Abstract
One-pot, three-component condensation reaction between (phenylsulfonyl)acetonitrile, aromatic aldehydes, and α-naphthol for preparation of 4-(aryl)-3-(phenylsulfonyl)-4H-benzo[h]chromen-2-amine derivatives has been reported. The method involves domino Knoevenagel condensation/Michael addition, and cyclization cascade. The reaction was performed in glycerol, which is a commercially available, inexpensive and non-toxic compound. High purity of the products, very high yields and wide scope of substrates are advantages of this protocol.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Green chemistry is a novel technique of looking at organic molecules to design drugs for pharmaceutical companies [1]. Green, environmentally benign processes offer several important economic advantages over traditional synthetic protocols, conveying high energy, hazardous, and wasteful processes towards the “ideal synthesis” that more useful for the economy, environment and society [2,3,4].
Multi-component reactions (MCRs) are a green approach towards the synthesis of various organic compounds, and for researchers there is a lot of scope to develop varieties of novel multi-component reactions used to synthesize diverse heterocycles, which is a key nucleus for different beneficial drugs [5, 6].
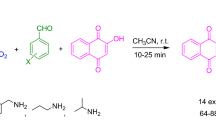
The design of novel and interesting materials with significant biological and pharmaceutical properties is a main target in medicine and drug design research [7, 8]. Chromene compounds represent an attractive medicinal scaffold in drug replacement systems [9,10,11,12,13]. These molecules and their analogs can exhibit significant effects such as antitumor [14], antimicrobial [15], antifungal [16], anticoagulant [17], anti-HIV [18], and anti-inflammatory activities [19]. Lipophilic characters of this class of compounds facilitate their delivery into the cell membrane [20]. Benzo[h]chromenes were designed, synthesized, and evaluated for cytotoxic activity and also exhibited promising cell growth inhibitory activity with ED50 values of 0.01–5.8 lM against all tested tumor cell lines [21]. They showed antitumor activity against a broad range of cancer cell lines with ED50 values of 0.01–76 lM [21]. Chromenes and their derivatives have been synthesized using various approaches, including multi-component reactions (MCRs) green synthesis routes using various techniques [22]. However, most of these methods suffer from such drawbacks as low yields, long reaction times, harsh reaction conditions, tedious work-up procedures, tedious steps for the preparation of catalyst, application of toxic and expensive catalysts, application of hazardous solvents for the work-up and lack of generality. Moreover, in most of the reported methods, catalysts are not recyclable. In this backdrop glycerol has emerged as an attractive solvent. It possesses the benefits of both water and ionic liquids like low toxicity, relatively low vapor pressure, easy availability, reusability, inexpensiveness, renewability, high boiling point and ability to dissolve a wide range of organic and inorganic compounds [23,24,25,26]. Development of catalyst-free reactions is another area that has attracted much attention in green chemistry due to the inherent advantages involved in terms of cost and environment [27, 28]. Literature survey revealed that there is only one report involving a three-component condensation between aldehydes, (phenylsulfonyl)acetonitrile, and α-naphthol [29]. Indeed, to the best of our knowledge, there are no reports on the diversity-oriented, catalyst-free synthesis of a library of this type of biologically active compound. In continuation of our research on multi-component reactions [30, 31], a simple, new, and efficient protocol for the preparation of 4-(aryl)-3-(phenylsulfonyl)-4H-benzo[h]chromen-2-amine derivatives in glycerol media is described for the first time (Scheme 1).
Experimental
General
All reagents were purchased from Merck or Aldrich companies and were used without further purification. All yields refer to separated products after purification. The FT-IR spectra were recorded on a FT-IR spectroscopy Perkin Elmer BX-II. 1H NMR and 13C NMR spectra were recorded on Bruker 300 MHz spectrometer in DMSO-d6 with TMS as an internal standard. Mass spectra were recorded using Agilent Technologies Model: 5975C VL MSD with Tripe-Axis Detector; EI mode at 70 eV. The spectral and analytical data for the selected compounds are presented below. Melting points were determined in open capillaries using a BUCHI510 melting point apparatus. Thin-layer chromatography (TLC) was performed on silica-gel Poly Gram SIL G/UV 254 plates.
General procedure for the asymmetric synthesis of 4-(aryl)-3-(phenylsulfonyl)-4H-benzo[h]chromen-2-amine derivatives
(Phenylsulfonyl)acetonitrile (1 mmol) was added to a round-bottom flask containing glycerol (5 mL) and benzaldehyde (1 mmol). The mixture was stirred at 80 °C. After formation of the cyano-olefin (verified with TLC), α-naphthol (1 mmol) was added to the reaction mixture, and it was allowed to stir until completion (TLC). Then, for the work-up procedure, warm water was added to the reaction mixture, glycerol dissolved and the insoluble solid crude product was separated by simple filtration. The desired solid product was washed by warm water. Experimental observation showed us that pure isolated product was obtained. The filtrate containing glycerol was extracted with methyl t-butyl ether (3 × 10 mL) to remove any organic compounds dissolved in the aqueous phase. The aqueous layer was separated and the water was evaporated under reduced pressure to give pure glycerol which was used for the next run under similar reaction conditions.
4-(4-chlorophenyl)-3-(phenylsulfonyl)-4H-benzo[h]chromen-2-amine (4i)
Solid, yield: 92%, m.p. 223–225 °C; IR (KBr) (ν, cm−1): 3466, 3340, 3060, 1652, 1625, 1261, 1185; 1H NMR (300 MHz, DMSO-d6) (δ, ppm): 8.10 (d, J = 12 Hz, 1H), 7.86 (dd, J = 9 & 6 Hz, 2H), 7.80–7.68 (m, 2H), 7.61–7.20 (m, 9H), 7.09 (t, J = 9 Hz, 2H), 6.99 (t, J = 9 Hz, 1H), 5.45 (s, 1H); 13C NMR (76 MHz, DMSO) (δ, ppm): 158.71, 158.67, 145.26, 144.15, 143.05, 133.03, 132.65, 131.42, 129.52, 129.28, 128.69, 128.10, 127.19, 127.15, 126.22, 126.17, 124.66, 123.16, 121.28, 121.11, 82.70; MS (EI): m/z 447.1.
4-(2,6-dichlorophenyl)-3-(phenylsulfonyl)-4H-benzo[h]chromen-2-amine (4j)
Solid, yield: 93%, m.p. 229–231 °C; IR (KBr) (ν, cm−1): 3448, 3325, 1654, 1625, 1263, 1196; 1H NMR (300 MHz, DMSO-d6) (δ, ppm): 8.31 (d, J = 9 Hz, 1H), 7.84 (d, J = 9 Hz, 1H), 7.68–7.58 (m, 5H), 7.58–7.45 (m, 4H), 7.37 (t, J = 6 Hz, 2H), 7.14 (t, J = 9 Hz, 1H), 7.03–6.88 (m, 2H), 6.11 (s, 1H); 13C NMR (76 MHz, DMSO) (δ, ppm): 158.83, 143.65, 143.46, 137.77, 135.64, 135.47, 133.32, 132.61, 130.60, 129.64, 129.12, 128.98, 128.09, 127.42, 127.24, 125.81, 124.90, 124.52, 122.61, 121.40, 115.79, 78.59, 36.82; MS (EI): m/z 481.1.
4-(2-chlorophenyl)-3-(phenylsulfonyl)-4H-benzo[h]chromen-2-amine (4k)
Solid, yield: 92%, m.p. 206–208 °C; IR (KBr) (ν, cm−1): 3466, 3348, 3059, 1653, 1627, 1262, 1199; 1H NMR (300 MHz, DMSO-d6) (δ, ppm): 8.33 (d, J = 9 Hz, 1H), 7.85 (d, J = 6 Hz, 1H), 7.71 (d, J = 6 Hz, 2H), 7.66–7.47 (m, 6H), 7.39 (t, J = 6 Hz, 2H), 7.27–7.17 (m, 3H), 7.10–7.06 (m, 2H), 5.55 (s, 1H); 13C NMR (76 MHz, DMSO) (δ, ppm): 158.70, 143.73, 142.96, 142.88, 133.14, 132.69, 131.71, 130.87, 129.83, 129.24, 128.62, 128.09, 128.02, 127.30, 127.23, 126.07, 125.26, 124.76, 123.10, 121.36, 119.78, 81.73, 38.19; MS (EI): m/z 447.1.
3-(phenylsulfonyl)-4-(o-tolyl)-4H-benzo[h]chromen-2-amine (4l)
Solid, yield: 90%, m.p. 219–221 °C; IR (KBr) (ν, cm−1): 3462, 3335, 3066, 1654, 1625, 1261, 1189; 1H NMR (300 MHz, DMSO-d6) (δ, ppm): 8.32 (d, J = 9 Hz, 1H), 7.86 (d, J = 9 Hz, 1H), 7.66–7.44 (m, 8H), 7.33 (t, J = 9 Hz, 2H), 7.12 (d, J = 9 Hz, 1H), 7.01–6.85 (m, 4H), 5.33 (s, 1H), 2.46 (s, 3H); 13C NMR (76 MHz, DMSO) (δ, ppm): 158.27, 158.22, 144.28, 144.01, 142.74, 134.81, 132.97, 132.47, 130.73, 129.51, 129.06, 128.07, 127.13, 126.77, 126.69, 126.09, 125.93, 124.56, 123.10, 121.30, 121.08, 83.10, 37.72, 19.67; MS (EI): m/z 427.2.
4-(2-fluorophenyl)-3-(phenylsulfonyl)-4H-benzo[h]chromen-2-amine (4m)
Solid, yield: 91%, m.p. 209–211 °C; IR (KBr) (ν, cm−1): 3446, 3317, 1653, 1627, 1262, 1188; 1H NMR (300 MHz, DMSO-d6) (δ, ppm): 8.32 (d, J = 9 Hz, 1H), 7.85 (d, J = 9.0 Hz, 1H), 7.72 (d, J = 9.0 Hz, 2H), 7.65–7.47 (m, 6H), 7.39 (t, J = 9.0 Hz, 2H), 7.27–7.20 (m, 2H), 7.16–7.08 (m, 1H), 7.01–6.90 (m, 2H), 5.29 (s, 1H); 13C NMR (76 MHz, DMSO) (δ, ppm): δ 161.54, 158.89, 158.30, 143.95, 143.23, 133.10, 132.67, 132.55, 132.38, 130.30, 130.25, 129.27, 129.09, 128.98, 128.10, 127.25, 127.18, 126.04, 125.87, 124.88, 124.69, 123.07, 121.30, 119.64, 115.95, 115.66, 81.04, 35.72; MS (EI): m/z 431.2.
4-(3-chlorophenyl)-3-(phenylsulfonyl)-4H-benzo[h]chromen-2-amine (4n)
Solid, yield: 91%, m.p. 267–269 °C; IR (KBr) (ν, cm−1): 3429, 3325, 3062, 1650, 1622, 1262, 1190; 1H NMR (300 MHz, DMSO-d6) (δ, ppm): 8.30 (d, J = 9.0 Hz, 1H), 7.87 (d, J = 9.0 Hz, 1H), 7.77–7.74 (m, 2H), 7.65–7.49 (m, 6H), 7.45–7.38 (m, 3H), 7.23–7.11 (m, 4H), 5.11 (s, 1H); 13C NMR (76 MHz, DMSO) (δ, ppm): 158.82, 148.54, 144.07, 143.10, 133.40, 133.07, 132.76, 130.73, 129.27, 128.11, 127.42, 127.24, 127.18, 126.80, 126.37, 126.18, 126.13, 124.72, 123.14, 121.30, 120.95, 82.40, 41.05; MS (EI): m/z 447.2.
3-(phenylsulfonyl)-4-(p-tolyl)-4H-benzo[h]chromen-2-amine (4o)
Solid; yield: 90%, m.p. 267–269 °C; IR (KBr) (ν, cm−1): 3457, 3338, 3019, 2917, 1652, 1625, 1260, 1184; 1H NMR (300 MHz, DMSO-d6) (δ, ppm): 8.30 (d, J = 9.0 Hz, 1H), 7.85 (d, J = 9.0 Hz, 1H), 7.73 (d, J = 6.0 Hz, 2H), 7.47 (m, 9H), 7.10 (d, J = 9.0 Hz, 2H), 6.94 (d, J = 6.0 Hz, 2H), 5.01 (s, 1H), 2.18 (s, 3H); 13C NMR (76 MHz, DMSO) (δ, ppm): 158.65, 144.25, 143.35, 142.99, 135.83, 132.92, 132.51, 129.29, 129.20, 128.08, 127.56, 127.05, 127.03, 126.38, 126.17, 124.49, 123.17, 121.87, 121.24, 83.20, 41.18, 20.98; MS (EI): m/z 427.2.
4-(4-nitrophenyl)-3-(phenylsulfonyl)-4H-benzo[h]chromen-2-amine (4p)
Solid, yield: 95%, m.p. 208–210 °C; IR (KBr) (ν, cm−1): 3463, 3337, 3071, 1652, 1627, 1263, 1186; 1H NMR (300 MHz, DMSO-d6) (δ, ppm): 8.33 (d, J = 9.0 Hz, 1H), 8.01 (d, J = 9.0 Hz, 2H), 7.86 (d, J = 6.0 Hz, 1H), 7.78 (d, J = 9.0 Hz, 2H), 7.69–7.44 (m, 8H), 7.47–7.31 (m, 3H), 5.26 (s, 1H); 13C NMR (76 MHz, DMSO) (δ, ppm): 158.76, 153.85, 146.42, 144.01, 143.17, 133.19, 132.79, 129.36, 129.01, 128.13, 127.39, 127.26, 126.18, 126.10, 124.81, 124.10, 123.14, 121.35, 120.10, 82.14, 41.18; MS (EI): m/z 458.2.
Results and discussion
At first, to optimize reaction conditions for the synthesis of 4-(aryl)-3-(phenylsulfonyl)-4H-benzo[h]chromen-2-amine, the pilot reaction was subjected using (phenylsulfonyl)acetonitrile, α-naphthol and benzaldehyde (reaction model). In a model reaction, a mixture of (phenylsulfonyl)acetonitrile (1 mmol), α-naphthol (1 mmol) and benzaldehyde (1 mmol) was stirred in different amounts of glycerol and also at various temperatures (Tables 1, 2). To find the best efficiency, we carried out this reaction in different common solvents such as acetonitrile, DMF, toluene, DMSO, and neat conditions at 80 °C; no product was observed (Table 3, entries 1, 2, 3, 4 and 7). Only a trace amount of products was detected in ethanol and water (Table 3, entries 5 and 6). In summary, the best results were obtained in glycerin (5 mL) at 80 °C in 1.5 h, and glycerol shows higher reactivity than other solvents because of multiple hydrogen bonds.

With these results in hand, three-component condensation of substituted aromatic aldehydes, (phenylsulfonyl)acetonitrile, and α-naphthol was investigated under the optimized reaction conditions for preparation of 4-(aryl)-3-(phenylsulfonyl)-4H-benzo[h]chromen-2-amines (Table 4). The substrate scope of the reaction was then evaluated using a variety of structurally diverse aldehydes. The presence of electron-withdrawing groups relate to electron-donating groups afforded the corresponding products in shorter reaction times with higher yield (Table 4).
The mechanism proposed for preparation of 4-(aryl)-3-(phenylsulfonyl)-4H-benzo[h]chromen-2-amine from benzaldehyde, (phenylsulfonyl)acetonitrile, and α-naphthol in the presence of glycerol is depicted in Scheme 2. According to the literature [32, 33], glycerol activated staring materials and intermediates by hydrogen bonding characters. Benzylidene (phenylsulfonyl)acetonitrile, containing an electron-poor C=C double bond, is formed quantitatively by Knoevenagel addition of (phenylsulfonyl)acetonitrile to the aromatic aldehyde in glycerol media. C-alkylation of α-naphthol occurs by the nucleophilic attack on electron-poor C=C double and gave intermediate (I), which is then cyclized by intramolecular nucleophilic attack of an OH group on the cyano (CN) moiety to give intermediate (II). Subsequent tautomerization produced the 4-(aryl)-3-(phenylsulfonyl)-4H-benzo[h]chromen-2-amine.
We also investigated recycling of the glycerol. Glycerol was dissolved in warm water while the product remained insoluble in water. The solid product was separated by simple filtration. The filtrate containing glycerol was extracted with methyl t-butyl ether (3 × 10 mL) to remove any organic compounds dissolved in the aqueous phase. The aqueous layer was separated and the water was evaporated under reduced pressure to give pure glycerol, which was used for the next run under similar reaction conditions. The recovered glycerol was reused for five runs without significant loss of activity. The findings of this study are shown in Fig. 1.
Conclusions
In summary, an efficient protocol for the one-pot preparation of 4-(aryl)-3-(phenylsulfonyl)-4H-benzo[h]chromen-2-amine derivatives via three-component reaction of aromatic aldehydes, (phenylsulfonyl)acetonitrile, and α-naphthol in glycerol media as a commercially available, environmental friendly and reusable compound was described. The reactions were carried out under thermal conditions with short reaction times and produced the corresponding products in good to excellent yields. Also, glycerol could be successfully recovered and recycled for at least five runs without significant loss of activity. The one-pot nature and the use of glycerol as an environmentally and eco-friendly material make it an interesting alternative towards diversity-oriented synthesis of novel 4-(aryl)-3-(phenylsulfonyl)-4H-benzo[h]chromen-2-amine derivatives.
References
H.J. Federsel, Green Chem. 15, 3105 (2013)
R.V.A. Orru, E. Ruijter, Synthesis of Heterocycles Via Multicomponent Reactions II (Springer, Berlin, 2010)
P.A. Wender, J.M. Kee, J.M. Warrington, Science 320, 649 (2008)
D. Chappell, A.T. Russell, Org. Biomol. Chem. 4, 4409 (2006)
J. Zhu, H. Bienayme (eds.), Multicomponent Reactions (Wiley-VCH, Wienheim, 2005)
D.J. Ramon, Y. Miguel, Angew. Chem. Int. Ed. 44, 1602 (2005)
J.N. Denis, A.E. Greene, D. Gu’enard, P. Potier, J. Am. Chem. Soc. 110, 5917 (1988)
R.A. Holton, R.J. Biediger, P.D. Boatman, M. Suffness, Semisynthesis of Taxol and Taxotere, Taxol: Science and Applications (CRC Press, Boca Raton, 1995), p. 97
M. Curini, G. Cravotto, F. Epifano, G. Giannone, Curr. Med. Chem. 13, 199 (2006)
R.D.H. Murray, J. Mendez, R.A. Brown, The Natural Coumarins (Wiley, New York, 1982)
K.C. Fylaktaki-dou, D.J. Hadjipavlou-Litina, K.E. Litinas, D.N. Nicolaides, Curr. Pharm. Des. 10, 3813 (2004)
J.R.S. Hoult, M. Paya, Gen. Pharmacol. 27, 713 (1996)
E. Melliou, P. Magiatis, S. Mitaku, A.L. Skaltsounis, E. Chinou, I.J. Chinou, Nat. Prod. 68, 78 (2005)
A.M. El-Agrody, A.M. Fouda, E.S.A.E.H. Khattab, Med. Chem. Res. 26, 691 (2017)
P.N. Kalaria, S.P. Satasia, D.K. Raval, New J. Chem. 38, 1512 (2014)
H. Xu, X. Zeng, Bioorg. Med. Chem. Lett. 20, 4193 (2010)
Y. Abrouki, A. Anouzla, H. Loukili, A. Chakir, M. Idrissi, A. Abrouki, A. Rayadh, M. Zahouily, K. Kacemi, J. Bessiere, B. Marouf, S. Sebti, Am. J. Biol. Chem. Pharm. Sci. 1, 28 (2013)
H.M. Kasralikar, S.C. Jadhavar, S.R. Bhusare, Bioorg. Med. Chem. Lett. 25, 3882 (2015)
S.T. Chung, W.H. Huang, C.K. Huang, F.C. Liu, R.Y. Huang, C.C. Wu, A.R. Lee, Res. Chem. Intermed. 42, 1195 (2016)
K.C. Nicolaou, J.A. Pfefferkorn, A.J. Roecker, G.Q. Cao, S. Barluenga, H.J. Mitchell, J. Am. Chem. Soc. 122, 9939 (2000)
Y. Dong, K. Nakagawa-Goto, C.Y. Lai, S.L. Morris-Natschke, K.F. Bastow, K.H. Lee, Bioorg. Med. Chem. 20, 4085 (2010)
K.U. Sadek, R.A.H. Mekheimer, M. Abd-Elmonem, A. Abdel-Hameed, M.H. Elnagdi, Tetrahedron Asymmetry 28, 1462 (2017)
S. Zhu, Y. Zhu, S. Hao, H. Zheng, T. Mo, Y. Li, Green Chem. 14, 2607 (2012)
J.I. Garcia, H. Garcia-Marín, E. Pires, Green Chem. 16, 1007 (2014)
Y. Gu, F. Jerome, Green Chem. 12, 1127 (2010)
C. Vidal, J. Garcia-Alvarez, Green Chem. 16, 3515 (2014)
H. Yang, W. Hu, S. Deng, T. Wu, H. Cen, Y. Chen, D. Zhang, B. Wang, New J. Chem. 39, 5912 (2015)
J. Liu, M. Lei, L. Hu, Green Chem. 14, 2534 (2012)
K.S. Pandit, R.V. Kupwade, P.V. Chavan, U.V. Desai, P.P. Wadgaonkar, K.M. Kodam, ACS Sustain Chem. Eng. 4, 3450 (2016)
Z. Arabpoor, H.R. Shaterian, RSC Adv. 6, 44459 (2016)
H.R. Shateria, S. Noura, RSC Adv. 4, 60543 (2014)
S. Singh, M. Saquib, M. Singh, J. Tiwari, F. Tufail, J. Singh, J. Singh, New J. Chem. 40, 63 (2016)
H.R. Shaterian, M. Mohammadnia, Res. Chem. Intermed. 41, 1301 (2015)
Acknowledgements
We gratefully appreciate the University of Sistan and Baluchestan Research Councils for the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Morshedi, A., Shaterian, H.R. Green approach to synthesis of novel and broad-range diversity of 4-(aryl)-3-(phenylsulfonyl)-4H-benzo[h]chromen-2-amine derivatives. Res Chem Intermed 44, 7219–7230 (2018). https://doi.org/10.1007/s11164-018-3552-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-018-3552-4