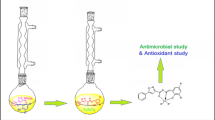
Abstract
A new series of diorganotin(IV) complexes of 4-((2-hydroxybenzylidene)amino)-[1,1′-biphenyl]-3-ol (H2L1), 4-((2-hydroxy-5-nitrobenzylidene)amino)-[1,1′-biphenyl]-3-ol (H2L2), 4-((3-bromo-2-hydroxy-5-nitrobenzylidene)amino)-[1,1′-biphenyl]-3-ol (H2L3) and 4-((3,5-dibromo-2-hydroxybenzylidene)amino)-[1,1′-biphenyl]-3-ol (H2L4) Schiff base ligands with general formula R2SnL (where R = Me, Et, Bu and Ph) were synthesized. Structural aspect of these synthesized compounds have been described with the help of elemental analysis and spectroscopic techniques like FT-IR, UV–Vis, NMR, Mass and fluorescence. Schiff bases coordinated to tin metal as dibasic tridentate ligand through imine nitrogen and two phenolic oxygen atoms forming pentacoordinated complexes. Metal complexes gave low molar conductance value describing their non-electrolytic nature. Thermal decomposition of the complexes resulted in the formation of SnO2 as end product. The in vitro antimicrobial activities have been evaluated against Gram positive bacteria, Gram-negative bacteria and two fungal strains. The screening evaluation showed that organotin(IV) complexes have better antimicrobial activity. The free radical scavenging ability of the compounds was invest by in vitro antioxidant assay involving DPPH radical and was found to be moderately good. The anti-inflammatory activity was done by egg albumin method and percentage inhibition of protein denaturation was calculated. By comparing the biological activities of the synthesized compounds it was found that metal complexes were more potent than the free ligands and compounds 16, 20 were having more potential as drugs.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The design of Schiff base ligands and organotin complexes represents a relevant and qualified objective in the development of coordination chemistry. Due to synthetic flexibility, ease of synthesis and very good binding ability of azomethine group, Schiff bases play an important role in inorganic chemistry to form stable complexes with most of the metal ions [1,2,3,4,5,6,7]. Organotin compounds have been exploited widely in drug design and development due to their continuous increase in the field of pharmaceuticals, industrials, biological, analytical and medicinal areas [8,9,10,11,12]. Recently, the study of organotin complexes derived from Schiff bases has stemmed from their antimicrobial, antihelminthic, antitumour, antituberculorsis, nematicidal, insecticidal activities [12,13,14,15,16,17,18,19,20]. Several organotin compounds have been found to exhibit potent anti-inflammatory activity and also used to relieve the oxidative stress of body by scavenging the free radicals [21, 22]. The biochemical action of organotin(IV) complexes were influenced by coordination number of metal atom, geometry, oxidation states, thermodynamic and kinetic stability of complexes. It is well known that tin metal form stable bond with the carbon as well as with heteroatoms and also exits in different coordination number from four to eight [23, 24]. The presence of different organic groups attached to tin atom influences the fat solubility of compounds and plays an essential role in the transportation of these compounds to specific sites in biological system [25].
Recently, a flourishing body of literature has highlighted the dynamic nature of antimicrobial resistance in pathogens. To conquer the problem of microbial resistance to antibiotics, the finding of active Schiff base derivatives has attracted considerable interest in the coordination chemistry. In view of this, and our continued interest towards the design and development of biologically active materials [26,27,28], the present article describes the synthesis of diorganotin(IV) complexes of Schiff bases containing salicylaldehyde derivatives and 4-amino-[1,1ʹ-biphenyl]-3-ol. The structure of synthesised compounds were elucidated using elemental analysis, FT-IR, 1H, 13C, 119Sn NMR, UV–Vis, mass spectroscopic studies. Further, antimicrobial activity of synthetic compounds was evaluated. Antioxidant efficiency and anti-inflammatory activity were demonstrated using by calculating their IC50 values.
Experimental
Materials
All chemicals and solvents used were of analytical grade. Salicylaldehyde, 5-nitrosalicylaldehyde, 3-bromo-5-nitrosalicylaldehyde, 3,5-dibromosalicylaldehyde and diorganotin dichloride were obtained from Sigma-Aldrich Company. Solvents were used after drying with standard procedure [29] and safety was taken to maintain chemicals, glass apparatus and synthesized compounds free from any type of moisture.
Instrumentation
The elemental analyses of the synthesized compounds were carried out on Perkin Elmer 2400 elemental analyzer. Tin content in the complexes was determined gravimetrically as SnO2 after decomposition of complexes with concentrated HNO3. FT-IR spectra were recorded using a Shimadzu IR affinity-I 8000 FT-IR spectrophotometer at 400–4000 cm−1 by using KBr pressed pellets in which powdered KBr and solid samples were mixed. NMR spectra (1H, 13C and 119Sn) of ligands and complexes were recorded on Bruker Avance II 400 MHz NMR spectrometer using tetramethylsilane and tetramethyltin as internal reference in DMSO-d6 and CDCl3 and chemical shifts (δ) and coupling constants (J) were in ppm and Hz. The melting of compounds were recorded on an electrical heating coil apparatus. Electronic spectra were recorded in DMSO on UV–Vis–NIR Varian Cary—5000 spectrometer at room temperature. Fluorescence spectra were recorded on SHIMADZU RF-5301PC spectrometer in DMSO and CHCl3. Molar conductance was measured in DMF using conductivity bridge type model- 306 Systronic at room temperature. Thermal gravimetric analysis (TGA) was carried out by using EXSTAR TG/DTG 6300 at a heating rate of 10 °C/min under high purity nitrogen atmosphere. Mass spectra were recorded in methanol solvent on SCIEX-QTOF Mass spectrometer at room temperature.
Synthesis of Schiff base ligands (1–4)
The Schiff base ligands were prepared by refluxing 30 mL methanolic solution of salicylaldehyde/5-nitro salicylaldehyde/3-bromo-5-nitrosalicylaldehyde/3,5-dibromosalicylaldehyde (0.5 mL/0.835 g/1.230 g/1.395 g, 5 mmol) and 4-amino-[1,1ʹ-biphenyl]-3-ol (0.925 g, 5 mmol) by adding 2–3 drops of glacial acetic acid (for activating the carbonyl carbon and to ease the attack of nucleophile) for about 1–2 h at room temperature. The completion of reaction was checked by thin layer chromatography (TLC).The solid product obtained was filtered, dried and recrystallised by using hot methanol to give pure product.
-
1.
4-((2-hydroxybenzylidene)amino)-[1,1ʹ-biphenyl]-3-ol, H2L1—Yield: (87%), Red solid; M.p.: 160–162οC, Conductivity: (ohm−1 cm2 mol−1) in DMF: 12.78, Mol.Wt: 289.33 Anal. Found. for C19H15NO2: C, 78.87; H, 5.23; N, 4.84. Calc.: C, 78.83; H, 5.27; N, 4.79. 1H NMR [400 MHz, CDCl3δ(ppm)]: 15.05 (s, 1H, C6′–OH), 12.29 (s, 1H, C2-OH), 8.79 (s, 1H,–N=C–H), 7.61–7.58 (2H, m, C3,6–Ar–H), 7.48–7.43 (5H, m, C2″-6″- Ar–H), 7.38–7.34 (2H, m, C5′,6′–Ar–H), 7.13–7.07 (2H, m, C4,5–Ar–H), 7.04–7.00 (1H, m, C2′ –Ar–H): 13C NMR [400 MHz, DMSO, δ(ppm)]: 160.63(HC=N), 149.35 (C–OH), 140.44, 134.58, 133.90, 132.83, 128.85, 128.62, 127.44, 127.08, 126.80, 119.67, 119.29, 117.33, 117.10, 116.20 (aromatic carbon). FT-IR (v, cm−1): 3550 (O–H, br), 1650 (C=N, m).
-
2.
4-((2-hydroxy-5-nitrobenzylidene)amino)-[1,1ʹ-biphenyl]-3-ol, H2L2—Yield: (89%), Yellowish red solid; M.p.: 166–168οC, Conductivity: (ohm−1 cm2 mol−1) in DMF: 12.50, Mol. Wt. —334.10, Anal. Found. for C19H14N2O4: C, 68.26; H, 4.22; N, 8.38; Calc.; C, 68.20; H, 4.25; N, 8.40;. 1H NMR [400 MHz, DMSO-d6δ(ppm)]: 15.71 (s, 1H, C6′–OH), 10.57 (s, 1H, C2-OH), 9.492 (s, 1H,–N=C–H), 8.62–8.61 (1H, d, C6–Ar–H, 4JHH = 3 Hz), 8.20–8.17 (1H, dd, C4–Ar–H, Jotho, meta = 9.44 Hz, 3 Hz), 7.9599–7.9545 (1H, d, C2′–Ar–H, 4JHH = 2.16 Hz), 7.72–7.07 (2H, m, C2″,6″–Ar–H), 7.58–7.55 (1H, dd, C4′–Ar–H, Jotho, meta= 8.48 Hz, 2.2 Hz), 7.49–7.45 (2H, m, C3″, 5″–Ar–H), 7.37–7.33 (1H, m, C4″–Ar–H), 7.12–7.09 (1H, d, C5′–Ar–H, 3JHH= 8.44 Hz), 6.93–6.91 (1H, d, C3-Ar–H, 3JHH= 9.48 Hz): 13C NMR [400 MHz, DMSO-d6, δ(ppm)]: 160.28 (HC=N), 152.98 (C- 5), 150.56 (C–OH), 139.78, 137.30, 132.48, 131.01, 130.60, 129.35, 127.91,127.52, 126.69, 121.37, 121.20, 121.11, 117.51, 117.32 116.98 (aromatic carbon). FT-IR (v, cm−1): 3357 (O–H, br), 1659 (C=N, m).
-
3.
4-((3-bromo-2-hydroxy-5-nitrobenzylidene)amino)-[1,1ʹ-biphenyl]-3-ol, H2L3—Yield: (95%), Yellowish red solid; M.p.: 178–182οC, Conductivity: (ohm−1 cm2 mol−1) in DMF: 12, Mol. Wt.—412.01, Anal. Found. for C19H13BrN2O4: C, 55.23; H, 3.17; Br, 19.34; N, 6.78; Calc.; C, 55.25; H, 3.18; N, 6.78; 1H NMR [400 MHz, DMSO-d6δ(ppm)]: 15.77 (s, 1H, C6′ –OH), 11.18 (s, 1H, C2-OH), 9.708 (s, 1H,–N=C–H), 8.60–8.59 (1H, d, C4–Ar–H, 4JHH= 2.96 Hz), 8.48–8.47 (1H, d, C6–Ar–H, 4JHH = 2.96 Hz), 8.166–8.161 (1H, d, C2′–Ar–H, 4JHH = 2.04 Hz), 7.75–7.72 (2H, d, C2″, 6″–Ar–H, 3JHH = 8.44 Hz), 7.65–7.63 (1H, dd, C4′–Ar–H, Jortho, meta= 8.52 Hz, 2.1 Hz), 7.52–7.48 (2H, m, C3″, 5″–Ar–H), 7.39–7.36 (1H, t, C4″–Ar–H, 3JHH = 7.4 Hz), 7.18–7.16 (1H, d, C5′–Ar–H, 3JHH = 8.52 Hz): 13C NMR [400 MHz, DMSO, δ(ppm)]: 160.17 (HC=N), 159.86 (C-5), 153.34 (C-3), 152.15 (C–OH), 149.33,146.25, 145.62, 138.72, 133.48, 132.96, 129.39, 127.77, 126.73, 118.41, 117.48, 116.73 (aromatic carbon). FT-IR (v, cm−1): 3360 (O–H, br), 1660 (C=N, m).
-
4.
4-((3,5-dibromo-2-hydroxybenzylidene)amino)-[1,1ʹ-biphenyl]-3-ol, H2L4—Yield: (94%), red solid; M.p.: 175–182οC, Conductivity: (ohm−1 cm2 mol−1) in DMF: 12.09, Mol. Wt.—444.93, Anal. found for C19H13Br2NO2: C, 51.04; H, 2.93; N, 3.13; Found; C, 51.14; H, 2.92; N, 3.10;. 1H NMR [400 MHz, DMSO-d6δ(ppm)]: 15.62 (s, 1H, C6′ -OH), 10.41 (s, 1H, C2-OH), 9.24 (s, 1H, –N=C–H), 7.9079–7.9018 (1H, d, C4-Ar–H, 4JHH = 2.44 Hz), 7.87–7.86 (1H, d, C2′–Ar–H, 4JHH = 2.12 Hz), 7.7967–7.7906 (1H, d, C6–Ar–H, 4JHH = 2.44 Hz), 7.71–7.69 (2H, m, C5′, 4′–Ar–H), 7.55–7.52 (1H, m, C6″–Ar–H), 7.48–7.44 (t, 2H, C3″, 5″–Ar–H, 3JHH = 7.48 Hz), 7.36–7.32 (1H, t, C4″–Ar–H, 3JHH = 7.3 Hz), 7.11–7.09 (1H, d, C2″–Ar–H, 3JHH = 8.48 Hz): 13C NMR [400 MHz, DMSO–d6, δ(ppm)]: 161.42 (HC=N), 161.39 (C-3,5), 150.99 (C–OH),139.89, 138.17, 134.57, 132.40, 131.89, 129.32, 127.71, 127.43, 126.68, 120.39, 117.62, 117.54, 113.96, 107.62 (aromatic carbon). FT-IR (v, cm−1): 3556 (O–H, br), 1684 (C=N, m).
Synthesis of diorganotin(IV) complexes (5–20)
Schiff base ligand H2L1 (0.28 g, 1 mmol) and triethylamine (0.278 mL, 2 mmol) were mixed and stirred in dried tetrahydrofuran (20 mL) for 30 min. Dimethyltin dichloride (2.19 g, 1 mmol) was added and resulting mixture was refluxed for 6 h. The Et3NHCl salt formed was filtered and solvent was evaporated on rotary evaporator under reduced pressure to get red colored product. The product was recrystallized from dry hexane and resulting compound was dried. The other tin complexes were synthesized by reacting the suitable amount of ligands, H2L2/H2L3/H2L4 with appropriate amount of R2SnCl2 in 1:1 molar ratio according to the above described procedure.
-
5.
Me2SnL1: Yield: (60%) red solid; M.p.: 140–145οC, Conductivity: (ohm−1 cm2 mol−1) in DMF: 12, Mol. Wt.—437.04, Anal. Found for C21H19NO2Sn: C, 57.84; H, 4.39; N, 3.21; Sn, 27.22. Calcd. C, 57.80; H, 4.29; N, 3.20; Sn, 27.25 1H NMR [400 MHz, CDCl3, δ(ppm)]: 8.78 (s, 1H, –N=C–H), 7.60–7.61 (d, 1H, Ar–H, 4JHH= 1.30 Hz), 7.58–7.59 (m, 2H, Ar–H), 7.49–7.46 (m, 3H, Ar–H), 7.44–7.43 (m, 1H, Ar–H), 7.36–7.31 (m, 2H, Ar–H), 6.94–6.92 (d, 1H, Ar–H, 3JHH = 8.48 Hz), 6.83–6.78 (m, 2H, Ar–H), 0.83 (s, 6H, Me). 13C NMR [400 MHz, CDCl3. δ(ppm)]: 150.81 (HC=N), 132.96 (C–OH), 7.01 (Me). 119Sn NMR [400 MHz, CDCl3, δ(ppm)]: −126.32. FT-IR (v, cm−1): 1600 (C=N, m), 480 (Sn-N), 601 (Sn–O).
-
6.
Et2SnL1: Yield: (63%) red solid; M.p.: 130–135οC, Conductivity: (ohm−1 cm2 mol−1) in DMF: 12, Mol. Wt.- 453.04, Anal. Found for C23H23NO2Sn: C, 59.52; H, 4.99; N, 3.02; Sn, 25.88. calcd. C, 59.55; H, 4.96; N, 3.07; Sn, 25.92. 1H NMR [400 MHz, CDCl3, δ(ppm)]: 8.74 (s, 1H, –N=C–H), 7.61–7.62 (d, 1H, Ar–H, 4JHH = 1.30 Hz), 7.58–7.59 (d, 1H, Ar–H, 4JHH = 2.14 Hz), 7.49–7.46 (m, 3H, Ar–H), 7.44–7.43 (m, 1H, Ar–H), 7.36–7.31 (m, 2H, Ar–H), 6.94–6.92 (d, 1H, Ar–H, 3JHH = 8.48 Hz), 6.83–6.78 (m, 2H, Ar–H), 1.40–1.36 (m, 4H), 0.88–0.90 (t, 6H, 3JHH = 7.32 Hz). 13C NMR [400 MHz, CDCl3. δ(ppm)]: 151.65 (HC=N), 143.06 (C–OH), 44.29 (Et-C), 7.59 (Et-C). 119Sn NMR [400 MHz, CDCl3, δ(ppm)]: −156.04. FT-IR (v, cm−1): 1607 (C=N, m), 467 (Sn–N), 651 (Sn–O).
-
7.
Bu2SnL1: Yield: (55%) red solid; M.p.: 142–147οC, Conductivity: (ohm−1 cm2 mol−1) in DMF: 13, Mol. Wt.—521.14, Anal. Found for C27H31NO2Sn: C, 62.33; H, 6.01; N, 2.69; Sn, 22.82. calcd. C, 62.35; H, 6.07; N, 2.68; Sn, 22.83. 1H NMR [400 MHz, CDCl3, δ(ppm)]: 8.77 (s, 1H, –N=C–H), 7.61- 7.56 (m, 3H, Ar–H), 7.48–7.40 (m, 5H, Ar–H), 7.35–7.31 (m, 1H, Ar–H), 6.95–6.93 (d, 1H, Ar–H, 3JHH= 8.48 Hz), 6.84–6.82 (d, 1H, Ar–H, 3JHH = 8.45 Hz), 6.79–6.75 (m, 1H, Ar–H), 1.55–151 (m, 4H), 1.40–1.31 (m, 7H), 0.89–0.85 (t, 7H, 3JHH = 7.32 Hz). 13C NMR [400 MHz, CDCl3. δ(ppm)]: 159.65 (HC=N), 146.40 (C–OH), 89.16 (Bu–C), 56.50 (Bu–C), 25.16 (Bu–C), 8.70 (Bu–C). 119Sn NMR [400 MHz, CDCl3, δ(ppm)]: −208.49. FT-IR (v, cm−1): 1606 (C=N, m), 487 (Sn–N), 608 (Sn–O).
-
8.
Ph2SnL1: Yield: (67%) red solid; M.p.: 154–157οC, Conductivity: (ohm−1 cm2 mol−1) in DMF: 12.80, Mol. Wt.- 561.08, Anal. Found for C31H23NO2Sn: C, 66.46; H, 4.14; N, 2.50; O, 5.71; Sn, 21.19. calcd. C, 66.47; H, 4.16; N, 2.53; Sn, 21.20. 1H NMR [400 MHz, CDCl3, δ(ppm)]: 8.79 (s, 1H, –N=C–H), 7.98–7.96 (m, 4H, Ar–H), 7.58–7.56 (d, 3H, Ar–H, 3JHH = 7.55 Hz), 7.50–7.53 (m, 2H, Ar–H), 7.41–7.46 (m, 8H, Ar–H), 7.30–7.34 (t, 2H, Ar–H, 3JHH = 7.14 Hz)), 7.19–7.21 (d, 1H, Ar–H, 3JHH = 8.43 Hz), 7.16–7.14 (d, 1H, Ar–H, 3JHH = 8.37 Hz), 6.79–6.83 (t, 1H, 3JHH = 7.49 Hz). 13C NMR [400 MHz, CDCl3. δ(ppm)]: 156.84 (HC=N), 141.02 (C–OH), 130–122 (Ph-C). 119Sn NMR [400 MHz, CDCl3, δ(ppm)]: −315.04. FT-IR (v, cm−1): 1603 (C=N, m), 473 (Sn–N), 607 (Sn–O).
-
9.
Me2SnL2: Yield: (70%) red solid; M.p.: 155–157οC, Conductivity: (ohm−1 cm2 mol−1) in DMF: 11, Mol. Wt.—482.03, Anal. Found for C21H18N2O4Sn: C, 52.43; H, 3.77; N, 5.82; Sn, 24.68. calcd. C, 52.42; H, 3.80; N, 5.85; Sn, 24.70. 1H NMR [400 MHz, CDCl3, δ(ppm)]: 8.86 (s, 1H, –N=C–H), 8.09–8.10 (d, 1H, Ar–H, 4JHH = 2.39 Hz), 7.74–7.75 (d, 1H, Ar–H, 4JHH = 2.35 Hz), 7.61–7.58 (m, 4H, Ar–H), 7.540–7.544 (d, 1H, Ar–H, 4JHH = 1.80 Hz), 7.51–7.52 (d, 1H, Ar–H, 4JHH = 1.84 Hz), 7.44–7.48 (t, 3H, Ar–H, 3JHH = 7.64 Hz), 0.94 (s, 6H, Me). 13C NMR [400 MHz, CDCl3. δ(ppm)]: 157.43 (HC=N), 146.23 (C–OH), 8.79 (Me). 119Sn NMR [400 MHz, CDCl3, δ(ppm)]: −136.14. FT-IR (v, cm−1): 1647 (C=N, m), 443 (Sn–N), 667 (Sn–O).
-
10.
Et2SnL2: Yield: (68%) red solid; M.p.: 146–150οC, Conductivity: (ohm−1 cm2 mol−1) in DMF: 12, Mol. Wt. —510.06, Anal. Found for C23H22N2O4Sn: C, 54.26; H, 4.36; N, 5.50; Sn, 23.32. calcd. C, 54.28; H, 4.40; N, 5.53; Sn, 23.37. 1H NMR [400 MHz, CDCl3, δ(ppm)]: 8.83 (s, 1H, –N=C–H), 8.29–8.30 (d, 1H, Ar–H, 4JHH = 2.18 Hz), 7.78–7.79 (d, 1H, Ar–H, 4JHH = 2.14 Hz), 7.60–7.61 (d, 1H, Ar–H, 4JHH = 1.63 Hz), 7.59 (s, 1H, Ar–H), 7.55 (s, 1H, Ar–H), 7.54 (d, 1H, Ar–H, 4JHH = 1.75 Hz), 7.53–7.52 (d, 3H, Ar–H, 4JHH = 1.80 Hz), 7.49–7.46 (t, 1H, Ar–H, 3JHH = 7.30 Hz), 1.53–1.43 (m, 6H, Me), 0.78–0.76 (t, 6H, 3JHH = 7.2 Hz). 13C NMR [400 MHz, CDCl3. δ(ppm)]: 155.75 (HC=N), 147 (C–OH), 44.27 (Et-C), 8.29 (Et-C). 119Sn NMR [400 MHz, CDCl3, δ(ppm)]: −157. FT-IR (v, cm−1): 1645 (C=N, m), 447 (Sn–N), 661 (Sn–O).
-
11.
Bu2SnL2: Yield: (55%) red solid; M.p.: 155–157οC, Conductivity: (ohm−1 cm2 mol−1) in DMF: 12.66, Mol. Wt. − 566.12, Anal. Found For C27H30N2O4Sn: C, 57.37; H, 5.35; N, 4.96; Sn, 21.00. calcd. C, 57.39; H, 5.37; N, 4.99; Sn, 21.07. 1H NMR [400 MHz, CDCl3, δ(ppm)]: 8.86 (s, 1H, –N=C–H), 8.39–8.38 (d, 1H, Ar–H, 4JHH = 2.64 Hz), 8.26–8.22 (dd,1H, Jortho,meta= 9.40, 2.68 Hz), 7.60 (s, 2H, Ar–H), 7.54–7.52 (m, 1H, Ar–H), 7.49–7.45 (t, 3H, Ar–H, 3JHH = 7.59 Hz), 7.37–7.33 (t, 3H, Ar–H, 3JHH = 7.60 Hz), 1.68–1.58 (m, 10H), 0.90–0.86 (t, 8H, 3JHH = 7.31 Hz). 13C NMR [300 MHz, CDCl3. δ(ppm)]: 154.00 (HC=N), 146.32 (C–OH) 46.45 (Bu–C), 32.83 (Bu–C), 28.36 (Bu–C), 9.02 (Bu–C). 119Sn NMR [400 MHz, CDCl3, δ(ppm)]: −237.14. FT-IR (v, cm−1): 1646 (C=N, m), 446 (Sn–N), 670 (Sn–O).
-
12.
Ph2SnL2: Yield: (68%) red solid; M.p.: 155–157οC, Conductivity: (ohm−1 cm2 mol−1) in DMF: 13, Mol. Wt.—606.06 Anal. Found for C31H22N2O4Sn: C, 61.52; H, 3.66; N, 4.63; Sn, 19.61. calcd. C, 61.55; H, 3.69; N, 4.61; Sn, 19.62. 1H NMR [400 MHz, CDCl3, δ(ppm)]: 8.78 (s, 1H, –N=C–H), 8.27–8.08 (m, 6H, Ar–H), 7.70–7.68 (d, 2H, Ar–H, 3JHH = 6.6 Hz), 7.56–7.50 (m, 2H, Ar–H), 6.98 (s, 6H, Ar–H), 6.88–6.86 (d, 1H, Ar–H, 3JHH = 8.2 Hz), 6.79–6.77 (d, 1H, Ar–H, 3JHH = 9.24 Hz), 6.06–6.05 (m, 3H). 13C NMR [400 MHz, CDCl3. δ(ppm)]: 156.70 (HC=N), 145.35 (C–OH). 119Sn NMR [400 MHz, CDCl3, δ(ppm)]: −317. FT-IR (v, cm−1): 1646 (C=N, m), 450 (Sn–N), 636 (Sn–O).
-
13.
Me2SnL3: Yield: (70%) red solid; M.p.: 155–157οC, Conductivity: (ohm−1 cm2 mol−1) in DMF: 11, Mol. Wt.- 555.94, Anal. Found for C21H17BrN2O4Sn: C, 45.04; H, 3.06; N, 5.00; Sn, 21.20. calcd. C, 45.07; H, 3.09; N, 5.00; Sn, 21.22. 1H NMR [400 MHz, CDCl3, δ(ppm)]: 8.90 (s, 1H, –N=C–H), 8.61–8.60 (d, 1H, Ar–H, 4JHH = 2.64 Hz), 8.40–8.39 (d, 1H, Ar–H, 4JHH = 2.68 Hz), 7.65–7.64 (d, 1H, Ar–H, 4JHH = 1.92 Hz), 7.6044–7.6013 (d, 1H, Ar–H, 4JHH = 1.22 Hz), 7.58 (s, 1H, Ar–H), 7.56–7.55 (d, 1H, Ar–H, 4JHH = 1.96 Hz), 7.54–7.53 (d, 1H, Ar–H, 4JHH = 1.96 Hz), 7.48–7.44 (t, 3H, Ar–H, 3JHH = 7.64 Hz), 0.94 (s, 6H, Me). 13C NMR [400 MHz, CDCl3. δ(ppm)]: 160.08 (HC=N), 144.78 (C–OH), 137.51, 8.65 (Me). 119Sn NMR [400 MHz, CDCl3, δ(ppm)]: −140.12. FT-IR (v, cm−1): 1593 (C=N, m), 418 (Sn–N), 705 (Sn–O).
-
14.
Et2SnL3: Yield: (69%) red solid; M.p.: 150–155οC, Conductivity: (ohm−1 cm2 mol−1) in DMF: 12, Mol. Wt.- 588.03, Anal. Found for C23H21BrN2O4Sn: C, 46.98; H, 3.60; N, 4.76; Sn, 20.19.cald. C, 46.99; H, 3.63; N, 4.75; Sn, 20.20. 1H NMR [400 MHz, CDCl3, δ(ppm)]: 8.91 (s, 1H, –N=C–H), 8.61–8.60 (d, 1H, Ar–H, 4JHHJ = 2.67 Hz), 8.38–8.37 (d, 1H, Ar–H, 4JHH = 2.69 Hz), 7.62–7.61 (d, 1H, Ar–H, 4JHH = 1.83 Hz), 7.59 (s, 1H, Ar–H), 7.56–7.55 (d, 1H, Ar–H, 4JHH = 2.00), 7.55–7.54 (d, 1H, Ar–H, 4JHH = 2.00 Hz), 1.37–1.33 (m, 4H), 0.87 (t, 6H, 3JHH = 7.2 Hz). 13C NMR [400 MHz, CDCl3. δ(ppm)]: 162.21 (HC=N), 142 (C–OH), 43.17 (Et–C), 9.02 (Et-C). 119Sn NMR [400 MHz, CDCl3, δ(ppm)]: −169. FT-IR (v, cm−1): 1595 (C=N, m), 420 (Sn–N), 693 (Sn–O).
-
15.
Bu2SnL3: Yield: (70%) red solid; M.p.: 155–157οC, Conductivity: (ohm−1 cm2 mol−1) in DMF: 13, Mol. Wt.—644.03, Anal. Found for C27H29BrN2O4Sn: C, 50.34; H, 4.54; N, 4.35Sn, 18.43. calcd. C, 50.40; H, 4.50; N, 4.33Sn, 18.42. 1H NMR [400 MHz, CDCl3, δ(ppm)]: 8.86 (s, 1H, –N=C–H), 8.62–8.61 (d, 1H, Ar–H, 4JHH = 2.77 Hz), 8.37–8.36 (d, 1H, Ar–H, 4JHH = 2.77 Hz), 7.61–7.60 (d, 2H, Ar–H, 4JHH = 1.69 Hz), 7.58 (s, 1H, Ar–H), 7.56 (d, 1H, Ar–H, 4JHH = 2.07), 7.54–7.53 (d, 1H, Ar–H, 4JHH = 2.00 Hz), 7.49–7.45 (t, 3H, 3JHH = 7.92 Hz), 1.69–1.65 (m,8H), 0.90–0.88 (t, 10H, 3JHH = 7.33 Hz). 13C NMR [400 MHz, CDCl3. δ(ppm)]: 159.52 (HC=N), 140.29 (C–OH), 45.88 (Bu–C), 30.32 (Bu–C), 8.71 (Bu–C). 119Sn NMR [400 MHz, CDCl3, δ(ppm)]: −217. FT-IR (v, cm−1): 1593 (C=N, m), 418 (Sn–N), 667 (Sn–O).
-
16.
Ph2SnL3: Yield: (72%) red solid; M.p.: 155–157οC, Conductivity: (ohm−1 cm2 mol−1) in DMF: 12.70, Mol. Wt.—683.97, Anal. Found for C31H22BrN2O4Sn: C, 54.42; H, 3.09; N, 4.09; Sn, 17.35. calcd. C, 54.40; H, 3.10; N, 4.07; Sn, 17.37. 1H NMR [400 MHz, CDCl3, δ(ppm)]: 8.89 (s, 1H, –N=C–H), 8.68 (s, 1H, Ar–H), 8.37–8.39 (m, 1H, Ar–H), 8.00–7.98 (m, 3H, Ar–H), 7.62–7.61(d, 2H, Ar–H, 3JHH = 6.03 Hz), 7.58–7.56 (d, 3H, Ar–H, 3JHH = 7.25 Hz), 7.47–7.43 (dd, 10H, Ar–H, Jortho= 7.42, 5.80 Hz). 13C NMR [400 MHz, CDCl3. δ(ppm)]: 159.53 (HC=N), 140.23 (C–OH), 129–116 (Ph–C). 119Sn NMR [400 MHz, CDCl3, δ(ppm)]: −324.13. FT-IR (v, cm−1): 1595 (C=N, m), 479 (Sn–N), 700 (Sn–O).
-
17.
Me2SnL4: Yield: (60%) red solid; M.p.: 155–157οC, Conductivity: (ohm−1 cm2 mol−1) in DMF: 12.01, Mol. Wt.—592.86, Anal. Found For C21H17Br2NO2Sn: C, 42.47; H, 2.89; N, 2.36; Sn, 19.99. calcd., 42.45; H, 2.87; N, 2.38; Sn, 20.01. 1H NMR [400 MHz, CDCl3, δ(ppm)]: 8.69 (s, 1H, –N=C–H), 7.83–7.82 (d, 1H, Ar–H, 4JHH = 2.04 Hz), 7.59–7.58 (d, 1H, Ar–H, 4JHH = 1.32 Hz), 7.56 (s, 1H, Ar–H), 7.55 (s, 1H, Ar–H), 7.53–7.52 (d, 1H, Ar–H, 4JHH = 2.04 Hz), 7.51–7.50 (d, 1H, Ar–H, 4JHH = 2.04 Hz), 7.46 (s, 1H, Ar–H), 7.44–7.43 (d, 1H, Ar–H, 4JHH = 2.68 Hz), 7.36–7.33 (t, 1H, Ar–H, 3JHH = 7.28 Hz), 6.95–6.93 (d, 1H, Ar–H, 3JHH = 8.48 Hz) 0.87 (s, 6H, Me). 13C NMR [400 MHz, CDCl3. δ(ppm)]: 158.81 (HC=N), 140.90(C–OH), 9.01 (Me). 119Sn NMR [400 MHz, CDCl3, δ(ppm)]: −127. FT-IR (v, cm−1): 1680 (C=N, m), 471 (Sn–N), 671 (Sn–O).
-
18.
Et2SnL4: Yield: (63%) red solid; M.p.: 153–157οC, Conductivity: (ohm−1 cm2 mol−1) in DMF: 12, Mol. Wt.—621.96, Anal. Found for C23H21Br2NO2Sn: C, 44.42; H, 3.40; N, 2.25; Sn, 19.09. calcd. C, 44.44; H, 3.43; N, 2.27; Sn, 19.10. 1H NMR [400 MHz, CDCl3, δ(ppm)]: 8.68 (s, 1H, –N=C–H), 7.81–7.80 (d, 1H, Ar–H, 4JHH = 2.28 Hz), 7.52 (d, 1H, Ar–H, 4JHH = 1.20 Hz), 7.47 (d, 1H, Ar–H, 4JHH = 1.16 Hz), 7.47–7.44 (m, 2H, Ar–H), 1.36–1.34 (m, 4H), 0.83–0.81 (t, 6H, 3JHH = 7.2 Hz). 13C NMR [400 MHz, CDCl3. δ(ppm)]: 155.56 (HC=N), 140.34 (C–OH), 47.13 (Et-C), 9.26 (Et-C). 119Sn NMR [400 MHz, CDCl3, δ(ppm)]: −165. FT-IR (v, cm−1): 1667 (C=N, m), 465 (Sn–N), 663 (Sn–O).
-
19.
Bu2SnL4: Yield: (55%) red solid; M.p.: 155–157οC, Conductivity: (ohm−1 cm2 mol−1) in DMF: 12.86, Mol. Wt.- 676.96, Anal. Found for C27H29Br2NO2Sn: C, 47.83; H, 4.31; N, 2.07; Sn, 17.51. calcd. C, 47.85; H, 4.30; N, 2.09; Sn, 17.54. 1H NMR [400 MHz, CDCl3, δ(ppm)]: 8.68 (s, 1H, –N=C–H), 7.82–7.81 (d, 1H, Ar–H, 4JHH = 2.48 Hz), 7.595–7.591 (d, 1H, Ar–H, 4JHH = 1.32 Hz), 7.574–7.571 (d, 1H, Ar–H, 4JHH = 1 Hz), 7.55–7.54 (m, 1H, Ar–H), 7.52–7.51 (d, 1H, Ar–H, 4JHH = 2.12), 7.50–7.49 (d, 1H, Ar–H, 4JHH = 2.12 Hz), 7.47 (s, 1H, Ar–H), 7.46 (s, 1H, Ar–H), 7.44 (s, 1H, Ar–H), 7.43–7.42 (d, 1H, Ar–H, 4JHH = 2.48 Hz), 1.71– 1.69 (m,5H, Bu–H), 1.60–1.56 (m, 6H, Bu–H), 0.91–0.90 (t, 7H, 3JHH = 7.32 Hz). 13C NMR [400 MHz, CDCl3. δ(ppm)]: 152.00 (HC=N), 141.14 (C–OH) 51.40 (Bu–C), 43.33 (Bu–C), 25.26 (Bu–C), 9.40 (Bu–C). 119Sn NMR [400 MHz, CDCl3, δ(ppm)]: −254. FT-IR (v, cm−1): 1651 (C=N, m), 462 (Sn–N), 646 (Sn–O).
-
20.
Ph2SnL4: Yield: (67%) red solid; M.p.: 155–157οC, Conductivity: (ohm−1 cm2 mol−1) in DMF: 13, Mol. Wt.- 716.90, Anal. Found for C31H21Br2NO2Sn: C, 51.86; H, 2.95; N, 1.95; Sn, 16.53. calcd. C, 51.88; H, 2.97; N, 1.93; Sn, 16.55. 1H NMR [400 MHz, CDCl3, δ(ppm)]: 8.69(s, 1H, –N=C–H), 8.00–7.99 (m, 4H, Ar–H), 7.91–7.90 (d, 1H, Ar–H, 4JHH = 2.2 Hz), 7.71–7.68 (m, 3H, Ar–H), 7.56 (s, 2H, Ar–H), 7.44–7.42 (m, 10H, Ar–H). 13C NMR [400 MHz, CDCl3. δ(ppm)]: 159.59 (HC=N), 147.53 (C–OH), 129.15–126.59 (Ph–C). 119Sn NMR [400 MHz, CDCl3, δ(ppm)]: −322.89. FT-IR (v, cm−1): 1645 (C=N, m), 474 (Sn–N), 669 (Sn–O).
Pharmacology
Antimicrobial activity
Schiff bases and their diorganotin(IV) complexes were screened for in vitro antibacterial activity against four pathogens viz. two Gram positive bacteria (Staphylococcus aureus (NCIM 5021) and Bacillus subtillus (NCIM 2063); two Gram-negative bacteria (Escherichia coli (MTCC 723) and Pseudomonas aeruginosa (MTCC 7093)) by taking ciprofloxacin as positive and DMSO as a negative control. Agar well diffusion method was performed to calculate the zone of inhibition where bacterial strains were subcultured in the nutrient broth and different concentration of compounds 200, 100, 50 and 25 µg/mL were prepared [30]. Bacterial strains were spread on the agar plate with the help of spreader and 4 mm diameter wells were dug with the help of sterile metallic borer. Above concentration of compounds were introduced into the well with the help of sterilised tips of micropipette and incubated it at 37 °C for 24 h. The zones of inhibition were noted at different concentration in triplicate and compared with the standard drug.
Serial dilution method was used to know the minimum inhibitory concentration (MIC) of synthesized compounds where the same bacteria culture was used and antifungal activity was assessed against fungal strains, namely Aspergillus niger (MTCC 9933) and Candida albicans (MTCC 227) by taking fluconazole as standard drug [30]. For determining MICs, solution of screened compound was prepared in DMSO by mixing 5 mg of compounds in 5 mL of DMSO. Stock solution of 100 µg/mL concentrations was prepared by taking 1 mL of above prepared solution in 9 mL DMSO [31]. 1 mL of this stock solution was added into the test tubes containing 1 mL nutrient broth/PDB for bacterial and fungal stains which were serially diluted up to 3.125 µg/mL concentration. Then, inoculated 1 mL of bacterial/fungal culture with the help of sterilized tip into each serially diluted test tube. The bacterial test tubes were incubated at 37 °C for 24 h and at 31 °C for 7 days in case of fungus. All experiments were performed as triplicate and data reported were the mean values.
Antioxidant assay
The 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity of Schiff base ligands and their diorganotin(IV) complexes (1–20) was determined by measuring the change in absorbance of DPPH at 517 nm using spectrophotometer [32, 33]. The variable concentration of compounds (200, 100, 50, 25, 12.5 µg/mL) was prepared in DMSO and 1 mL of prepared solution was added into the 1 mL solution containing DPPH in DMSO (5 mg in 100 ml) and made the resulting solution up to 3.0 mL with DMSO. The mixture was shaken vigorously and allowed to stand for 30 min in darkness at room temperature. The absorbance of the mixture was measured at 517 nm by taking DPPH with DMSO as blank and percentage of radical scavenging effect was calculated using ascorbic acid as a standard for comparison of activity by the following equation:
where A is absorbance of the blank and B is absorbance of the sample.
The percentage inhibition and IC50 values of the screened compounds were calculated by plotting the graph between the % radical scavenging activity and concentration.
Anti-inflammatory activity
For analyzing anti-inflammatory activity of compounds egg albumin method was used [34, 35] where different concentration of ligands and their diorganotin(IV) complexes (200, 100, 50, 25, 12.5 µg/mL) in DMSO were prepared and 2 mL of resulting solutions were added into the test tube containing 2.8 mL phosphate buffer (pH = 6.4) and 0.2 mL egg albumin. The resulting mixtures were incubated at 37οC ± 2 in a BOD for 15 min and heated for 5 min at 70οC. The absorbance of reaction mixture after cooling was recorded at 660 nm by taking double distilled water as control. The percentage inhibitions of protein denaturation were calculated and compared it with standard sodium diclofenac by using the formula:
where A and B is absorbance of the control and sample.
The IC50 value was calculated and compared with the standard drug.
Results and discussion
Synthetic aspect
In the present investigation, the Schiff base ligands (1–4) were obtained as red solid by the reaction of 4-amino-[1,1ʹ-biphenyl]-3-ol with salicylaldehyde and its derivatives using methanol as a solvent followed by their reaction with dichlorodiorganotin(IV) in the presence of trimethyl amine in dry tetrahydrofuran to form complexes of type R2SnL1−4 (5–20). Complexes obtained (Scheme 1) were red solid formed by evaporation of solvent under high vacuum pressure and homogenenity was checked by TLC. The synthesized complexes were soluble in DMF, DMSO, CHCl3, methanol, ethanol but were insoluble in water. The analytical data expressed in experimental part indicates that the metal to ligand ratio is 1:1 for all the complexes with general formulae R2SnL1−4 and spectroscopic data suggested pentacoordinated geometry for the complexes. The elemental analysis is in good agreement with the calculated results from the empirical formulae. The molar conductivity of the complexes was found to be less than 14 Ohm−1cm2mol−1 suggesting their non electrolytic nature.
Electronic spectra
The UV–Vis spectra of ligands and their diorganotin(IV) complexes gives information regarding the arrangement of ligands around the central tin atom. The electronic spectra of Schiff base ligands exhibit bands at 276 and 403 nm assigned to π–π* and n–π* transition of azomethine group. A red shift in complexes was observed at 280 and 410 nm due to donation of electron pair of nitrogen atom of azomethine group to central tin atom [36]. Some lower intensity band appear at 216 nm due to π–π* transition of benzene ring which remains unaltered on complex formation.
IR spectra
The binding of Schiff base ligands with metal ions were confirmed with the identification and comparing of characteristic vibrational frequencies of ligands and their complexes. In FT-IR spectrum of ligands, a characteristic broad adsorption band was observed at 3350–3357 cm−1 due to the presence of a hydroxyl group in the Schiff base ligands. In diorganotin(IV) complexes this broad absorption band gets disappeared due to the deprotonation and coordination of oxygen atom of phenolic group to the central tin atom. The absorption band assigned for azomethine group ν(C=N) was observed at 1650–1684 cm−1, gets shifted to lower values by 10–50 cm−1 in diorganotin(IV) complexes [37, 38]. On complexation the electron density from the nitrogen of azomethine group gets shifted to tin atom and suggests the coordination of nitrogen to the metallic ion. In all the synthesized complexes some new absorption bands were observed in the regions of 601–673 cm−1 and 426–478 cm−1 assigned to ν(Sn–O) and ν(Sn–N) bond vibration frequencies, respectively. The emergence of these IR bands supports the formation of complexes and are further investigated by 1H, 13C and 119Sn NMR spectroscopic studies [39].
NMR spectra
1H NMR spectra
The formation of organotin(IV) complexes from Schiff base ligands was supported by the 1H NMR study. The 1H NMR spectra were recorded in Bruker Avance II 400 MHz NMR spectrometer in CDCl3 and DMSO-d6. The obtained chemical shifts values were found to be in the expected range. The disappearance of OH signals in the spectra of diorganotin(IV) complexes which were present in the spectra of Schiff base ligands at δ 15.05–15.77 ppm (C6′ –OH) and δ 10.57–12.29 ppm (C2–OH) indicates the deprotonation of phenolic group and involvement of both oxygen of these group in chelation. A signal in the range of δ 8.79–9.70 assigned to azomethine proton (CH=N) was shifted upfield to δ 8.69–8.90 ppm in diorganotin(IV) complexes, thereby confirming its bond formation with metal centre. This Sn–N coordination is further confirmed by tin satellite peaks which is due to 3J(119Sn-1H) spin–spin coupling (J = 39–52 Hz) [40, 41]. The signals due to aromatic protons in the region δ 7.61–8.60 ppm in ligands remains not much affected due to their no direct involvement in complexation. Some new signals appeared in the complexes due to presence of methyl, ethyl, butyl and phenyl protons at δ 0.83–0.94, δ 0.76–1.53, δ 1.71–0.85, δ 6.86–7.46 ppm, respectively [42]. These new signals in spectra confirmed the complexation processes. The coupling constant value for methyl, ethyl, butyl and phenyl complexes gives information of coordination environment of central metal atom. The 2J(1H–119Sn) coupling constant value for methyl, ethyl, butyl complexes are in the range of 72.4–68.3 Hz, and for phenyl complexes 3J(1 H–119Sn) value occurs in the range of 64.4–62.1 Hz which lies in the range of pentacoordinated geometry [43, 44].
13C NMR spectra
In the 13C NMR spectra of ligands, appearance of singlet at δ 159.86–160.40 ppm confirmed the presence of azomethine (–CH=N–) carbon which gets shifted towards lower value in the complexes suggested the participation of azomethine nitrogen atom in complexation as observed in IR and 1H NMR spectra [45, 46]. The signal at δ 149.33–150.99 ppm due to hydroxyl carbon gets shifted to lower value in the complexes proposed its coordination with central tin atom. Aromatic carbon atoms of ligands appeared in the range of δ 107–132 ppm that remained unchanged in the complexes, indicating their non participation in bond formation with central atom. In the spectra of complexes some new signals due to carbon atom attached to tin metal were present at δ 8.06–9.01 due to methyl group, whereas signals at δ 7.26–9.02 and δ 43.17–47.13 due to ethyl group, for n-butyl group at δ 8.70–9.40, 25.16–28.36, 32.83–45.83 and 58.33–46.43 ppm and phenyl group appeared in the range of δ 119–126.07 ppm.
Sn NMR spectra
119Sn NMR spectra were recorded in CDCl3 and spectra displayed one sharp singlet in every complex indicating the presence of a single species. A sharp singlet appeared at δ − 127 to − 140.12 ppm for methyl, − 156 to − 169 ppm for ethyl, − 207 to − 254 ppm for butyl, − 315 to − 322.89 ppm for phenyl complexes, respectively. These chemical shifts indicated the formation of penta coordinated tin complexes [47, 48].
Mass spectra
The mass spectra provides highly valuable information about the molecular weight of compounds. The synthesised Schiff base ligands and their diorganotin(IV) complexes were characterized by mass spectrometry. Figure 1 shows that the molecular ion peaks of compounds are very close to the expected values. The possible molecular ion fragments obtained from the mass spectrum of Schiff base H2L2 are given in Scheme 2 which is measured in positive mode and its MS spectrum is given in Fig. 1a. H2L2 showed a molecular ion peak (parent peak) of maximum intensity at m/z 335 assigned for [M + H] ion which agree with its molecular weight. The molecular ion peak undergoes three major fragmentation pathways with m/z = 316.35, 302.33 and 288.31 by the loss of salicylaldehydic –OH and hydrogen radical ion, both –OH group and third path is by the loss of –NO2 group in the moiety. The m/z = 316.35 undergoes further fragmentation to give a mass peak at m/z = 274.29 by the elimination of nitro group. The m/z = 288.31 undergoes further two cleavage at m/z = 241.00 and 186.09 [49].
In the mass spectra of their diorganotin(IV) complexes, [Ph2SnL1] and [Me2SnL2] shows molecular ion peak at m/z 562.13 and 483 due to [M + H] ion. In [Me2SnL2] complex, the removal of both methyl groups attached to tin metal takes place at m/z 453.38. Fragmentation loss of m/z 453.38 take place at m/z = 335 due to cleavage of metal bond (Scheme 3). All the complexes have the same fragmentation pattern. Some peaks of similar molecular mass appeared as present in ligands which confirm the coordination of ligand moiety with the central tin atom. From the mass spectral data it was evident that Schiff bases form complexes with diorganotin(IV) in 1:1 molar ratio which was same as concluded from the other spectroscopic techniques.
Fluorescence spectra
The fluorescence spectra of the Schiff base ligands and their diorganotin(IV) complexes were recorded in CHCl3 and DMSO with an excitation wavelength of 300 nm. On complexation of ligands some significant changes in intensity, quenching of spectra, appearance of some new bands, or shifting of wavelength in emission bands were observed. Figure 2 represents the fluorescence spectra of Schiff base ligand H2L3 and its dibutyltin complex (Bu2SnL3). Ligand shows the fluorescent emission band at 495 nm whereas on complexation shifting of emission bands at 345 nm take place with increase in intensity of fluorescence spectra resulting in hyperchromic and blue shift. This confirms coordination of Schiff base with tin metal centre. From fluorescent data it was revealed that there is complexation between Schiff base and metal centre and compounds have fluorescence properties which make them to be used in some photochemical applications [50].
Thermal studies
Thermal analysis of the synthesized compounds was carried out in the temperature ranging from 30οC to 500οC and mass loss at particular temperature range was calculated [51]. In TGA curves the decomposition of all the diorganotin(IV) complexes exhibited similar behaviour and occurred in two steps from 130 to 500 οC which indicates that complexes were thermally stable up to 130οC and absence of lattice water molecule in complexes as seen in Fig. 3 [52, 53]. First step of decomposition occurred from 130 to 320 οC with mass loss 29.48%, indicated the removal of alkyl or aryl group attached to tin metal. Similarly in the second step the mass loss of 49.01% at temperature range 330–400 οC take place due to removal of ligand moiety and at last ended with the formation of SnO2 residue [54].
The results from the TG data concluded that the complexes are stable up to 130–140 οC which indicated their non volatile nature and absence lattice water molecule in complexes. The end product of the decomposition was SnO2.
Molar conductance
The molar conductance data of diorganotin(IV) complexes measured in DMF for 1 × 10−3M solution at room temperature are given in “experimental section”. The values fall in the range of 6–14 Ω−1cm2mol−1, which is the expected range for the complexes to behave as non electrolytes [55].
Antimicrobial activity
The main aim of any antimicrobial drug is to inhibit the microbes without any side effects on human. The newly synthesized compounds and standard drugs were evaluated for the in vitro antibacterial and antifungal activity against two Gram positive bacteria (Staphylococcus aureus and Bacillus subtillus); two Gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa) and two fungus (Aspergillus niger, and Candida albicans). Agar well diffusion method was used for calculating the zone of inhibition and serial dilution method was used for calculating the MICs using ciprofloxacin and fluconazole as antibacterial and antifungal agents. DMSO was used as negative control which showed no inhibitory effect for tested bacterial and fungal strains. The graphical representation of activity data is given in Fig. 4 and antimicrobial data is given in Tables 1 and 2.
From the given antimicrobial table it is concluded that:
-
1.
The activity of synthesized compounds is due to the fact that Schiff bases and their organotin complexes possess azomethine (–CH=N) group. Schiff base ligands functions well against the microbes, but after complexation with the tin metal the complexes are found to be more potent in inhibiting the growth of microorganism due to chelation therapy [56]. The process of chelation reduces the polarity of the metal atom by partial sharing of the positive charge by donor atom on the ligand and there is electron delocalisation over the whole chelate ring. This increases the lipophilic character of the metal complexes and favours the impermeability through the lipid bilayer of microbes, blocking the metal binding site of enzymes of the microorganism. [57].
-
2.
Among synthesized Schiff bases, the ligand H2L3 and H2L4 were found to be most active than other ligands for all the tested bacterial and fungal strains. The general activity order of Schiff base ligands was: H2L3 > H2L4 > H2L2 > H2L1 due to the reason that ligands with more electronegative atom were more active which resulted in increase in the lipophilicity of the compounds [58].
-
3.
Diorganotin(IV) complexes (5–20) exhibited enhanced antimicrobial activity than their respective Schiff base ligands. Phenyl derivatives were more potent having maximum zone of inhibition (10–26 mm) and lowest MIC value (0.0111–0.0021 µM/mL) as compared to the other complexes due to the delocalisation of π electron cloud which increases the lipophilicity of central atom and crosses the lipid bilayer of the cell. The general trend of diorganotin(IV) complexes against used strains was: Ph > Bu > Et ≥ Me [59].
-
4.
From the pharmacological data, it was revealed that the compounds were more active against the gram positive bacteria than gram negative bacteria as they have more lipopolysaccharide and lipoprotein contents and they do not penetrate into the cell wall easily [60].
-
5.
Compounds 15, 16, 19, 20 were found to be more potent than the standard drugs and compound 20 (Ph2SnL4) and 16 (Ph2SnL3) were most active complexes with maximum zone of inhibition (23–16 mm) and minimum MIC value (0.0021–0.0043 µM/mL) (Fig. 5). The importance of this activity lies in the fact that these compounds could be used in the treatment of some common diseases caused by these microbes and some hospital acquired infections.
Fig. 5 Microbial growth inhibition tests using agar plate diffusion method. a Compound 18 (at 100 µg/mL) and 20 (at 200 µg/mL) against S. aureusb compound 4 (at 200 µg/mL) and 17 (at 100 µg/mL) against E. coli c compound 16 (at 200 µg/mL) and 15 (at 50 µg/mL) against S. aureusd compound 19 (at 200 µg/mL) and 4 (at 200 µg/mL) against P. aregunoisa. The clear zone in plate shows inhibition of bacteria
Antioxidant activity
Many of the human diseases takes place due to oxidative damage like diabetes, heart disease, cancer, Parkinson, Alzheimer disease etc. so the use of antioxidant substances are necessary which prevent or slow down the process of oxidative damage to our body. In vitro, antioxidant activity is commonly carried out by using DPPH which is a stable free radical with purple colour that become a diamagnetic molecule by accepting an electron or hydrogen radical from other compounds and change in colour takes place which results in decrease in absorbance of 517 nm [61,62,63]. It is evident from Fig. 6 that the percentage scavenging activity of compounds increases with the increase in concentration in the range tested and the Schiff base ligand H2L4 (4) have 30.14% scavenging activity but on complexation scavenging activity increases up to 45.95% at a investigated concentration of 200 µg/mL which may be due to availability of atoms for more hydrogen abstraction. The antioxidant activity data expressed in Table 3 and Fig. 7 with IC50 value revealed that the free ligands have IC50 values in the range of 9.11–6.93 µM due to hydrogen abstraction of OH group which react with DPPH radical to form stable diamagnetic molecule but on complexation the antioxidant activity of compounds increases with IC50 value ranging between 5.82–3.56 µM [64]. On complexation the abstraction of azomethine hydrogen was easy due to its more acidic nature and stabilisation of negative charge due to presence of benzene ring and other electron withdrawing groups. In ligands, the antioxidant activity increases with the increase in the electron withdrawing group so the order followed by ligands was H2L3 > H2L4 > H2L2 > H2L1 and order of antioxidant activity for complexes are diphenyl > dibutyl > diethyl ≥ dimethyl. Compound 15, 16, 19, 20 was most active with lower IC50 value ranging between 4.46 and 3.56 µM. So these complexes have a strong potential to be used as scavenger to eliminate free radicals.
Anti-inflammatory activity
Inflammation is a body response to injury which is characterized by redness, heat, pain and disturbed physiological function. Inflammation is a protective response to tissue injury caused by chemical, physical trauma or any microbial agents. It is response of the body to inactivate the invading organism to remove the irritant and to allow the body for repair of tissues [65]. Inflammatory inhibition of synthesized compounds and reference drug sodium diclofenac was calculated for percentage inhibition of protein denaturation in fresh egg albumin and results are expressed in terms of IC50 values and presented in Table 4, Fig. 8.
The following conclusions were obtained from the data:
-
1.
The Schiff base ligands exhibit a varying degree of the percentage inhibition from 10.34 to 18.68% at 200 µg/mL concentrations and order of inhibition for ligands was: H2L3 > H2L4 > H2L2 > H2L1 which depends on electronegative group attached to the ligands. More is the electronegativity of group, more is percentage inhibition and lower is IC50 value. On complexation with tin metal there is increase in the inhibition percentage from 13.06 to 28.27% with further decrease in IC50 value suggesting more potency of complexes as compared to ligands as anti-inflammatory agents.
-
2.
From the anti-inflammatory data it was revealed that the organic groups attached to the tin metal also influence the activity. Diphenyl diorganotin(IV) complexes are most active having IC50 value in the range 13.06–7.57 µM [66, 67]. The general trend of anti-inflammatory activity for the complexes was: Ph > Bu > Et ≥ Me
-
3.
Anti inflammatory activity is dependent more or less on the concentration of compounds. As the concentration increases there is increase in the inhibition percentage of denaturation but somewhere after the 200 µg/mL concentration there is slight increase in the curve showing saturation (Fig. 9).
-
4.
Compounds 15, 16, 19, 20 have IC50 value very near to the standard drug (6.44 µM). Compound 16 with IC50 value 7.57 µM was most potent anti-inflammatory compound and might be beneficial for the treatment of inflammation related diseases.
-
5.
The exact mechanism of action was not known but according to the proposed mechanism these compounds inhibit the protein denaturation which results in the inhibition of water retention and adema formation. Thus the inhibition of adema formation leads to the inhibition of inflammation [68].
References
N. Galic, Z. Cimerman, V. Tomisic, Spectrochim. Acta A 71, 1274 (2008)
M.H.S.A. Hamid, A.N.A.H. Said, A.H. Mirza, M.R. Karim, Md Arifuzzaman, MdA Ali, P.V. Bernhardt, Inorg. Chim. Acta 453, 742 (2016)
T.S.B. Baul, P. Kehie, A. Duthie, N. Guchhait, N. Raviprakash, R.B. Mokhamatam, S.K. Manna, N. Armata, M. Scopelliti, R. Wang, U. Englert, J. Inorg. Biochem. 168, 76 (2017)
S. Shujah, N. Khalid, S. Ali, Russ. J. Gen. Chem. 87(3), 515 (2017)
S. Nazneen, S. Ali, S. Shahzadi, S. Shujah, Russ. J. Gen. Chem. 87(12), 2970 (2017)
P. Bhatra, J. Sharma, R.A. Sharma, Y. Singh, Appl. Organomet. Chem. 31(7), e3639 (2017)
N. Neelofar, N. Ali, A. Khan, S. Amir, N.A. Khan, M. Bilal, Bull. Chem. Soc. Ethiopia 31(3), 445 (2017)
M. Jain, V. Singh, R.V. Singh, J. Iran. Chem. Soc. 1, 20 (2004)
Z. Asadi, Int. J. Chem. Kin. 43, 247 (2011)
L. Pellerito, L. Nagy, Coord. Chem. Rev. 224, 111 (2002)
S.K. Bharti, S.K. Patel, G. Nath, R. Tilak, S.K. Singh, Trans. Met. Chem. 35, 917 (2010)
N.A. Oztas, G. Yenisehirli, N. Ancınc, S.G. Oztas, Y. Ozcand, Spectrochim. Acta A 72, 929 (2009)
J. Devi, J. Yadav, Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry—Anti-Cancer Agents), 18(3), 335 (2018)
J. Devi, S. Pachwania, Inorg. Chem. Commun. 91, 44 (2018)
R. Joshi, N. Pandey, R. Tilak, S.K. Yadav, H. Mishra, S. Pokharia, Appl. Organomet. Chem. 32(5), e4324 (2018)
F. Shaheen, M. Sirajuddin, S. Ali, Z. Rehman, P.J. Dyson, N. Ali Shah, M.N. Tahir, J. Organomet. Chem. 186, 13 (2018)
J.O. Adeyemi, D.C. Onwudiwe, Molecule 23(10), 2571 (2018)
M. Sankarganesh, J.D. Raja, K. Sakthikumar, R.V. Solomon, J. Rajesh, S. Athimoolam, V. Vijayakumar, Bioorg. Chem. 81, 144 (2018)
M.S. Agiorgiti, A. Evangelou, P. Vezyraki, S.K. Hadjikakou, V. Kalfakakou, I.T. Sanaktsidi, A. Batistatou, J. Zelovitis, Y.V. Simos, V. Ragos, S. Karkabounas, D. Peschos, Med. Chem. Res. 27(4), 1122 (2018)
P.G. Avaji, C.V. Kumar, S.A. Patil, K. Shivananda, C. Nagaraju, Eur. J. Med. Chem. 44, 3552 (2009)
J.O. Adeyemi, D.C. Onwudiwe, A.C. Ekennia, C.P. Anokwuru, N. Nundkumar, M. Singh, E.C. Hosten, Inorg. Chim. Acta 485, 64 (2019)
Q.K. Panhwar, S. Memon, Inorg. Chim. Acta 407, 252 (2013)
M. Carcelli, G. Corazzari, S. Ianelli, G. Pelizzi, C. Solinas, Inorg. Chim. Acta 353, 310 (2003)
M. Nardelli, C. Pelizzi, G. Pelizzi, P. Tarasconi, J. Chem. Soc., Dalton Trans. 0(2), 321 (1985)
M.A. Girasolo, A. Attanzio, P. Sabatino, L. Tesoriere, S. Rubino, G. Stocco, Inorg. Chim. Acta 423(B), 168 (2014)
J. Devi, S. Devi, A. Kumar, Med. Chem. Comm. 5 (2016)
J. Devi, S. Devi, A. Kumar, Heterocyc. Chem. 27(6), 361 (2016)
J. Devi, S. Kumari, S. Devi, R. Malhotra, P. Kumar, B. Narasimhan, Monatsh. für Chem. 146(12), 1995 (2014)
A.I. Vogel, Text Book of Quantitative Chemical Analysis, 5th edn. (Longmans, Edison, 1999)
J. Devi, N. Batra, Spectrochim. Acta A Mol. Biomol. Spectrosc. 135, 710 (2015)
J. Devi, M. Yadav, D. Kumar, L.S. Naik, D.K. Jindal, J. App. Organomet. Chem. 33(2), e4693 (2019)
A. Braca, N.D. Tommasi, L.D. Bari, C. Pizza, M. Politi, I. Morelli, J. Nat. Prod. 64, 892 (2001)
B. Bozin, N.M. Dukic, I. Samojlik, A. Goran, R. Igic, Food Chem. 111, 925 (2008)
S. Chandra, P. Chatterjee, P. Dey, S. Bhattacharya, Asian Pacific J. Trop. Biomed. S178 (2012)
S. Chandra, P. Chatterjee, P. Dey, S. Bhattacharya, J. Adv. Pharm. Edu. Res. 2, 25 (2012)
H.L. Singh, J.B. Singh, K.P. Sharma, Res. Chem. Intermed. 38, 53 (2012)
J. Devi, M. Yadav, A. Kumar, A. Kumar, Chem. pap. (2018)
H.L. Singh, J.B. Singh, S. Bhanuka, Res. Chem. Intermed. 42, 997 (2016)
M. Hong, H.D. Yin, X.Y. Zhang, C. Li, C.H. Yue, S. Cheng, J. Organomet. Chem. 724, 23 (2013)
S. Shujah, Z. Urrehman, S. Ali, N. Khalid, N. Muhammad, M.N. Tahir, J. Organomet. Chem. 696, 2772 (2011)
S. Shujah, S. Ali, N. Khalid, M.J. Alam, S. Ahmad, A. Meetsma, Chem. Pap. 72, 903 (2017)
Yu-Xing Tan, Zhi-Jian Zhang, Yang Liu, Yu. Jiang-Xi, Xiao-Ming Zhu, Dai-Zhi Kuang, Wu-Jiu Jiang, J. Mol. Struct. 1149, 874 (2017)
T.P. Lockhart, W.F. Manders, Inorg. Chem. 25, 892 (1986)
T.P. Lockhart, W.F. Manders, J. Am. Chem. Soc. 109, 7015 (1987)
H.D. Yin, S.W. Chen, L.W. Li, D.Q. Wang, Inorg. Chim. Acta 360, 2215 (2007)
L.S. Mun, M.A. Hapipah, S.K. Shin, A.M. Sri Nurestri, L.K. Mun, Appl. Organomet. Chem. 26, 310–311 (2012)
K. SinghDharampal, Dharampal, V. Parkash, Phosphorus, Sulfur Silicon Relat. Elem. 183, 2784 (2008)
J. Wagler, U. Bohme, E. Brendler, B. Thomas, S. Goutal, H. Mayr, B. Kempf, G.Y. Remennikov, G. Roewer, Inorg. Chim. Acta 358, 4270 (2005)
G.G. Mohamed, C.M. Sharaby, Spectrochim. Acta A 66, 949 (2007)
M. Gulcan, Y. Karatas, S. Isik, G. Ozturk, E. Akbas, E. Sahin, J. Fluoresc. 24, 1679 (2014)
Y. Han-dong, C. Ji-Chun, Q. Yan-Ling, Polyhedron 27, 2157 (2008)
A. Chilwal, P. Malhotra, A.K. Narula, Phosphorus, Sulfur Silicon Relat. Elem. 189, 410 (2014)
S. Mun Lee, H.M.A. Kae, S. Sim, S.N.A. Malek, K.M. Lo, Inorg. Chim. Acta 406, 272 (2013)
K.S. Prasad, L.S. Kumar, M. Prasad, H.D. Revanasiddappa, Bioinorg. Chem. Appl. 2010, 1 (2010)
J. Devi, S. Kumari, S. Asijaa, R. Malhotra, Phosphorus, Sulfur Silicon Relat. Elem. 187, 1409 (2012)
Y. Anjaneyula, R.P. Rao, Synth. React. Inorg. Met. Org. Chem. 16, 257 (1986)
Z.H. Chohan, A. Scozzafava, C.T. Supuran, J. Enzy. Inhib. Med. Chem. 18, 259 (2003)
R. Raman, A. Selvan, J. Coord. Chem. 64, 534 (2011)
A. Chilwal, P. Malhotra, A.K. Narula, Phosphorus, Sulfur Silicon Relat. Elem. 189, 410 (2014)
M.A. Affan, M.A. Salam, F.B. Ahmad, R.B. Hitam, F. White, Polyhedron 33, 19 (2012)
D.B. Shpakovsky, C.N. Banti, E.M. Mukhatova, YuA Gracheva, V.P. Osipova, N.T. Berberova, D.V. Albov, T.A. Antonenko, L.A. Aslanov, E.R. Milaev, S.K. Hadjikakoub, Dalton Trans. 43, 6880 (2014)
S. Jabbar, I. Shahzadi, R. Rehman, H. Iqbal, Q. Ul-Ain, A. Jamil, R. Kousar, S. Ali, S. Shahzadi, M.A. Choudhary, M. Shahid, Q.M. Khan, S.K. Sharma, K. Qanungo, J. Coord. Chem. 65(4), 572 (2012)
A. Ramírez-Jiménez, R. Luna-García, A. Cortés-Lozada, S. Hernández, T.R. írez-Apan, A. Nieto-Camacho, E. Gómez, J. Organomet. Chem. 738, 10 (2013)
M. Sirajuddin, S. Ali, F.A. Shah, M. Ahmad, M.N. Tahir, J. Iran. Chem. Soc. 11, 297 (2014)
F. Diaz-Gonzalez, I. Gonzalez-Alvaro, M.R. Campanero, F. Mollinedo, M.A. del Pozo, C. Munoz, J.P. Pivel, F. Sinchez-Madrid, J. Clin. Invest. 95, 1756 (1995)
M. Nath, P.K. Saini, A. Kumar, J. Organomet. Chem. 695, 1353 (2010)
M. Nath, R. Yadav, G. Eng, T. Nguyen, A. Kumar, J. Organomet. Chem. 577, 1 (1999)
G. A. Kaysen, J. A. Dubin, Hans-Georg M¨Uller, L. Rosales, N. W. Levin, W. E. Mitch, The Hemo Study Group, Kidney International, 65, 1408 (2004)
Acknowledgements
One of the author Ms. Jyoti Yadav is highly thankful to the HSCST Panchkula for the financial support and also thankful to A. P. J. Abdul Kalam Central Instrumentation Laboratory GJUS&T, Hisar for carrying the spectral data and Department of Chemistry, GJUS&T, Hisar for financial support in the form of research project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Devi, J., Yadav, J. & Singh, N. Synthesis, characterisation, in vitro antimicrobial, antioxidant and anti-inflammatory activities of diorganotin(IV) complexes derived from salicylaldehyde Schiff bases. Res Chem Intermed 45, 3943–3968 (2019). https://doi.org/10.1007/s11164-019-03830-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-019-03830-3