 4-(aryldiazenyl)phenols are a convenient alternative to the approaches based on 5-(aryldiazenyl)salicylic aldehydes discussed above. Despite the exceptional accessibility of phenol-based azo coupling products, the examples of the syntheses of 4H-chromenes based on 4-(aryldiazenyl)phenols are few and are published almost exclusively recently. Thus, derivatives of flavone 7, which have a pronounced antioxidant and antibacterial effect, were obtained by oxidative cyclization of unsaturated ketones 8.33

Intensely colored 4H-benzo[h]chromenes 9 were synthesized by the reaction of aldehydes, active methylene nitriles, and 4-[(4-ethoxyphenyl)diazenyl]-α-naphthol 10.34 Compounds 9 also exhibit pronounced antimicrobial and antitumor activity.

The first examples of the use of 4-(aryldiazenyl)resorcinols 11 in the synthesis of 6-(aryldiazenyl)-4H-chromenes appeared in the literature in 2017.35,36 Products 12 have antibacterial, fungicidal, and anticancer effects.

Further modification of the substituents led to the preparation of azo compounds 13 with an improved pharmacological profile.37

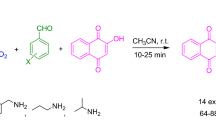
The introduction of a zinc-binding sulfamide fragment into the diazo component and subsequently into the resorcinol derivative led to azosulfonamides/4H-chromenes 14, which are strong inhibitors of class I zinc-dependent histone deacetylases with anticancer activity.38 Compounds 14 have absorption maxima in the range of 387–445 nm and also show antimicrobial activity.


Synthesis on the basis of 4
H
-chromenes

Upon a possibility of modification, 4H-chromenes can also serve as starting compounds for the preparation of 6-(aryldiazenyl)-4H-chromenes. For example, 7-hydroxy-4-(4-hydroxyphenyl)-4H-chromene 15 underwent regioselective azo coupling to form azochromenes 16 which exhibit antioxidant activity.39 Sequential treatment with stearoyl chloride and excess propylene oxide gave colored surfactants 17 suitable for use as antioxidants for lubricating oils.

The preparation of azochromene 18, an analog of sodium cromoglycate 19, an anti-allergic and anti-asthma chromene drug, was described.40 The presence of an azo bridge allows cis/trans photoisomerization and controlled inhibition of mast cell activation (only the cis-form is biologically active). It was noted that the novel photoswitchable inhibitor 18 has a much higher activity than the original dichromene 19. The key step in the preparation of azochromene 18 is the reaction of 6-aminochromene 20 and 6-nitrosochromene 21.


References
Litvinov, Yu. M.; Shestopalov, A. M. In Advances in Heterocyclic Chemistry; Katritzky, A. R., Ed.; Elsevier: New York, 2011, Vol. 103, p. 175.
Patil, S. A.; Patil, R.; Pfeffer, L. M.; Miller, D. D. Future Med. Chem. 2013, 5, 1647.
El-Agrody, A. M.; Afifi, T. H. Heterocycles 2014, 89, 1557.
Sonsona, I. G.; Marqués-López, E.; Herrera, R. P. Symmetry 2015, 7, 1519.
Elnagdi, M. H.; Moustafa, M. S.; Al-Mousawi, S. M.; Mekheimer, R. A.; Sadek, K. U. Mol. Diversity 2015, 19, 625.
Patil, S. A.; Patil, S. A.; Patil, R. Future Med. Chem. 2015, 7, 893.
Costa, M.; Dias, T. A.; Brito, A.; Proença, F. Eur. J. Med. Chem. 2016, 123, 487.
Maleki, B. Org. Prep. Proced. Int. 2016, 48, 81.
Sadek, K. U.; Mekheimer, R. A. H.; Abd-Elmonem, M.; Abdel-Hameed, A.; Elnagdi, M. H. Tetrahedron Asymmetry 2017, 28, 1462.
Mamaghani, M.; Nia, R. H.; Tavakoli, F.; Jahanshahi, P. Curr. Org. Chem. 2018, 22, 1704.
Tashrifi, Z.; Mohammadi-Khanaposhtani, M.; Hamedifar, H.; Larijani, B.; Ansari, S.; Mahdavi, M. Mol. Diversity 2020, 24, 1385.
Raj, V.; Lee, J. Front. Chem. 2020, 8, 623.
Chatterjee, R.; Bhukta, S.; Dandela, R. J. Heterocycl. Chem. 2022, 59, 633.
Krivenko, A. P.; Vasilkova, N. O.; Nikulin, A. V.; Sorokin, V. V. ChemChemTech 2022, 65(9), 13.
Nawaz, A.; Aslam, S.; Ahmad, M.; Zahoor, A. F.; Naqvi, S. A. R. J. Iran. Chem. Soc. 2022, 19, 3721.
Di Martino, M.; Sessa, L.; Di Matteo, M.; Panunzi, B.; Piotto, S.; Concilio, S. Molecules 2022, 27, 5643.
Kaur, H.; Narasimhan, B. Curr. Top. Med. Chem. 2018, 18(1), 3.
Khan, M. N.; Parmar, D. K.; Das, D. Mini-Rev. Med. Chem. 2021, 21, 1071.
Leippe, P.; Frank, J. A. Curr. Opin. Struct. Biol. 2019, 57, 23.
Kumari, R.; Sunil, D.; Ningthoujam, R. S.; Kumar, N. A. Chem.-Biol. Interact. 2019, 307, 91.
Benkhaya, S.; M'rabet, S.; El Harfi, A. Heliyon 2020, 6, e03271.
Crespi, S.; Simeth, N. A.; König, B. Nat. Rev. Chem. 2019, 3, 133.
Ghanavatkar, C. W.; Mishra, V. R.; Sekar, N. Dyes Pigm. 2021, 191, 109367.
Ziarani, G. M.; Moradi, R.; Lashgari, N.; Kruger, H. G. In Metal-Free Synthetic Organic Dyes; Ziarani, G. M.; Moradi, R.; Lashgari, N.; Kruger, H. G., Eds.; Elsevier: New York, 2018, Chapter 4, p. 47.
Arbabi, H. A.; Soltani, S. S.; Salehi, H.; Rezazadeh, S.; Zonouzi, A.; Toosibashi, M. J. Chem. Res. 2018, 42(2), 68.
Fouad, S. A.; Hessein, S. A.; Abbas, S. Y.; Farrag, A. M.; Ammar, Y. A. Croat. Chem. Acta 2018, 91(1), 99.
Bhuvaneswari, K.; Sivaguru, P.; Lalitha, A. J. Chin. Chem. Soc. 2020, 67, 1877.
Korade, S. N.; Patil, J. D.; Gaikwad, D. S.; Sonawane, S. A.; Vibhute, S. P.; Dige, N. C.; Mhaldar, P. M.; Pore, D. M. Org. Prep. Proced. Int. 2020, 52, 147.
Abdolmohammadi, S.; Dahi-Azar, S. J. Heterocycl. Chem. 2021, 58, 2181.
Gaikwad, D. S.; Undale, K. A.; Patravale, A. A.; Choudhari, P. B. Res. Chem. Intermed. 2020, 46, 621.
Korade, S. N.; Mhaldar, P. M.; Kulkarni, P. P.; Rashinkar, G. S.; Pore, D. M. Synth. Commun. 2021, 51, 2336.
Nikpassand, M.; Keyhani, A.; Fekri, L. Z.; Varma, R. S. J. Mol. Struct. 2022, 1251, 132065.
Sharma, P. K.; Bandyopadhyay, P.; Sharma, P.; Kumar, A. Med. Chem. Res. 2014, 23, 3569.
Abd-El-Aziz, A. S.; Alsaggaf, A.; Assirey, E.; Naqvi, A.; Okasha, R. M.; Afifi, T. H.; Hagar, M. Int. J. Mol. Sci. 2021, 22, 2807.
Afifi, T. H.; Okasha, R. M.; Alsherif, H.; Ahmed, H. E. A.; Abd-El-Aziz, A. S. Curr. Org. Synth. 2017, 14(7), 1036.
Afifi, T. H.; Okasha, R. M.; Ahmed, H. E. A.; Ilaš, J.; Saleh, T.; Abd-El-Aziz, A. S. EXCLI J. 2017, 16, 868.
Afifi, T. H.; Riyadh, S. M.; Deawaly, A. A.; Naqvi, A. Med. Chem. Res. 2019, 28, 1471.
Okasha, R. M.; Alsehli, M.; Ihmaid, S.; Althagfan, S. S.; El-Gaby, M. S. A.; Ahmed, H. E. A.; Afifi, T. H. Bioorg. Chem. 2019, 92, 103262.
El-Sayed, R.; Mohamed, K. S.; Fadda, A. A. Afinidad 2018, 75, 581.
Velema, W. A.; van der Toorn, M.; Szymanski, W.; Feringa, B. L. J. Med. Chem. 2013, 56, 4456.
The study was supported financially by the Kuban Science Foundation within the framework of the scientific project H-21.1/15 “Highly functionalized 4H-pyrans: synthesis, properties, and biological activity”.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2022, 58(12), 681–683
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dotsenko, V.V., Varzieva, E.А.  Synthesis of 6-(aryldiazenyl)-4H-chromene derivatives (microreview).
Chem Heterocycl Comp 58, 681–683 (2022). https://doi.org/10.1007/s10593-023-03143-9
Synthesis of 6-(aryldiazenyl)-4H-chromene derivatives (microreview).
Chem Heterocycl Comp 58, 681–683 (2022). https://doi.org/10.1007/s10593-023-03143-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-023-03143-9