Abstract
In Iraq, like most developing countries, attempts are being made to synthesize new compounds with several pharmacological properties. (E)-2-(3-(2-imino-1-methylimidazolidin-4-ylidene)-1-methylguanidino)acetic acid (L) has been synthesized and used as a ligand for the formation of Cr(III), Co(II), Ni(II), and Cu(II) complexes. The chemical structures of synthesized compounds were characterized using different spectroscopic methods. All chelates except Ni(II) chelate are found to be octahedral structures, Ni(II) chelate was square planar. The stability for the prepared complexes was studied theoretically using density function theory. The total energy for the complexes was calculated and it was shown that the copper complex is the most stable one. Ligand and complexes were tested against selected types of microbial organisms and showed significant activities. The free-radical scavenging activity of ligand and metal complexes have been determined by their interaction with the stable free-radical DPPH and all the compounds have shown encouraging antioxidant activities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schiff base compounds have been used as fine chemicals and medical substrates [1]. Schiff bases are well known for their biological applications including antibacterial, antifungal, anti-inflammatory, and antitumor activity [2]. Schiff bases are important intermediates for the synthesis of some bioactive compounds such as β-lactams [3], and employed as ligands for the complexation of metal ions [4–7]. Antioxidants are extensively studied for their capacity to protect organisms and cells from damage that is induced by oxidative stress. Scientists in many different disciplines became more interested in new compounds, either synthesized or obtained from natural sources that could provide active components to prevent or reduce the impact of oxidative stress on cells [8]. Bioorganometallic chemistry is dedicated to the study of metallic complexes as well as their biological applications, to designing new drugs offering better performance than those already known. Molecules have been designed for the treatment of cancer [9], Alzheimer’s [10], neurodegenerative diseases [11], as therapeutic and diagnosis agents [12], microbicide [13], chelators [14]. Schiff base metal complexes have a key role in the development of coordination chemistry, resulting in an enormous number of publications, ranging from pure synthetic work to modern physicochemical and biochemically relevant studies of metal complexes [15]. The synthesis of the coordination compounds of the Schiff’s base ligands having N,S-donor binding sites has attracted considerable attention because of their potential biological activities [16]. The main features of these compounds are their preparative accessibility, diversity, structural variability, and versatile coordinating properties. These compounds have also been widely investigated to examine the effect of metalation on the antipathogenic activities of such ligand systems. These compounds have been found to be more effective when they are administered as metal complexes [17]. Many hundreds of neutral complexes and complex ions containing imidazoles have been prepared and characterized. Imidazoles are particularly interesting ligands in bioinorganic and metallic supramolecular [18] chemistry. Creatine is synthesized endogenously and is stored in skeletal muscle in a high-energy phosphorylated form. During muscle contraction, creatine, and creatine phosphate are spontaneously converted to creatinine. Creatinine is eliminated from the body by renal excretion at a relatively constant rate, making it a clinically chosen measurement to show renal function and a forensically chosen measurement to detect dilute urine and potentially adulterated specimens [19]. Some nitrogen heterocyclic compounds were reported to be antiparkinsonian [20], anticancer [21], antimicrobial [22]. Recently, density functional theory (DFT) has been accepted by the quantum chemistry community as a cost-effective approach for the computation of molecular structure, vibration frequencies, and energies of chemical reactions. Many studies have shown that molecular structures and vibration frequencies calculated by DFT methods are more reliable than MP2 methods [23]. While there is sufficient evidence that DFT provides an accurate description of the electronic and structural properties of solids, interfaces, and small molecules, relatively little is known about the symmetric performance of DFT applications to molecular associates. The preparation of a (E)-2-(3-(2-imino-1-methylimidazolidin-4-ylidene)-1-methylguanidino)acetic acid, used as a ligand for the formation of Cr(III), Co(II), Ni(II), and Cu(II) complexes, is presented in this study. The chemical structures of the newly synthesized complexes were confirmed. The microbial activities of all synthesized compounds and their in vitro antioxidant activities were also investigated. It was envisaged that these two active pharmacological molecules, (creatine and creatinine) if linked together would generate novel molecular templates, which are likely to exhibit interesting biological properties.
Results and discussion
Synthesis of the ligand was done by the reflux of creatine with creatinin. The structure of ligand was confirmed from its spectral data (Scheme 1).
The complexes were synthesized by reaction of the ligand with the metal ions in 2:1 molar ratio in ethanolic medium. The ligand behaves as bidentate coordinate through oxygen and nitrogen donor atoms. All the complexes are colored, stable towards air and moisture, and decompose at high temperatures. The molecular weights were determined cryoscopically in freezing DMF, which show them to be monomeric in nature (Table 1).
The IR bands are shown in Table 2.
-
1.
C=O stretching vibration: the band at 1,725 cm−1 of the ligand due to C=O stretching decreased to 1,687–1,705 cm−1 on complexation.
-
2.
C=N stretching vibration: the band at 1,589 cm−1 in the spectrum of the ligand due to ν(C=N) stretching decreased to 1,576 cm−1 or increased to 1,596 cm−1 on complexation.
-
3.
The low-frequency bands of complexes: new bands which appeared at low frequencies in the spectra of the synthesized complexes were probably due to (metal–nitrogen) and (metal–oxygen) bond vibration frequencies. On the basis of the above evidence, there is a good indication of complex formation.
Electronic absorption spectrum
Generally, the bands of the newly synthesized complexes are either shifted to shorter or longer wavelengths than that of ligand, but the high intensity of the bands is an indication for complex formation. The origin of the band observed about 500–700 nm in the electronic spectra of complexes has been identified as d–d transition. In these complexes, the bands observed at 300–400 nm could be assigned to nitrogen–metal charge transfer absorption. The electronic absorption bands for the ligand and complexes are classified into two distinct groups: those that belong with ligand transitions appeared in the UV region while d–d transitions appeared in the visible region. These transitions are assigned in relevance to the structures of complexes.
The UV–Vis spectrum of ligand in DMF shows two distinct peaks at 275 and 320 nm (36,363, 31,250 cm−1), which were assignable to π → π* and n → π* transitions. The Cr(III) complex showed magnetic moments 3.37 BM, which supports the high-spin octahedral geometry. The Cr(III) complex showed three bands with the absorbance maxima at 610 nm (16,393 cm−1), 505 nm (19,801 cm−1), and 315 nm (31,746 cm−1) which were considered as respectively 4A2g(F) → 4T2g(F), 4A2g(F) → 4T1g(F), 4A2g(F) → 4T1g(P) absorption bands. These transitions suggest an octahedral geometry for the Cr(III) complex. The magnetic susceptibility measurement after diamagnetic corrections yielded a magnetic moment of 4.70 BM, which is close to that expected for an octahedral Co(II) complex. The high-spin octahedral Co(II) complex showed two spin allowed 555 nm (18,018 cm−1), and 445 nm (22,471 cm−1) assignable to 4T1g(F) → 4A2g(F) and 4T1g 4(F)→ T1g(P) transitions, respectively, are in agreement with octahedral arrangements for Co(II) ion. The appearance of a band at 575 nm (17,391 cm−1) due to 1A1g → 1A2g transition favors the square planar structure for the Ni(II) complex. The complex has a room-temperature magnetic moment of 1.39 BM, which corresponds to distorted octahedral structure for the Cu(II) ion. Only one broad band is observed at 505 nm (19,801 cm−1) in the electronic spectrum of the Cu(II) complex assigned to 2Eg → 2T2g transition, which is in conformity with octahedral geometry (Table 3).
The parameters β, B and Dq have been evaluated from the electronic spectral absorptions of the complexes. The β values are found to be <1.0, indicating that the M–L bond is covalent.
Stability study
The stability for the prepared complexes was studied theoretically by the density function theory (DFT). The total energy for the complexes was calculated and it was shown that the copper complex is the most stable and the chromium complex is the least stable, as follows: Cu-complex > Ni-complex > Co-complex > Cr-complex.
Density function theory
Density function theory calculations were performed for ligand (energy; −41.20951849 Hartree). Optimized molecular structures of the most stable forms are shown in Fig. 1. Molecular orbital calculations provide a detailed description of orbitals including spatial characteristics, nodal patterns, and individual atom contributions. The contour plots of the frontier orbitals for the ground state of ligand is shown in Fig. 2, including the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO). It is interesting to see that both orbitals are substantially distributed over the conjugation plane. It can be seen that the HOMO orbitals are located on the substituted molecule while LUMO orbitals resemble those obtained for the unsubstituted molecule.
Stereo-suggested structures of complexes
Antibacterial activity
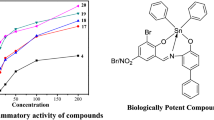
The antibacterial screening data show that the ligand and metal complexes exhibit antibacterial properties, and note that the metal chelates exhibit more inhibitory affects than the parent ligand (Figs. 4, 5, 6, 7, and 8). The increased activity of the metal chelates can be explained because of chelation theory. It is known that chelation tends to make the ligand act as more powerful and potent bactericidal agents, thus killing more of the bacteria than the ligand. It is observed that, in a complex, the positive charge of the metal is partly shared with the donor atoms present in the ligand, and there may be π-electron delocalization over the whole chelating [24]. This increases the lipophilic character of the metal chelate and favors its permeation through the lipoid layer of the bacterial membranes. The increased lipophilic character of these complexes seems to be responsible for their enhanced potent antibacterial activity. It may be suggested that these complexes deactivate various cellular enzymes, which play a vital role in various metabolic pathways of these microorganisms. It has also been proposed that the ultimate action of the toxicant is the denaturation of one or more proteins of the cell, which, as a result, impairs normal cellular processes. There are other factors which also increase the activity, which are solubility, conductivity, and bond length among the metal and the ligand.
As a result from the study of antibacterial of the prepared metal complexes (Figs. 4, 5, 6 and 7), the following conclusions may be stated:
-
1.
The results of antibacterial activity study for the (E)-2-(3-(2-imino-1-methylimidazolidin-4-ylidene)-1-methylguanidino)acetic acid showed that the new ligand exhibited antibacterial activity against the studied bacteria at low and high concentration.
-
2.
The study of antibacterial activity revealed that the ligand exhibited a greater activity against the studied bacteria E. coli, and Klebsiella.
-
3.
Generally, the result of prepared complexes exhibited that antibacterial activity toward E. coli bacteria and Klebsiella was more than the complexes inhibition on Staphylococcus aureus (Figs. 4, 5, 6, 7, 8).
Antifungal activity of ligand and metal complexes was between 10 and 100 μg mL−1, with high potency noted for complex C4. Also, good antifungal activity was observed for C2, the ligand showed lower potency against tested fungal strain (Fig. 9).
Radical scavenging activity
2,2″-diphenyl-1-picrylhydrazyl (DPPH) is a stable free-radical and accepts an electron or hydrogen radical to become a stable diamagnetic molecule [25]. DPPH is a stable free radical containing an odd electron in its structure and usually used for detection of the radical scavenging activity in chemical analysis [26]. The reduction capability of DPPH radicals was determined by decrease in its absorbance at 517 nm induced by antioxidants. The absorption maximum of a stable DPPH radical in ethanol was at 517 nm. With this method, it is possible to determine the antiradical power of an antioxidant by measurement of the decrease in the absorbance of DPPH at 517 nm. Resulting from a color change from purple to yellow, the absorbance decreased when the DPPH is scavenged by an antioxidant, through donation of hydrogen to form a stable DPPH molecule. In the radical form, this molecule shows an absorbance at 517 nm, which disappeared after acceptance of an electron or hydrogen radical from an antioxidant compound to become a stable diamagnetic spin-paired molecule [27]. The graph was plotted with percentage scavenging effects on the y-axis and concentration (μg/mL) on the x-axis. The scavenging ability of the ligand and metal complexes was compared with ascorbic acid as a standard. The ligand and metal complexes showed good activities as a radical scavenger compared with ascorbic acid. There are literature reports of metallic complexes where the ligand has the antioxidant activity and it is expected that the metal moiety will increase its activity [28].
In this study, the ligand displayed better scavenging ability than the complexes. This may be due to the presence of free amino and hydroxyl groups (Fig. 10).
Conclusions
In this study, (E)-2-(3-(2-imino-1-methylimidazolidin-4-ylidene)-1-methylguanidino)acetic acid (L) was synthesized by the reaction of creatinine and creatine, the structurally modified of creatinine and creatine naturally occurring compounds make them potential dietary antioxidants. Cr(III), Co(II), Ni(II), and Cu(II) complexes of L have been successfully synthesized and characterized by using various spectroscopic methods, elemental analysis, magnetic moment, and molar conductance studies. The synthesized complexes were tested for antioxidant and antimicrobial activities. Out of these complexes, Cu(II) indicated significant antimicrobial activities as compared to either Cr(III) or Ni(II). In addition, the Cu(II) complex is also found to be a superior antioxidant complex as compared to ascorbic acid.
Experimental
All chemicals used were of reagent grade (supplied by Sigma-Aldrich, Merck, and Fluka) and used as supplied. FT-IR spectra were recorded using shimadzu-8300 spectrophotometer using KBr (and CsI discs in the range 4,000–200 cm−1). Electronic spectra were recorded using shimadzu UV–Vis spectrophotometer type 160 Å in the range 200–800 nm. 1H-NMR spectra were recorded on a Bruker-DPX 300-MHz spectrometer. Elemental microanalysis was carried out using C.H.N elemental analyzer model 5500-Carlo Erba instrument (Italy). Furthermore, magnetic susceptibility was measured by Bruker Magnetic M60. Conductivity measurements of the complexes in DMF solution (10−3 M) were done using a Siemens digital conductivity meter.
Synthesis of ligand
A mixture of hot ethanolic solution of creatine (2.62 g, 0.02 mol) (2-(1-methylguanidino)acetic acid) and creatinine (2.26 g, 0.02 mol) (2-imino-1-methylimidazolidin-4-one) were refluxed with stirring for 3 h, then added a few drops of concentrated HCl, then reflux for 20 h. The completion of the reaction was confirmed by the TLC. The reaction mass was degassed on a rotatory evaporator, over a water bath. The ligand was (L) filtered, washed with cold ethanol, and dried under vacuum over P4O10. IR (cm−1) in KBr, 1725 (C=O), 1,589 (C=N)), 3,200 (O–H) and 3,425 (NH2); 1H-NMR (dichloromethane) [3.1 s (2H) for CH2 (C-6); 2.7 s (3H) for CH3 (C-5); 3.7 s (3H) for CH3 (C-13); the CH2 protons (C-8) were observed down field as a singlet (2H) at 4.61 ppm, and d (2H) at 4.71 and 4.73; 9.5 for NH (N-12); 7.9 for OH (O-15), 5.8 for NH (N-1)]. Anal. Calcd for C8H14N6O2, formula weight: 226.23576: C, 42.47; H, 6.24; N, 37.15. Found: C, 41.98; H, 5.89; N, 36.82.
Study of complex formation in solution
Complexes of L with metal ions were studied, to determine (M:L) ratio in the complex following molar ratio method. A series of solutions were prepared having a constant concentration 10−3 M of metal ion and L. The [M/L] ratio was determined from the relationship among absorption of the absorbed light and mole ratio of [M/L].
Synthesis of metal complexes
Bis(E)-2-(3-(2-imino-1-methylimidazolidin-4-ylidene)-1-methylguanidino)acetic acid) metal complexes were obtained by refluxing the mixture of corresponding metal chloride (0.005 mol) (CrCl3·6H2O, CoCl2·6H2O, NiCl2·6H2O, and CuCl2·2H2O) (1 mmol) and 2 mmol of the ligand in 50 mL of ethanol until the complexes precipitated out. The colored complexes were filtered, washed with water, ethanol, and dried under vacuum. The results of complex formation are listed in Table 1.
Antibacterial activities
The in vitro antibacterial screening effects of the ligand and complexes were evaluated against species of Gram-positive bacteria (S. aureus) and four Gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumonia, and Proteus vulgaris) by disc diffusion method [6] using nutrient agar medium. All test microorganisms were collected from the Biotechnology division, Department of Applied Science, University of Technology. The identity of all the strains was confirmed. The bacteria were sub-cultured in the agar medium and were incubated for 24 h at 37 °C. The discs, having a diameter of 4 mm, were soaked in the test solutions (sterile filter paper discs (Whatman’s No. 1.6 mm in diameter) were soaked with the appropriate equivalent amount of the ligand and metal complexes dissolved in sterile dimethylformamide (DMF) at concentrations of 1–10 mg/disc) and were placed on an appropriate medium previously seeded with organisms in Petri plates and stored in an incubator at the above-mentioned period of time. The inhibition zone around each disc was measured and the results have been recorded as inhibition zones (diameter, mm) to clarify any affect of DMF on the biological screening. Separate studies were carried out with solutions alone of DMF and they showed no activity against any microbial strains. The stock solutions (1 mg/mL) of the test compounds were prepared in DMF.
Antifungal assay
Antifungal activity was carried out according to Daw et al. [29], and was based on the measure of the inhibition of linear growth mycelia of different mold strains (Aspergillus niger and Candida albicans) in potato dextrose broth medium (PDB). Under aseptic conditions, 1 mL of spore suspension (5 × 106 cfu/mL) of tested fungi was added to 50 mL PDB medium in a 100-mL Erlenmeyer flask. Appropriate volumes of tested ligand and complexes were added to produce concentrations ranging from 10 to 100 μg mL−1. Flasks were incubated at 27 ± 1 °C in the dark for 5 days and then the mycelium was collected on filter paper (5.5 cm in diameter). The filter papers were dried to a constant weight and the level of inhibition, relative to the control flasks was calculated from the following formula:
Evaluation of antioxidant activity
Stock solution (1 mg/mL) was diluted to final concentrations of 20–100 μg/mL. Ethanolic DPPH solution (1 mL, 0.3 mmol) was added to sample solutions in DMSO (3 mL) at different concentrations (50–300 μg/mL) [30]. The mixture was shaken vigorously and allowed to stand at room temperature for 30 min. The absorbance was then measured at 517 nm in a UV–Vis spectrophotometer. The lower absorbance of the reaction mixture indicates higher free-radical scavenging activity. Ethanol was used as the solvent and ascorbic acid as the standard. The DPPH radical scavenger was calculated using the following equation:
where A 0 is the absorbance of the control reaction and A 1 is the absorbance in the presence of the samples or standards A 0 − A 1.
References
Y.Y. Yu, G.L. Zhao, Y.H. Wen, Chin. J. Struct. Chem. 26, 1359–1362 (2007)
M. Nath, P.K. Saini, A. Kumar, J. Organomet. Chem. 625, 1353–1362 (2010)
O.M. Walsh, M.J. Meegan, R.M. Prendergast, T.A. Nakib, Eur. J. Med. Chem. 31, 989–1000 (1996)
A. Venturini, J. Gonzalez, J. Org. Chem. 67, 9089–9092 (2002)
F. Aydogan, N. Öcal, Z. Turgut, C. Yolacan, Bull. Korean Chem. Soc. 22, 476–480 (2001)
A.H. Kadhum, A. Mohamad, A.A. Al-Amiery, M.S. Takriff, Molecules 16, 6969–6984 (2011)
A.A Al-Amiery, K Al-Majedy. H Abdulreazak, H Abood, Synthesis, characterization, theoretical crystal structure and antibacterial activities of some transition metal complexes of the thiosemicarbazone (Z)-2-(pyrrolidin-2-ylidene)hydrazinecarbothioamide. Bioinorg. Chem. Appl. (2011). doi:10.1155/2011/483101
Y.K. Choi, W.S. Kim, K.I. Chung, M.W. Chung, H.P. Nam, Microchem. J. 65, 3 (2000)
H.H. Hussain, G. Babic, T. Durst, J. Wright, M. Flueraru, A. Chichirau, L. Chepelev, J. Org. Chem. 68, 7023 (2003)
S.J. Berners, J. Biol. Inorg. Chem. 12, S7–S52 (2007)
C. Orving, J. Biol. Inorg. Chem. 12, S7–S52 (2007)
R. Crichton, J. Biol. Inorg. Chem. 12, S7–S52 (2007)
M.J. Clarke, J. Biol. Inorg. Chem. 12, S7–S52 (2007)
I.C. Mendes, J.P. Moreira, J.D. Ardisson, D.S.R. Gouvêa, P.R.O. Da Silva, I. Garcia, A. Castiñeiras, H. Beraldo, Eur. J. Med. Chem. 43, 1454–1461 (2008)
P. Bernhardt, J. Biol. Inorg. Chem. 12, S7–S52 (2007)
R. Silva, M.J.S. Monte, J.M. Gonçalves, É.M.R. Fernandes, J. Chem. Soc. Dalton Trans. 7, 1257–1262 (1997)
M.C. Rodríguez-Argüelles, P. Tourón-Touceda, R. Cao, J. Inorg. Biochem. 103, 35–42 (2009)
S. Adsule, V. Barve, D. Chen, J. Med. Chem. 49, 7242–7246 (2006)
P.J. Steel, Coord. Chem. Rev. 106, 227–265 (1990)
A. Whelton, A.J. Watson, R.C. Rock, C.A. Burtis, E.R. Ashwood, Tietz Textbook of Clinical Chemi s try, 2nd ed edn. (WB Saunders, Philadelphia, 1994), pp. 1513–1575
A.E. Amr, M.H. Abou-Ghalia, Amino Acids 26, 283–289 (2004)
M.F. Brana, J.M. Castellano, M. Mpran, M.J. Perez de Vega, X.D. Gian, C.A. Romerdahl, G. Keihauer, Eur. Med. Chem. 30, 235–239 (1995)
M. Monajjemi, M. Sayadian, K. Zare, A.R. Ilkhani, F. Mollaamin, Computational study of hydrogen bonding on calix [8] arene as nanostructure compound. Inter. J. Phys. Sci. 6(16), 4063–4066 (2011)
B.K. Gudasi, R.V. Shenoy, R.S. Vadavi, M.S. Patil, S.A. Patil, Chem. Pharm. Bull. 53, 1077 (2005)
J.R. Soares, T.C.P. Dinis, A.P. Cunha, L.M. Ameida, Free Rad. Res. 26, 469–478 (1997)
P.D. Duh, Y.Y. Tu, G.C. Yen, Lebn. Wissen. Technol. 32, 269 (1999)
B. Matthaus, J. Agri. Food Chem. 50, 3444–3452 (2002)
S.B. Bukhari, S. Memon, M. Mahroof-Tahir, M.I. Bhanger, Acta Part a-Mol. Biomol. Spec. 71, 1901–1906 (2009)
Z.Y. Daw, G.S. EL-Baroty, A.E. Mahmoud, Chem. Mikrobiol. Technol. Lebensm. 16, 129–135 (1995)
Y. Chen, M. Wong, R. Rosen, C. Ho, J. Agric. Food Chem. 47, 2226–2228 (1999)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Amiery, A.A. Synthesis and antioxidant, antimicrobial evaluation, DFT studies of novel metal complexes derivate from Schiff base. Res Chem Intermed 38, 745–759 (2012). https://doi.org/10.1007/s11164-011-0414-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-011-0414-8